Abstract
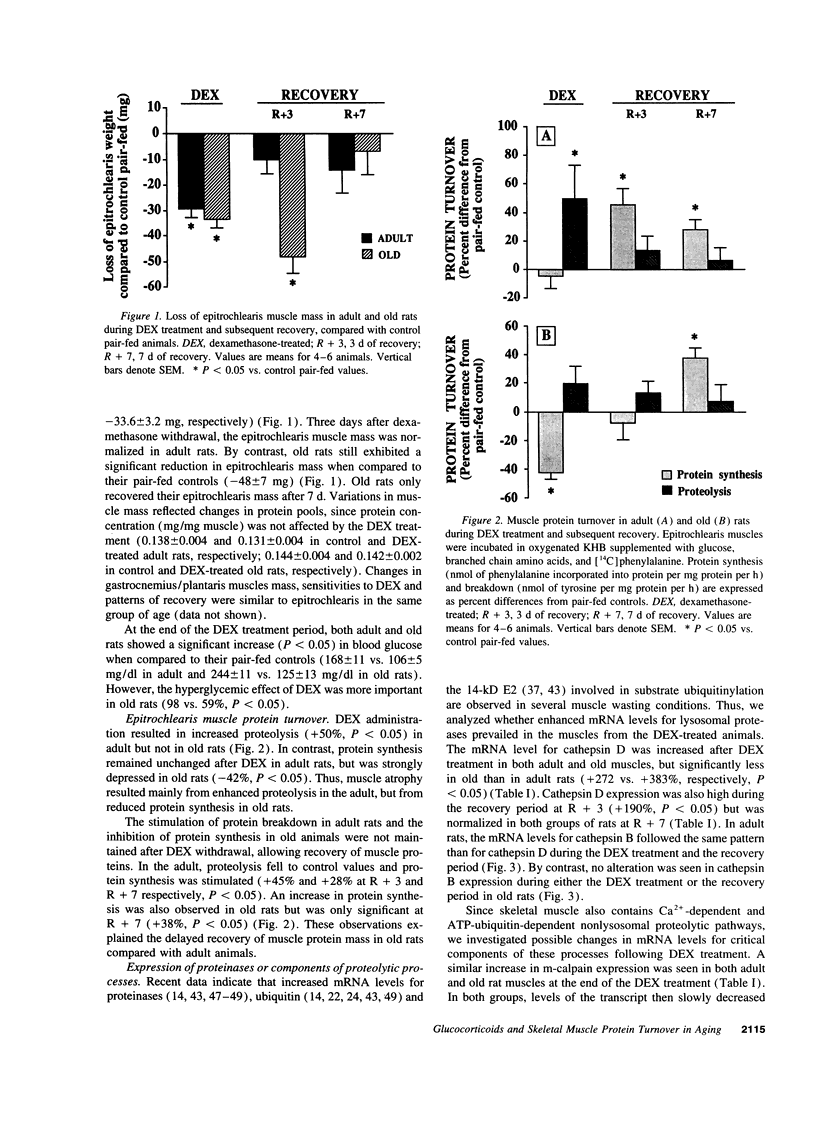
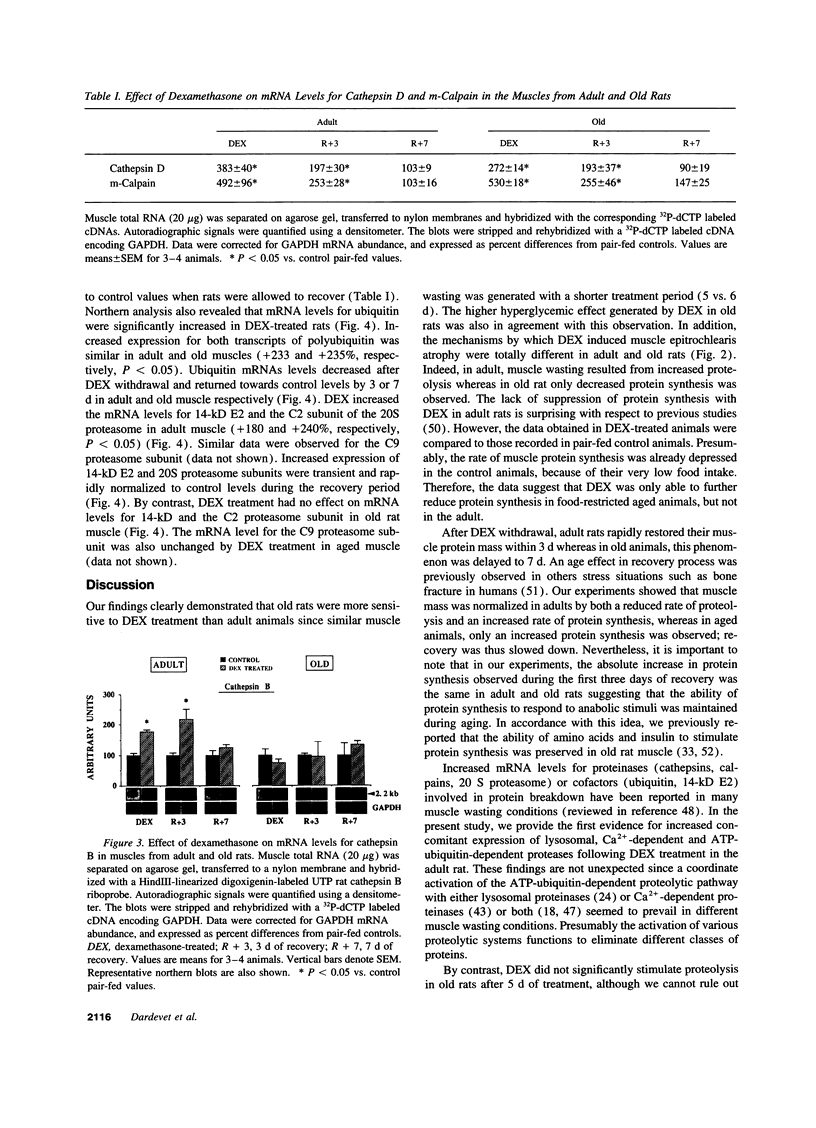
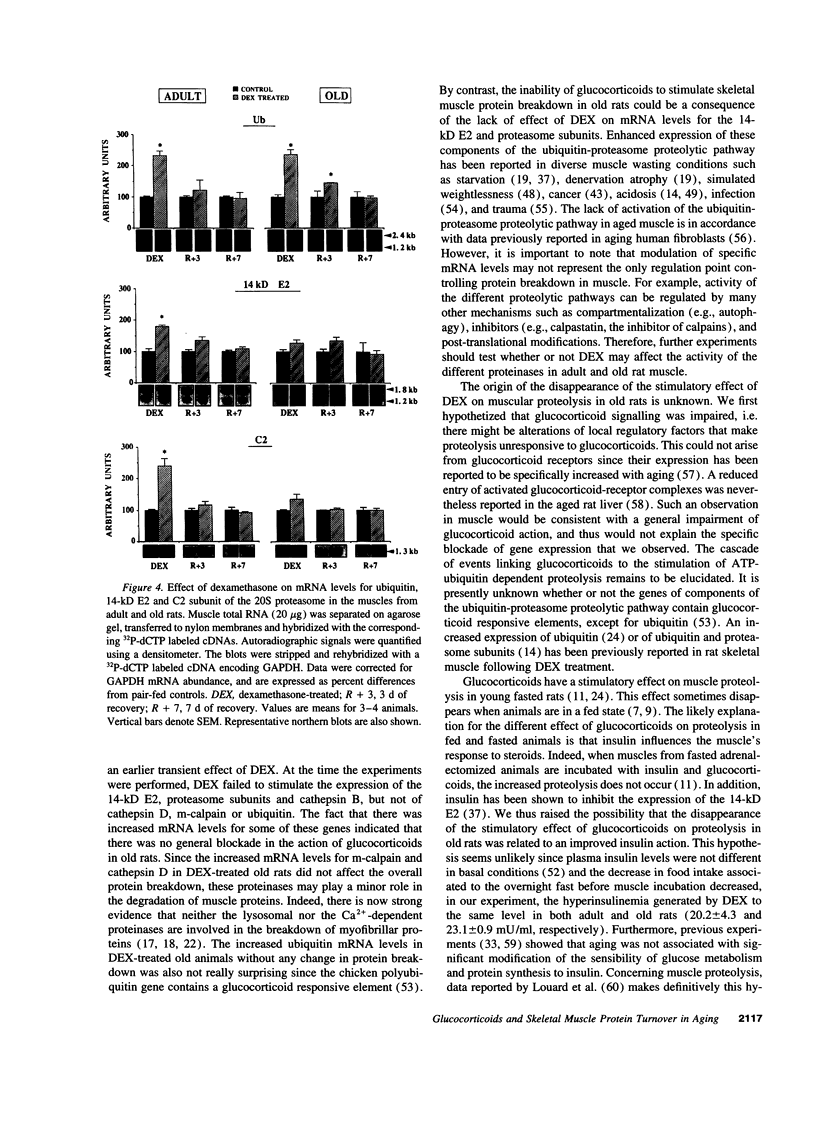
We studied glucocorticoid-induced muscle wasting and subsequent recovery in adult (7-mo-old) and old (22-mo-old) rats, since the increased incidence of various disease states may result in glucocorticoids hypersecretion in aging. Adult and old rats received dexamethasone in their drinking water and were then allowed to recover. Muscle wasting occurred more rapidly in old rats and the recovery of muscle mass was impaired, suggesting that glucocorticoids may be involved in the emergence of muscle atrophy with advancing age. According to measurements in incubated epitrochlearis muscles, dexamethasone-induced muscle wasting mainly resulted from increased protein breakdown in the adult, but from depressed protein synthesis in the aged animal. Increased expression of cathepsin D, m-calpain, and ubiquitin was observed in the muscles from both dexamethasone-treated adult and old rats. By contrast, the disappearance of the stimulatory effect of glucocorticoids on protein break-down in aging occurred along with a loss of ability of steroids to enhance the expression of the 14-kD ubiquitin carrier protein E2, which is involved in protein substrates ubiquitinylation, and of subunits of the 20 S proteasome (the proteolytic core of the 26 S proteasome that degrades ubiquitin conjugates). Thus, if glucocorticoids play any role in the progressive muscle atrophy seen in aging, this is unlikely to result from an activation of the ubiquitin-proteasome proteolytic pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agell N., Bond U., Schlesinger M. J. In vitro proteolytic processing of a diubiquitin and a truncated diubiquitin formed from in vitro-generated mRNAs. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3693–3697. doi: 10.1073/pnas.85.11.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaix D., Taillandier D., Temparis S., Larbaud D., Aurousseau E., Combaret L., Voisin L. Regulation of ATP-ubiquitin-dependent proteolysis in muscle wasting. Reprod Nutr Dev. 1994;34(6):583–597. doi: 10.1051/rnd:19940605. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994 Oct 7;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Clark A. S., Kelly R. A., Mitch W. E. Systemic response to thermal injury in rats. Accelerated protein degradation and altered glucose utilization in muscle. J Clin Invest. 1984 Sep;74(3):888–897. doi: 10.1172/JCI111506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardevet D., Komori K., Grunfeld C., Rosenzweig S. A., Buse M. G. Increased hepatic insulin proreceptor-to-receptor ratio in diabetes: a possible processing defect. Am J Physiol. 1991 Nov;261(5 Pt 1):E562–E571. doi: 10.1152/ajpendo.1991.261.5.E562. [DOI] [PubMed] [Google Scholar]
