Abstract
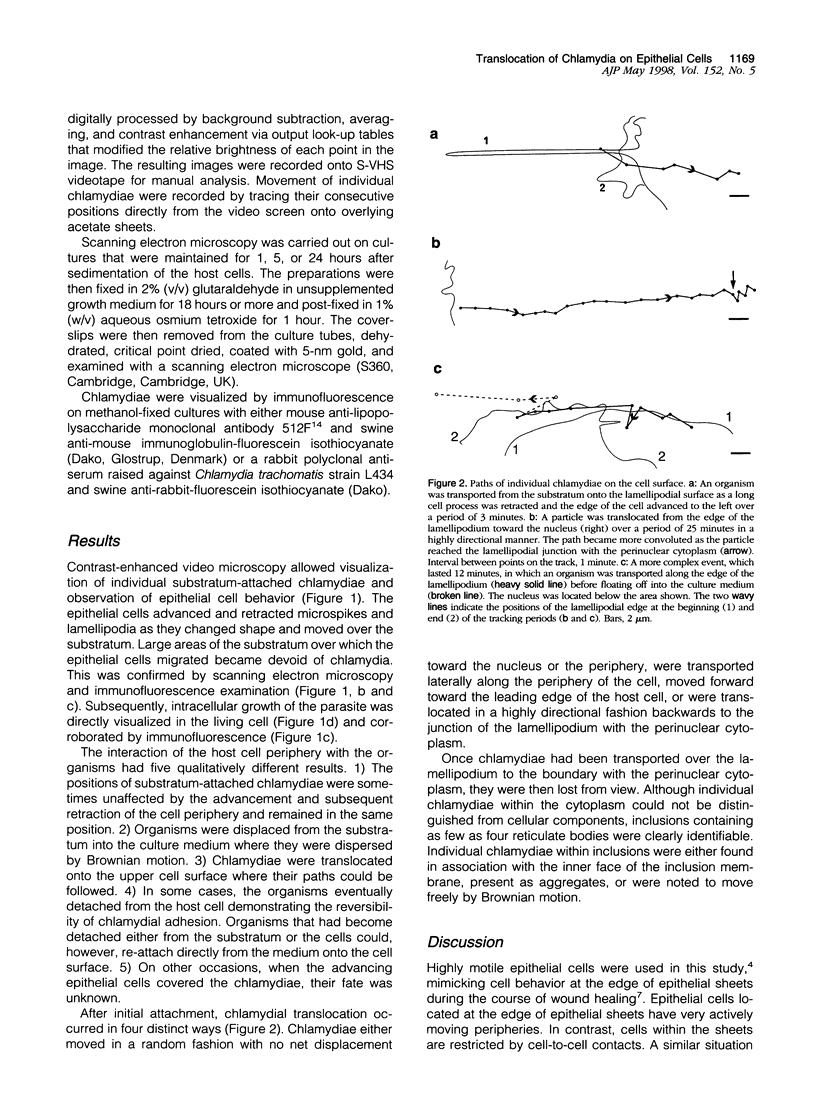
Infection of eukaryotic cells by intracellular pathogens such as chlamydia requires attachment to the host cell surface. Chlamydia are thought to attach to the tips of microvilli in confluent monolayers of polarized cells. In vitro evidence obtained from migrating epithelial cells suggested that during healing the route of pathogen uptake might be different from that in intact epithelia. The small size of infectious chlamydial elementary bodies (approximately 0.3 microm in diameter) has made it difficult, however, to analyze the early stages of pathogen-host cell interaction in living cells by conventional microscopy. Contrast-enhanced video microscopy was therefore used to examine the earliest events of host-pathogen interaction and test the hypothesis that chlamydial uptake into the healing epithelia can involve translocation over the host cell surface. Observations made in this way were validated by scanning and immunofluorescence microscopy. These studies revealed two fates for chlamydiae taken onto the lamellipodial surface: 1) some chlamydiae were moved in a random fashion on the cell surface or were detached into the culture medium, whereas 2) other chlamydiae were translocated across the lamellipodium in a highly directed manner toward the microvillous perinuclear region. After internalization, these latter chlamydiae were found within intracellular inclusions, which demonstrated that this route of attachment and location of uptake resulted in productive growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. The phagokinetic tracks of 3T3 cells. Cell. 1977 Jun;11(2):395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Campbell S., Richmond S. J., Haynes P., Gump D., Yates P., Allen T. D. An in vitro model of Chlamydia trachomatis infection in the regenerative phase of the human endometrial cycle. J Gen Microbiol. 1988 Jul;134(7):2077–2087. doi: 10.1099/00221287-134-7-2077. [DOI] [PubMed] [Google Scholar]
- Campbell S., Richmond S. J., Yates P. S., Storey C. C. Lipopolysaccharide in cells infected by Chlamydia trachomatis. Microbiology. 1994 Aug;140(Pt 8):1995–2002. doi: 10.1099/13500872-140-8-1995. [DOI] [PubMed] [Google Scholar]
- Campbell S., Yates P. S., Richmond S. J. Chlamydia trachomatis infection of cultured motile cells after uptake of chlamydiae from the substratum. J Gen Microbiol. 1993 Sep;139(9):2151–2158. doi: 10.1099/00221287-139-9-2151. [DOI] [PubMed] [Google Scholar]
- Campbell S., Yates P. S., Waters F., Richmond S. J. Purification of Chlamydia trachomatis by a simple and rapid filtration method. J Gen Microbiol. 1991 Jul;137(7):1565–1569. doi: 10.1099/00221287-137-7-1565. [DOI] [PubMed] [Google Scholar]
