Abstract
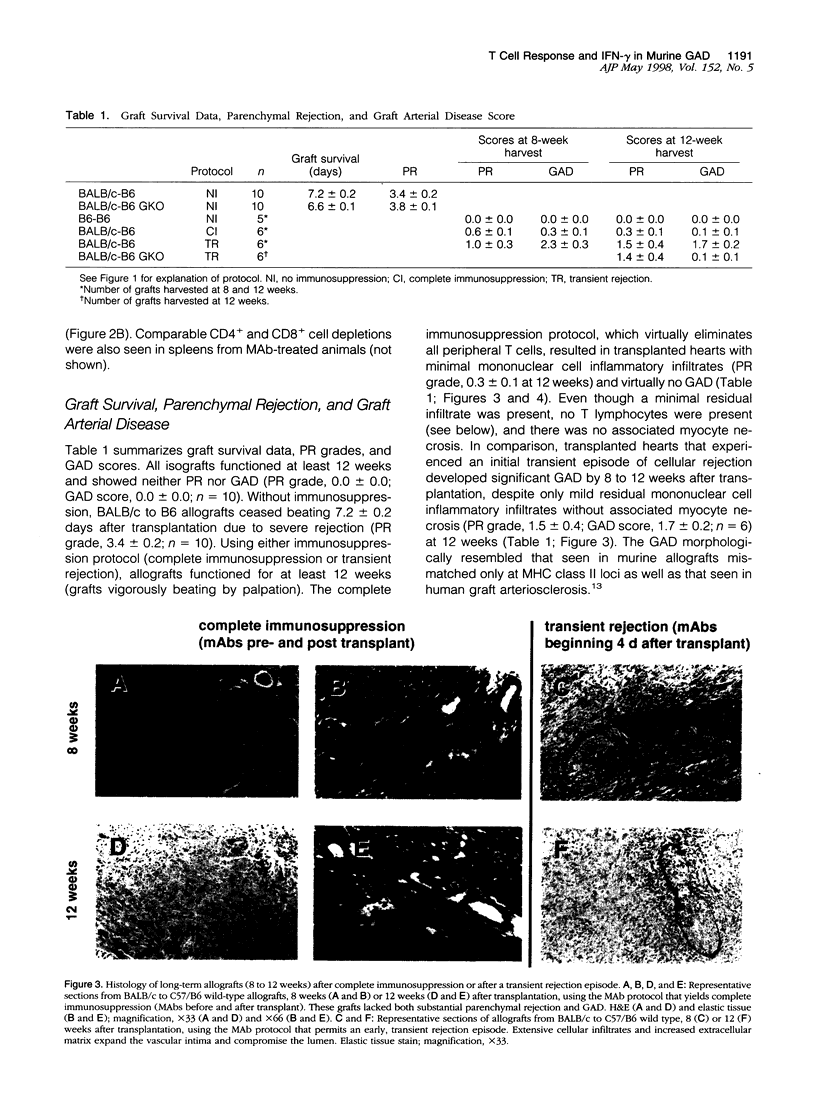
This study evaluated the contribution of acute parenchymal rejection and interferon (IFN)-gamma to the development of graft arterial disease (GAD) in totally allogeneic murine cardiac transplants. BALB/c (H-2d) hearts were transplanted into wild-type C57BL/6 (B6, H-2b) or B6 IFN-gamma-deficient (GKO) recipient mice. Assessing the role of acute parenchymal rejection in the GAD process involved two different immunosuppression protocols using anti-CD4 and -CD8 monoclonal antibodies (MAbs): virtually complete long-term immunosuppression (denoted as complete immunosuppression) was achieved by administering both MAbs 6, 3, and 1 day before transplantation and weekly thereafter; in contradistinction, a single, early, transient episode of rejection (transient rejection) was attained by administering MAbs beginning 4 days after transplant and then at weekly intervals. The extent and duration of T cell depletion under these two regimens were evaluated using flow cytometric analysis of peripheral blood lymphocytes. After a single injection of MAbs, peripheral blood CD4+ and CD8+ T cell depletion was approximately 98% at 1 week and approximately 88% at 2 weeks. After three injections (analogous to days 6, 3, and 1 before transplant), peripheral blood CD4+ and CD8+ T cell depletion was >98% at 2 weeks and approximately 87% at 4 weeks. Functioning cardiac allografts were removed at 8 and 12 weeks after transplant and analyzed by hematoxylin and eosin, elastic tissue, and immunohistochemical stains, and the severity of parenchymal rejection versus GAD was scored. With complete immunosuppression (antibody before and after transplant), BALB/c allografts showed little parenchymal rejection or GAD, suggesting that persistent depletion of T cells blocked subsequent development of GAD. However, even a single transient acute rejection episode allowed the subsequent development of GAD accompanied by augmented major histocompatibility complex (MHC) class II, VCAM-1, and ICAM-1 expression at 12 weeks; these allografts showed no residual CD4+ or CD8+ T cells. In comparison, allografts undergoing transient rejection in GKO recipients did not develop GAD, despite persistent macrophage and natural killer cell (NK) infiltrates comparable to those seen in wild-type recipients. Moreover, the arterioles of hearts transplanted into GKO recipients showed no or minimal increases in MHC class II, ICAM-1, and VCAM-1 relative to baseline expression. In conclusion, a single episode of allogeneic injury mediated by T cells suffices to evoke subsequent graft arteriosclerosis, even in the absence of additional T-cell-mediated injury, and the process appears to depend on IFN-gamma.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardehali A., Laks H., Drinkwater D. C., Ziv E., Drake T. A. Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac allograft vasculopathy. Circulation. 1995 Aug 1;92(3):450–456. doi: 10.1161/01.cir.92.3.450. [DOI] [PubMed] [Google Scholar]
- Barr C. L., Saunders G. F. Interferon-gamma-inducible regulation of the human invariant chain gene. J Biol Chem. 1991 Feb 25;266(6):3475–3481. [PubMed] [Google Scholar]
- Billingham M. E., Cary N. R., Hammond M. E., Kemnitz J., Marboe C., McCallister H. A., Snovar D. C., Winters G. L., Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990 Nov-Dec;9(6):587–593. [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J. C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Hanto D. W., Berens E., Schreiber R. D. Lack of an obligate role for IFN-gamma in the primary in vitro mixed lymphocyte response. J Immunol. 1988 Feb 15;140(4):1148–1152. [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Drew P. D., Lonergan M., Goldstein M. E., Lampson L. A., Ozato K., McFarlin D. E. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. J Immunol. 1993 Apr 15;150(8 Pt 1):3300–3310. [PubMed] [Google Scholar]