- Dardevet D., Sornet C., Attaix D., Baracos V. E., Grizard J. Insulin-like growth factor-1 and insulin resistance in skeletal muscles of adult and old rats. Endocrinology. 1994 Mar;134(3):1475–1484. doi: 10.1210/endo.134.3.8119189. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Nutrition, genetics, and aging. World Rev Nutr Diet. 1990;63:175–182. doi: 10.1159/000418507. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J. 1987 Apr 15;243(2):335–343. doi: 10.1042/bj2430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G. B., Halloran E. The adult decline in lean body mass. Hum Biol. 1976 Feb;48(1):161–173. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Kumatori A., Shin S., Yoshimura T., Ichihara A., Tokunaga F., Aruga R., Iwanaga S., Kakizuka A. Molecular cloning of cDNA for proteasomes (multicatalytic proteinase complexes) from rat liver: primary structure of the largest component (C2). Biochemistry. 1989 Sep 5;28(18):7332–7340. doi: 10.1021/bi00444a028. [DOI] [PubMed] [Google Scholar]
- Furuno K., Goodman M. N., Goldberg A. L. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem. 1990 May 25;265(15):8550–8557. [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., el Haj A. J., Lewis S. E., Merry B. J., Holehan A. M. The influence of chronic dietary intervention on protein turnover and growth of the diaphragm and extensor digitorum longus muscles of the rat. Exp Gerontol. 1987;22(1):67–78. doi: 10.1016/0531-5565(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Chen M., Cartee G. D., Young J. C. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev. 1991 Oct;60(2):199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Ilian M. A., Forsberg N. E. Gene expression of calpains and their specific endogenous inhibitor, calpastatin, in skeletal muscle of fed and fasted rabbits. Biochem J. 1992 Oct 1;287(Pt 1):163–171. doi: 10.1042/bj2870163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajoh S., Aoki K., Ohno S., Emori Y., Kawasaki H., Sugihara H., Suzuki K. Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease. Biochemistry. 1988 Oct 18;27(21):8122–8128. doi: 10.1021/bi00421a022. [DOI] [PubMed] [Google Scholar]
- Johnson P. Calpains (intracellular calcium-activated cysteine proteinases): structure-activity relationships and involvement in normal and abnormal cellular metabolism. Int J Biochem. 1990;22(8):811–822. doi: 10.1016/0020-711x(90)90284-a. [DOI] [PubMed] [Google Scholar]
