Abstract
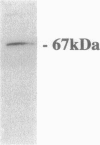
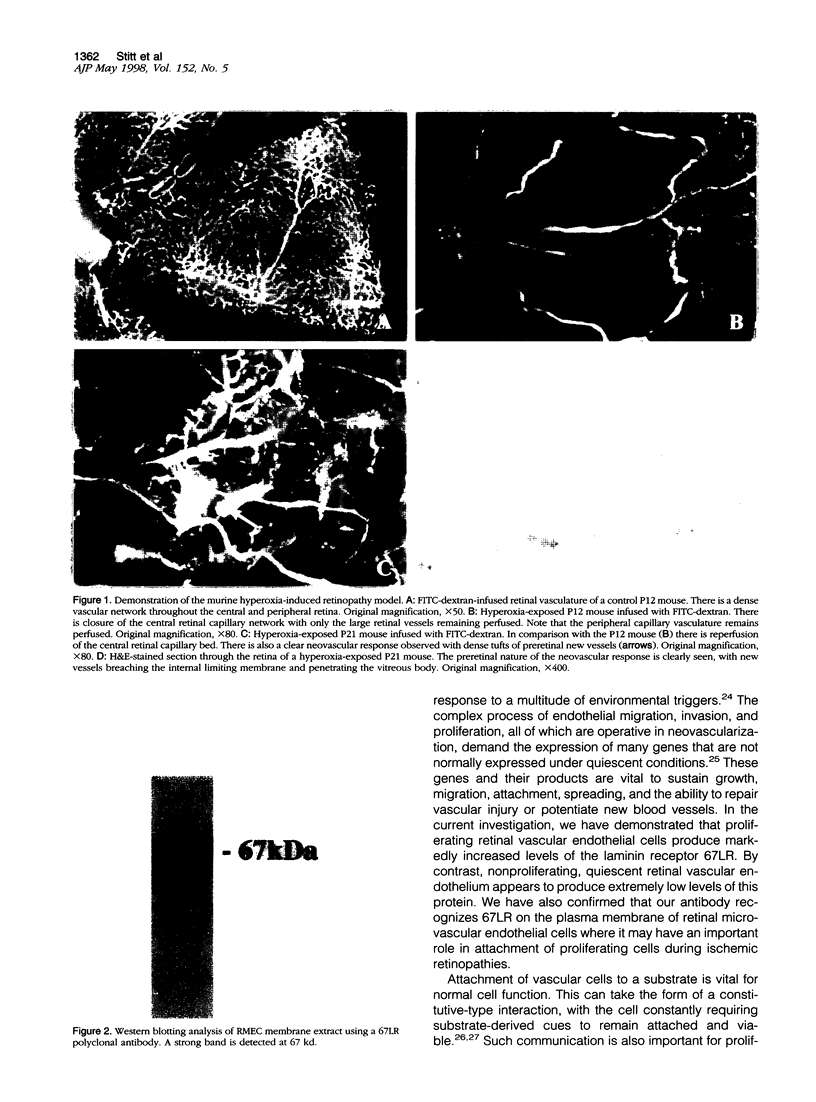
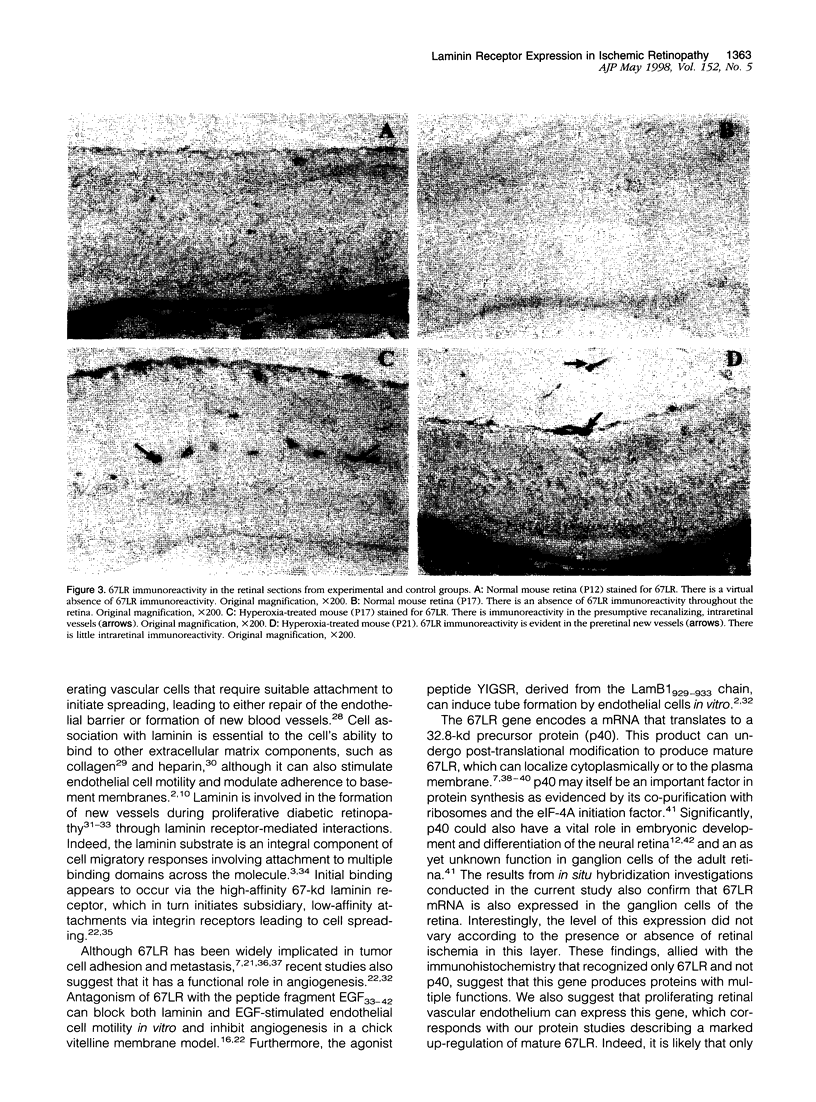
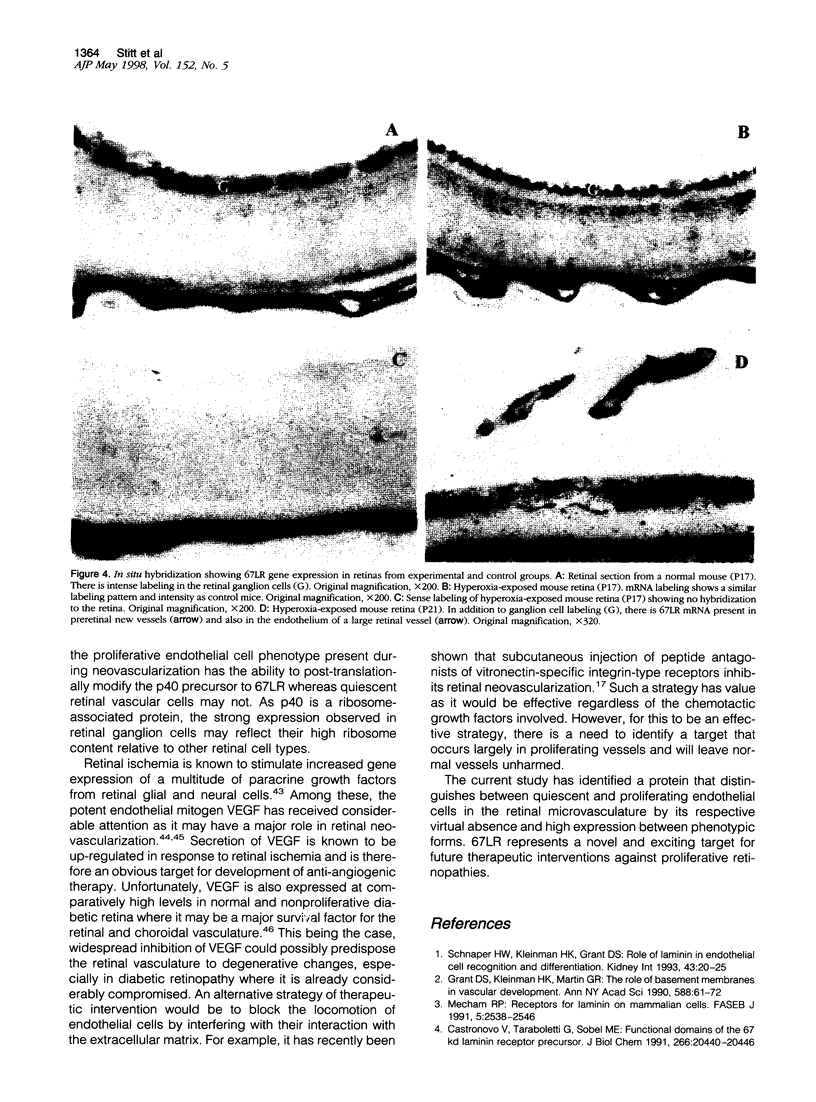
Endothelial cell association with vascular basement membranes is complex and plays a critical role in regulation of cell adhesion and proliferation. The interaction between the membrane-associated 67-kd receptor (67LR) and the basement membrane protein laminin has been studied in several cell systems where it was shown to be crucial for adhesion and attachment during angiogenesis. As angiogenesis in the pathological setting of proliferative retinopathy is a major cause of blindness in the Western world we examined the expression of 67LR in a murine model of hyperoxia-induced retinopathy that exhibits retinal neovascularization. Mice exposed to hyperoxia for 5 days starting at postnatal day 7 (P7) and returned to room air (at P12) showed closure of the central retinal vasculature. In response to the ensuing retinal ischemia, there was consistent preretinal neovascularization starting around P17, which persisted until P21, after which the new vessels regressed. Immunohistochemistry was performed on these retinas using an antibody specific for 67LR. At P12, immunoreactivity for 67LR was absent in the retina, but by P17 it was observed in preretinal proliferating vessels and also within the adjacent intraretinal vasculature. Intraretinal 67LR immunoreactivity diminished beyond P17 until by P21 immunoreactivity was almost completely absent, although it persisted in the preretinal vasculature. Control P17 mice (not exposed to hyperoxia) failed to demonstrate any 67LR immunoreactivity in their retinas. Parallel in situ hybridization studies demonstrated 67LR gene expression in the retinal ganglion cells of control and hyperoxia-exposed mice. In addition, the neovascular intra- and preretinal vessels of hyperoxia-treated P17 and P21 mice labeled strongly for 67LR mRNA. This study has characterized 67LR immunolocalization and gene expression in a murine model of ischemic retinopathy. Results suggest that, although the 67LR gene is expressed at high levels in the retinal ganglion cells, the mature receptor protein is preferentially localized to the proliferating retinal vasculature and is almost completely