- Dumont F. J., Dijkmans R., Palfree R. G., Boltz R. D., Coker L. Selective up-regulation by interferon-gamma of surface molecules of the Ly-6 complex in resting T cells: the Ly-6A/E and TAP antigens are preferentially enhanced. Eur J Immunol. 1987 Aug;17(8):1183–1191. doi: 10.1002/eji.1830170816. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Geerling R. A., de Bruin R. W., Scheringa M., Bonthuis F., Jeekel J., Ijzermans J. N., Marquet R. L. Suppression of acute rejection prevents graft arteriosclerosis after allogeneic aorta transplantation in the rat. Transplantation. 1994 Dec 15;58(11):1258–1263. [PubMed] [Google Scholar]
- Hauptman P. J., Davis S. F., Miller L., Yeung A. C. The role of nonimmune risk factors in the development and progression of graft arteriosclerosis: preliminary insights from a multicenter intravascular ultrasound study. Multicenter Intravascular Ultrasound Transplant Study Group. J Heart Lung Transplant. 1995 Nov-Dec;14(6 Pt 2):S238–S242. [PubMed] [Google Scholar]
- Hauptman P. J., Nakagawa T., Tanaka H., Libby P. Acute rejection: culprit or coincidence in the pathogenesis of cardiac graft vascular disease? J Heart Lung Transplant. 1995 Nov-Dec;14(6 Pt 2):S173–S180. [PubMed] [Google Scholar]
- Hosenpud J. D., Shipley G. D., Wagner C. R. Cardiac allograft vasculopathy: current concepts, recent developments, and future directions. J Heart Lung Transplant. 1992 Jan-Feb;11(1 Pt 1):9–23. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Hullett D. A., Geraghty J. G., Stoltenberg R. L., Sollinger H. W. The impact of acute rejection on the development of intimal hyperplasia associated with chronic rejection. Transplantation. 1996 Dec 27;62(12):1842–1846. doi: 10.1097/00007890-199612270-00028. [DOI] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Izutani H., Miyagawa S., Shirakura R., Matsumiya G., Nakata S., Shimazaki Y., Matsuda H. Evidence that graft coronary arteriosclerosis begins in the early phase after transplantation and progresses without chronic immunoreaction. Histopathological analysis using a retransplantation model. Transplantation. 1995 Nov 27;60(10):1073–1079. doi: 10.1097/00007890-199511270-00002. [DOI] [PubMed] [Google Scholar]
- Libby P., Salomon R. N., Payne D. D., Schoen F. J., Pober J. S. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989 Aug;21(4):3677–3684. [PubMed] [Google Scholar]
- Liu G., Butany J. Morphology of graft arteriosclerosis in cardiac transplant recipients. Hum Pathol. 1992 Jul;23(7):768–773. doi: 10.1016/0046-8177(92)90346-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H., Mitchell R. N., Taylor M. K., Hasegawa S., Tilney N. L., Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997 Aug 1;100(3):550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Sukhova G. K., Rabkin E., Winters G. L., Schoen F. J., Libby P. Acute rejection accelerates graft coronary disease in transplanted rabbit hearts. Circulation. 1995 Aug 15;92(4):987–993. doi: 10.1161/01.cir.92.4.987. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz C. G., Ohye R. G., Pelletier R. P., Van Buskirk A. M., Huang E., Morgan C., Kincade P. W., Ferguson R. M. Treatment with anti-vascular cell adhesion molecule 1 monoclonal antibody induces long-term murine cardiac allograft acceptance. Transplantation. 1993 Aug;56(2):453–460. doi: 10.1097/00007890-199308000-00039. [DOI] [PubMed] [Google Scholar]
