Abstract
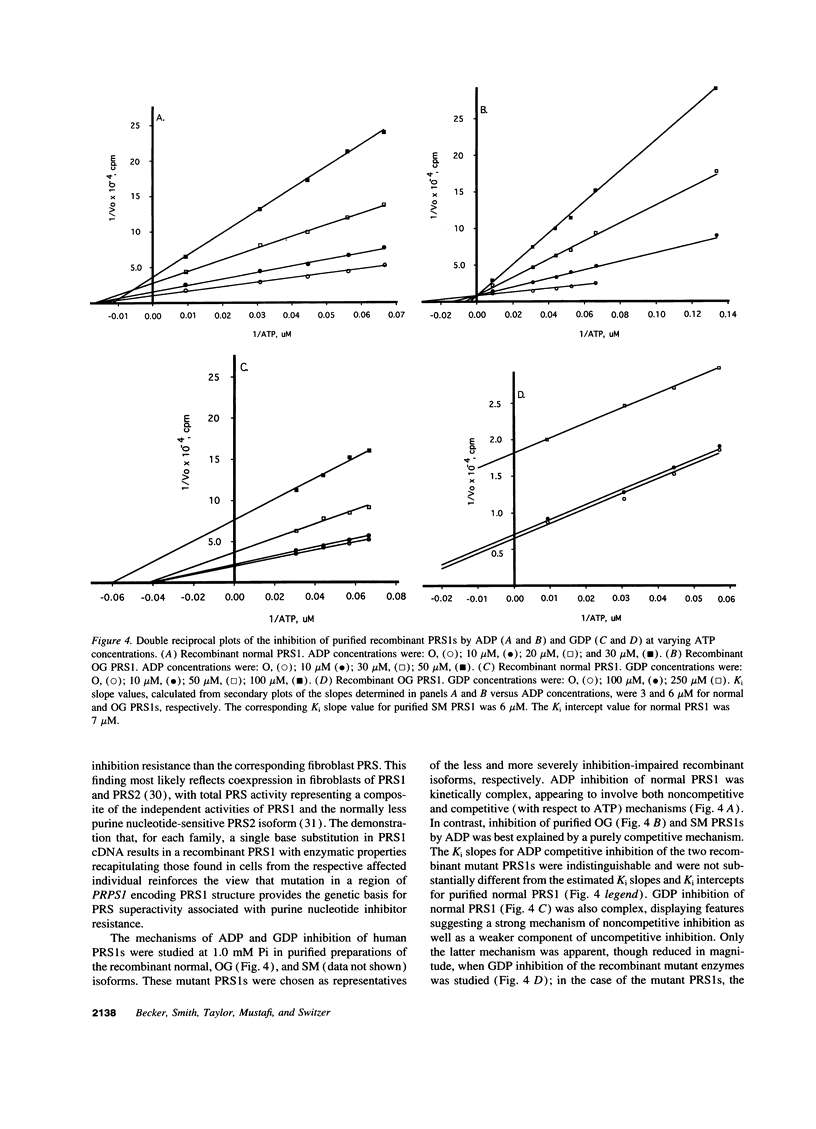
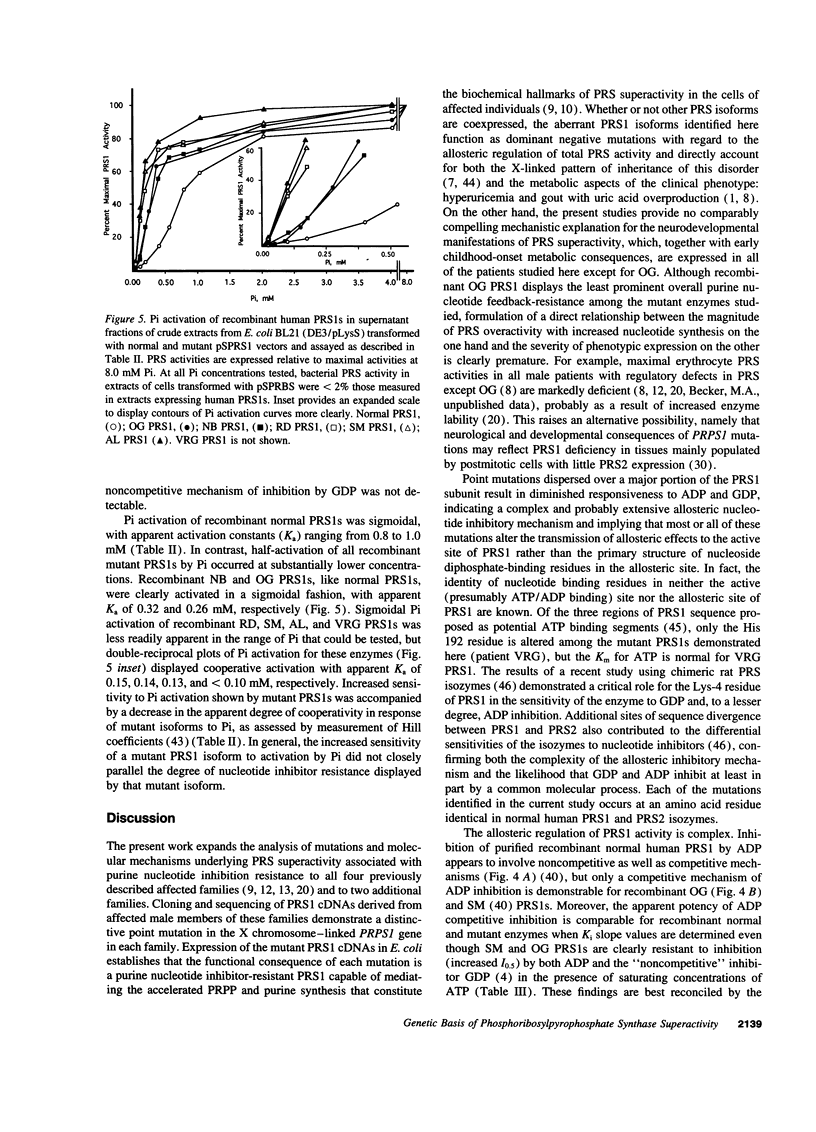
The genetic and functional basis of phosphoribosylpyrophosphate synthetase (PRS) superactivity associated with purine nucleotide inhibitor-resistance was studied in six families with this X chromosome-linked purine metabolic and neurodevelopmental disorder. Cloning and sequencing of PRS1 and PRS2 cDNAs, derived from fibroblast total RNA of affected male patients by reverse transcription and PCR amplification, demonstrated that each PRS1 cDNA contained a distinctive single base substitution predicting a corresponding amino acid substitution in the PRS1 