absent from quiescent vessels. The differential expression of 67LR between proliferating and quiescent retinal vessels suggests that this laminin receptor is an important and novel target for future chemotherapeutic intervention during proliferative vasculopathies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Shima D. T., Tolentino M. J., Gragoudas E. S., Ferrara N., Folkman J., D'Amore P. A., Miller J. W. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996 Jan;114(1):66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- Alon T., Hemo I., Itin A., Pe'er J., Stone J., Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995 Oct;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Ardini E., Tagliabue E., Magnifico A., Butò S., Castronovo V., Colnaghi M. I., Ménard S. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the alpha6beta4 integrin. J Biol Chem. 1997 Jan 24;272(4):2342–2345. doi: 10.1074/jbc.272.4.2342. [DOI] [PubMed] [Google Scholar]
- Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997 Feb 1;99(3):373–376. doi: 10.1172/JCI119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaroli Marano R. P., Preissner K. T., Vilaró S. Fibronectin, laminin, vitronectin and their receptors at newly-formed capillaries in proliferative diabetic retinopathy. Exp Eye Res. 1995 Jan;60(1):5–17. doi: 10.1016/s0014-4835(05)80079-x. [DOI] [PubMed] [Google Scholar]
- Castronovo V., Taraboletti G., Sobel M. E. Functional domains of the 67-kDa laminin receptor precursor. J Biol Chem. 1991 Oct 25;266(30):20440–20446. [PubMed] [Google Scholar]
- Grant D. S., Kibbey M. C., Kinsella J. L., Cid M. C., Kleinman H. K. The role of basement membrane in angiogenesis and tumor growth. Pathol Res Pract. 1994 Oct;190(9-10):854–863. doi: 10.1016/S0344-0338(11)80989-1. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Kinsella J. L., Fridman R., Auerbach R., Piasecki B. A., Yamada Y., Zain M., Kleinman H. K. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992 Dec;153(3):614–625. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Kleinman H. K., Martin G. R. The role of basement membranes in vascular development. Ann N Y Acad Sci. 1990;588:61–72. doi: 10.1111/j.1749-6632.1990.tb13197.x. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Grosso L. E., Park P. W., Mecham R. P. Characterization of a putative clone for the 67-kilodalton elastin/laminin receptor suggests that it encodes a cytoplasmic protein rather than a cell surface receptor. Biochemistry. 1991 Apr 2;30(13):3346–3350. doi: 10.1021/bi00227a026. [DOI] [PubMed] [Google Scholar]