- Kayali A. G., Young V. R., Goodman M. N. Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. Am J Physiol. 1987 May;252(5 Pt 1):E621–E626. doi: 10.1152/ajpendo.1987.252.5.E621. [DOI] [PubMed] [Google Scholar]
- Kelly F. J., Goldspink D. F. The differing responses of four muscle types to dexamethasone treatment in the rat. Biochem J. 1982 Oct 15;208(1):147–151. doi: 10.1042/bj2080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettelhut I. C., Wing S. S., Goldberg A. L. Endocrine regulation of protein breakdown in skeletal muscle. Diabetes Metab Rev. 1988 Dec;4(8):751–772. doi: 10.1002/dmr.5610040805. [DOI] [PubMed] [Google Scholar]
- Klitgaard H., Brunet A., Maton B., Lamaziere C., Lesty C., Monod H. Morphological and biochemical changes in old rat muscles: effect of increased use. J Appl Physiol (1985) 1989 Oct;67(4):1409–1417. doi: 10.1152/jappl.1989.67.4.1409. [DOI] [PubMed] [Google Scholar]
- Kumatori A., Tanaka K., Tamura T., Fujiwara T., Ichihara A., Tokunaga F., Onikura A., Iwanaga S. cDNA cloning and sequencing of component C9 of proteasomes from rat hepatoma cells. FEBS Lett. 1990 May 21;264(2):279–282. doi: 10.1016/0014-5793(90)80267-m. [DOI] [PubMed] [Google Scholar]
- Louard R. J., Bhushan R., Gelfand R. A., Barrett E. J., Sherwin R. S. Glucocorticoids antagonize insulin's antiproteolytic action on skeletal muscle in humans. J Clin Endocrinol Metab. 1994 Jul;79(1):278–284. doi: 10.1210/jcem.79.1.8027242. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., Ruderman N. B., Goodman M. N. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986 Feb 15;234(1):237–240. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides S. C. Protein synthesis and degradation during aging and senescence. Biol Rev Camb Philos Soc. 1983 Aug;58(3):343–422. doi: 10.1111/j.1469-185x.1983.tb00394.x. [DOI] [PubMed] [Google Scholar]
- May R. C., Kelly R. A., Mitch W. E. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986 Feb;77(2):614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. A., Goldspink D. F. Glucocorticoid action on protein synthesis and protein breakdown in isolated skeletal muscles. Biochem J. 1982 Sep 15;206(3):641–645. doi: 10.1042/bj2060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R., Wing S. S., Goldberg A. L. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995 May 1;307(Pt 3):631–637. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita J., López-Ibor B., Pau M., Mezquita C. Intron and intronless transcription of the chicken polyubiquitin gene UbII. FEBS Lett. 1993 Mar 22;319(3):244–248. doi: 10.1016/0014-5793(93)80555-9. [DOI] [PubMed] [Google Scholar]