- Paul L. C., Fellström B. Chronic vascular rejection of the heart and the kidney--have rational treatment options emerged? Transplantation. 1992 Jun;53(6):1169–1179. doi: 10.1097/00007890-199206000-00001. [DOI] [PubMed] [Google Scholar]
- Pelletier R. P., Ohye R. G., Vanbuskirk A., Sedmak D. D., Kincade P., Ferguson R. M., Orosz C. G. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J Immunol. 1992 Oct 1;149(7):2473–2481. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Russell M. E., Fujita M., Masek M. A., Rowan R. A., Billingham M. E. Cardiac graft vascular disease. Nonselective involvement of large and small vessels. Transplantation. 1993 Dec;56(6):1599–1601. [PubMed] [Google Scholar]
- Russell M. E., Hancock W. W., Akalin E., Wallace A. F., Glysing-Jensen T., Willett T. A., Sayegh M. H. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996 Feb 1;97(3):833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. IV Effects of treatment with monoclonal antibodies to intercellular adhesion molecule-1 and leukocyte function-associated antigen-1. Transplantation. 1995 Oct 15;60(7):724–729. [PubMed] [Google Scholar]
- Räisänen-Sokolowski A., Glysing-Jensen T., Koglin J., Russell M. E. Reduced transplant arteriosclerosis in murine cardiac allografts placed in interferon-gamma knockout recipients. Am J Pathol. 1998 Feb;152(2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Sadahiro M., McDonald T. O., Allen M. D. Reduction in cellular and vascular rejection by blocking leukocyte adhesion molecule receptors. Am J Pathol. 1993 Mar;142(3):675–683. [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Konieczny B. T., Lowry R. P., Baddoura F. K., Lakkis F. G. Acute rejection of vascularized heart allografts in the absence of IFNgamma. Transplantation. 1996 Dec 27;62(12):1908–1911. doi: 10.1097/00007890-199612270-00039. [DOI] [PubMed] [Google Scholar]
- Salomon R. N., Hughes C. C., Schoen F. J., Payne D. D., Pober J. S., Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991 Apr;138(4):791–798. [PMC free article] [PubMed] [Google Scholar]
- Shi C., Lee W. S., He Q., Zhang D., Fletcher D. L., Jr, Newell J. B., Haber E. Immunologic basis of transplant-associated arteriosclerosis. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4051–4056. doi: 10.1073/pnas.93.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Libby P. Interaction of the allogeneic state and hypercholesterolemia in arterial lesion formation in experimental cardiac allografts. Arterioscler Thromb. 1994 May;14(5):734–745. doi: 10.1161/01.atv.14.5.734. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Swanson S. J., Cybulsky M. I., Schoen F. J., Libby P. Endothelial and smooth muscle cells express leukocyte adhesion molecules heterogeneously during acute rejection of rabbit cardiac allografts. Am J Pathol. 1994 May;144(5):938–951. [PMC free article] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Haskard D. O. IL-4 increases human endothelial cell adhesiveness for T cells but not for neutrophils. J Immunol. 1990 Apr 15;144(8):3060–3065. [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Tullius S. G., Hancock W. W., Heemann U., Azuma H., Tilney N. L. Reversibility of chronic renal allograft rejection. Critical effect of time after transplantation suggests both host immune dependent and independent phases of progressive injury. Transplantation. 1994 Jul 15;58(1):93–99. [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Zerbe T., Uretsky B., Kormos R., Armitage J., Wolyn T., Griffith B., Hardesty R., Duquesnoy R. Graft atherosclerosis: effects of cellular rejection and human lymphocyte antigen. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S104–S110. [PubMed] [Google Scholar]