- Guo N. H., Krutzsch H. C., Vogel T., Roberts D. D. Interactions of a laminin-binding peptide from a 33-kDa protein related to the 67-kDa laminin receptor with laminin and melanoma cells are heparin-dependent. J Biol Chem. 1992 Sep 5;267(25):17743–17747. [PubMed] [Google Scholar]
- Hammes H. P., Brownlee M., Jonczyk A., Sutter A., Preissner K. T. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996 May;2(5):529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- Hilario E., Unda F., Pérez-Yarza G., Alvarez A., García-Sanz M., Allño S. F. Presence of laminin and 67KDa laminin-receptor on endothelial surface of lung capillaries. An immunocytochemical study. Histol Histopathol. 1996 Oct;11(4):915–918. [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Parr B. The body language of cells: the intimate connection between cell adhesion and behavior. Cell. 1995 Oct 6;83(1):5–8. doi: 10.1016/0092-8674(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Mizoguchi M. Modulation of morphological differentiation of human endothelial cells in culture by the synthetic peptide YIGSR and cytochalasin B. Clin Exp Dermatol. 1993 May;18(3):236–240. doi: 10.1111/j.1365-2230.1993.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Landowski T. H., Dratz E. A., Starkey J. R. Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry. 1995 Sep 5;34(35):11276–11287. doi: 10.1021/bi00035a037. [DOI] [PubMed] [Google Scholar]
- McCaffery P., Neve R. L., Dräger U. C. A dorso-ventral asymmetry in the embryonic retina defined by protein conformation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8570–8574. doi: 10.1073/pnas.87.21.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham R. P. Receptors for laminin on mammalian cells. FASEB J. 1991 Aug;5(11):2538–2546. doi: 10.1096/fasebj.5.11.1651264. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuori N., Sobel M. E. The 67-kDa laminin receptor and tumor progression. Curr Top Microbiol Immunol. 1996;213(Pt 1):205–214. doi: 10.1007/978-3-642-61107-0_13. [DOI] [PubMed] [Google Scholar]