- Mitch W. E., Medina R., Grieber S., May R. C., England B. K., Price S. R., Bailey J. L., Goldberg A. L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest. 1994 May;93(5):2127–2133. doi: 10.1172/JCI117208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoni L., Houlier M. L., Mirand P. P., Bayle G., Grizard J. Effect of amino acids alone or with insulin on muscle and liver protein synthesis in adult and old rats. Am J Physiol. 1993 Apr;264(4 Pt 1):E614–E620. doi: 10.1152/ajpendo.1993.264.4.E614. [DOI] [PubMed] [Google Scholar]
- Mosoni L., Valluy M. C., Serrurier B., Prugnaud J., Obled C., Guezennec C. Y., Mirand P. P. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995 Feb;268(2 Pt 1):E328–E335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kuzuya H., Okamoto M., Yoshimasa Y., Yamada K., Ida T., Kakehi T., Imura H. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol. 1988 Jan;254(1 Pt 1):E92–E98. doi: 10.1152/ajpendo.1988.254.1.E92. [DOI] [PubMed] [Google Scholar]
- Odedra B. R., Bates P. C., Millward D. J. Time course of the effect of catabolic doses of corticosterone on protein turnover in rat skeletal muscle and liver. Biochem J. 1983 Aug 15;214(2):617–627. doi: 10.1042/bj2140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. X., Short S. R., Goff S. A., Dice J. F. Ubiquitin pools, ubiquitin mRNA levels, and ubiquitin-mediated proteolysis in aging human fibroblasts. Exp Gerontol. 1993 Jan-Feb;28(1):39–49. doi: 10.1016/0531-5565(93)90018-9. [DOI] [PubMed] [Google Scholar]
- Price S. R., England B. K., Bailey J. L., Van Vreede K., Mitch W. E. Acidosis and glucocorticoids concomitantly increase ubiquitin and proteasome subunit mRNAs in rat muscle. Am J Physiol. 1994 Oct;267(4 Pt 1):C955–C960. doi: 10.1152/ajpcell.1994.267.4.C955. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Jefferson L. S. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol. 1980 Jun;238(6):E564–E572. doi: 10.1152/ajpendo.1980.238.6.E564. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Natural substrates of the ubiquitin proteolytic pathway. Cell. 1991 Aug 23;66(4):615–618. doi: 10.1016/0092-8674(91)90104-7. [DOI] [PubMed] [Google Scholar]
- Sabatino F., Masoro E. J., McMahan C. A., Kuhn R. W. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J Gerontol. 1991 Sep;46(5):B171–B179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- San Segundo B., Chan S. J., Steiner D. F. Identification of cDNA clones encoding a precursor of rat liver cathepsin B. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2320–2324. doi: 10.1073/pnas.82.8.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R., Armanini M., Packan D., Tombaugh G. Stress and glucocorticoids in aging. Endocrinol Metab Clin North Am. 1987 Dec;16(4):965–980. [PubMed] [Google Scholar]