- Coles A. M., Reynolds D. J., Harper A., Devitt A., Pearce J. H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis? FEMS Microbiol Lett. 1993 Jan 15;106(2):193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- Dembo M., Harris A. K. Motion of particles adhering to the leading lamella of crawling cells. J Cell Biol. 1981 Nov;91(2 Pt 1):528–536. doi: 10.1083/jcb.91.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale A. Locomotory activity of epithelial cells in culture. Exp Cell Res. 1975 Aug;94(1):191–215. doi: 10.1016/0014-4827(75)90545-5. [DOI] [PubMed] [Google Scholar]
- Ferenczy A. Studies on the cytodynamics of human endometrial regeneration. II. Transmission electron microscopy and histochemistry. Am J Obstet Gynecol. 1976 Mar 15;124(6):582–595. doi: 10.1016/0002-9378(76)90059-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997 May 2;276(5313):718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- Garrigues J., Anderson J., Hellström K. E., Hellström I. Anti-tumor antibody BR96 blocks cell migration and binds to a lysosomal membrane glycoprotein on cell surface microspikes and ruffled membranes. J Cell Biol. 1994 Apr;125(1):129–142. doi: 10.1083/jcb.125.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K. Locomotion of tissue culture cells considered in relation to ameboid locomotion. Int Rev Cytol. 1994;150:35–68. doi: 10.1016/s0074-7696(08)61536-3. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik D. F., Elson E. L., Sheetz M. P. Forward transport of glycoproteins on leading lamellipodia in locomoting cells. Nature. 1989 Jul 27;340(6231):315–317. doi: 10.1038/340315a0. [DOI] [PubMed] [Google Scholar]
- Kucik D. F., Kuo S. C., Elson E. L., Sheetz M. P. Preferential attachment of membrane glycoproteins to the cytoskeleton at the leading edge of lamella. J Cell Biol. 1991 Sep;114(5):1029–1036. doi: 10.1083/jcb.114.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov V. I., Gyoeva F. K., Tanaka E., Bershadsky A. D., Vasiliev J. M., Gelfand V. I. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993 Dec;123(6 Pt 2):1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon T., Walker R. A., Pryer N. K. Video-enhanced differential interference contrast light microscopy. Biotechniques. 1989 Jun;7(6):624–633. [PubMed] [Google Scholar]
- Sheetz M. P. Glycoprotein motility and dynamic domains in fluid plasma membranes. Annu Rev Biophys Biomol Struct. 1993;22:417–431. doi: 10.1146/annurev.bb.22.060193.002221. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Choong J., Davis C. H., Knight S. T., Royal M. O., Maslow A. S., Bagnell C. R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989 Aug;57(8):2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