- Nelson J., Allen W. E., Scott W. N., Bailie J. R., Walker B., McFerran N. V., Wilson D. J. Murine epidermal growth factor (EGF) fragment (33-42) inhibits both EGF- and laminin-dependent endothelial cell motility and angiogenesis. Cancer Res. 1995 Sep 1;55(17):3772–3776. [PubMed] [Google Scholar]
- Nelson J., Scott W. N., Allen W. E., Wilson D. J., Harriott P., McFerran N. V., Walker B. Murine epidermal growth factor peptide (33-42) binds to a YIGSR-specific laminin receptor on both tumor and endothelial cells. J Biol Chem. 1996 Oct 18;271(42):26179–26186. doi: 10.1074/jbc.271.42.26179. [DOI] [PubMed] [Google Scholar]
- Nelson J., Stewart R., McGivern M., Bailie J. R., Walker B., Murphy R. F., Wilson D. J. Synthetic murine epidermal growth factor sequence 20-31 is mitogenic and angiogenic. Carcinogenesis. 1991 Oct;12(10):1823–1829. doi: 10.1093/carcin/12.10.1823. [DOI] [PubMed] [Google Scholar]
- Paques M., Massin P., Gaudric A. Growth factors and diabetic retinopathy. Diabetes Metab. 1997 Apr;23(2):125–130. [PubMed] [Google Scholar]
- Pierce E. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabacchi S. A., Neve R. L., Dräger U. C. A positional marker for the dorsal embryonic retina is homologous to the high-affinity laminin receptor. Development. 1990 Jul;109(3):521–531. doi: 10.1242/dev.109.3.521. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Robinson G. S., Pierce E. A., Rook S. L., Foley E., Webb R., Smith L. E. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaper H. W., Kleinman H. K., Grant D. S. Role of laminin in endothelial cell recognition and differentiation. Kidney Int. 1993 Jan;43(1):20–25. doi: 10.1038/ki.1993.5. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D'Amato R., Sullivan R., D'Amore P. A. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):101–111. [PubMed] [Google Scholar]
- Sobel M. E. Differential expression of the 67 kDa laminin receptor in cancer. Semin Cancer Biol. 1993 Oct;4(5):311–317. [PubMed] [Google Scholar]
- Stitt A. W., Anderson H. R., Gardiner T. A., Bailie J. R., Archer D. B. Receptor-mediated endocytosis and intracellular trafficking of insulin and low-density lipoprotein by retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3384–3392. [PubMed] [Google Scholar]