- Spindler S. R., Grizzle J. M., Walford R. L., Mote P. L. Aging and restriction of dietary calories increases insulin receptor mRNA, and aging increases glucocorticoid receptor mRNA in the liver of female C3B10RF1 mice. J Gerontol. 1991 Nov;46(6):B233–B237. doi: 10.1093/geronj/46.6.b233. [DOI] [PubMed] [Google Scholar]
- Temparis S., Asensi M., Taillandier D., Aurousseau E., Larbaud D., Obled A., Béchet D., Ferrara M., Estrela J. M., Attaix D. Increased ATP-ubiquitin-dependent proteolysis in skeletal muscles of tumor-bearing rats. Cancer Res. 1994 Nov 1;54(21):5568–5573. [PubMed] [Google Scholar]
- Tessitore L., Costelli P., Baccino F. M. Humoral mediation for cachexia in tumour-bearing rats. Br J Cancer. 1993 Jan;67(1):15–23. doi: 10.1038/bjc.1993.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao G., Fagan J. M., Samuels N., James J. H., Hudson K., Lieberman M., Fischer J. E., Hasselgren P. O. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest. 1994 Dec;94(6):2255–2264. doi: 10.1172/JCI117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan G. M., Becker R. A., Allen J. P., Goodwin C. W., Jr, Pruitt B. A., Jr, Mason A. D., Jr Cortisol and corticotrophin in burned patients. J Trauma. 1982 Apr;22(4):263–273. doi: 10.1097/00005373-198204000-00001. [DOI] [PubMed] [Google Scholar]
- Vellas B. J., Albarede J. L., Garry P. J. Diseases and aging: patterns of morbidity with age; relationship between aging and age-associated diseases. Am J Clin Nutr. 1992 Jun;55(6 Suppl):1225S–1230S. doi: 10.1093/ajcn/55.6.1225S. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wing S. S., Banville D. 14-kDa ubiquitin-conjugating enzyme: structure of the rat gene and regulation upon fasting and by insulin. Am J Physiol. 1994 Jul;267(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1994.267.1.E39. [DOI] [PubMed] [Google Scholar]
- Wing S. S., Goldberg A. L. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol. 1993 Apr;264(4 Pt 1):E668–E676. doi: 10.1152/ajpendo.1993.264.4.E668. [DOI] [PubMed] [Google Scholar]
- el Haj A. J., Lewis S. E., Goldspink D. F., Merry B. J., Holehan A. M. The effect of chronic and acute dietary restriction on the growth and protein turnover of fast and slow types of rat skeletal muscle. Comp Biochem Physiol A Comp Physiol. 1986;85(2):281–287. doi: 10.1016/0300-9629(86)90251-3. [DOI] [PubMed] [Google Scholar]