- Tashiro K., Nagata I., Yamashita N., Okazaki K., Ogomori K., Tashiro N., Anai M. A synthetic peptide deduced from the sequence in the cross-region of laminin A chain mediates neurite outgrowth, cell attachment and heparin binding. Biochem J. 1994 Aug 15;302(Pt 1):73–79. doi: 10.1042/bj3020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Brown J. C. The laminins. Matrix Biol. 1994 Aug;14(4):275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Vacca A., Ribatti D., Roncali L., Lospalluti M., Serio G., Carrel S., Dammacco F. Melanocyte tumor progression is associated with changes in angiogenesis and expression of the 67-kilodalton laminin receptor. Cancer. 1993 Jul 15;72(2):455–461. doi: 10.1002/1097-0142(19930715)72:2<455::aid-cncr2820720222>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Van den Ouweland A. M., Van Duijnhoven H. L., Deichmann K. A., Van Groningen J. J., de Leij L., Van de Ven W. J. Characteristics of a multicopy gene family predominantly consisting of processed pseudogenes. Nucleic Acids Res. 1989 May 25;17(10):3829–3843. doi: 10.1093/nar/17.10.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Taraboletti G., Sobel M. E., Albrechtsen R., Liotta L. A. Role of laminin receptor in tumor cell migration. Cancer Res. 1987 Nov 1;47(21):5691–5698. [PubMed] [Google Scholar]
- Yang G., Douville P., Gee S., Carbonetto S. Nonintegrin laminin receptors in the nervous system: evidence for lack of a relationship to P40. J Neurobiol. 1992 Jul;23(5):491–506. doi: 10.1002/neu.480230505. [DOI] [PubMed] [Google Scholar]
- Yow H. K., Wong J. M., Chen H. S., Lee C. G., Davis S., Steele G. D., Jr, Chen L. B. Increased mRNA expression of a laminin-binding protein in human colon carcinoma: complete sequence of a full-length cDNA encoding the protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6394–6398. doi: 10.1073/pnas.85.17.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Saleh W., Delvenne P., van den Brule F. A., Menard S., Boniver J., Castronovo V. Expression of the 67 KD laminin receptor in human cervical preneoplastic and neoplastic squamous epithelial lesions: an immunohistochemical study. J Pathol. 1997 Mar;181(3):287–293. doi: 10.1002/(SICI)1096-9896(199703)181:3<287::AID-PATH762>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]