isoform. Overall, the array of substitutions encompassed a substantial portion of the translated sequence of PRS1 cDNA. Plasmid-mediated expression of variant PRS1 cDNAs in Escherichia coli BL21 (DE3/pLysS) yielded recombinant mutant PRS1s, which, in each case, displayed a pattern and magnitude of purine nucleoside diphosphate inhibitor-resistance comparable to that found in cells of the respective patient. Kinetic analysis of recombinant mutant PRS1s showed that widely dispersed point mutations in the X chromosome-linked PRPS1 gene encoding the PRS1 isoform result in alteration of the allosteric mechanisms regulating both enzyme inhibition by purine nucleotides and activation by inorganic phosphate. The functional consequences of these mutations provide a tenable basis for the enhanced production of phosphoribosylpyrophosphate, purine nucleotides, and uric acid that are the biochemical hallmarks of PRS superactivity.
Full text
PDF


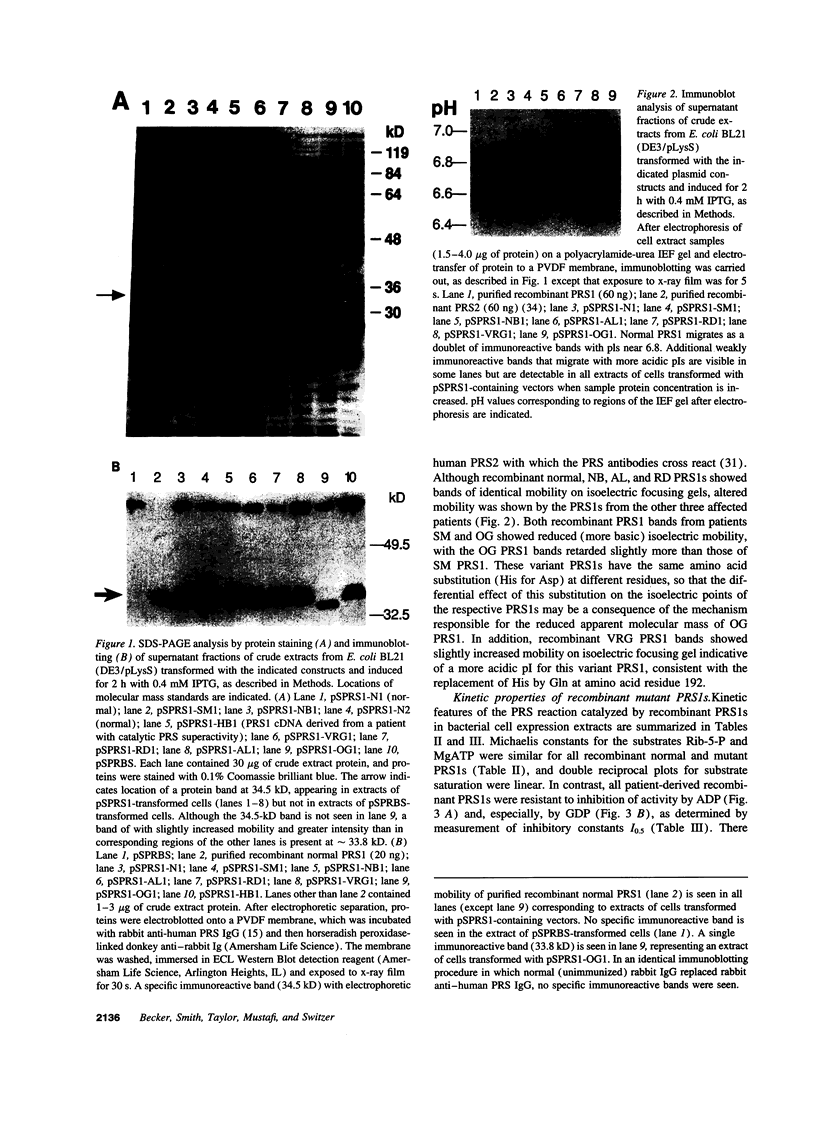
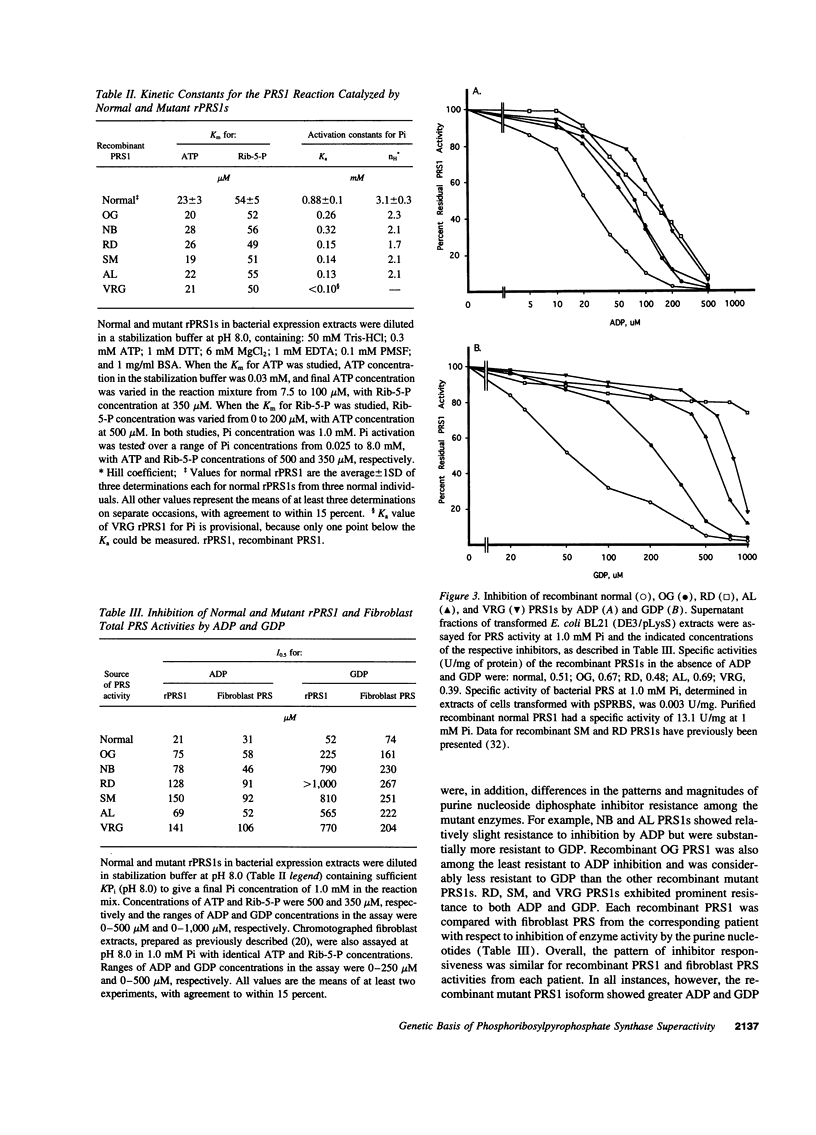


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Ishijima S., Kita K., Tatibana M. Identification of amino-acid residues linked to different properties of phosphoribosylpyrophosphate synthetase isoforms I and II. Biochim Biophys Acta. 1994 Jul 20;1207(1):126–133. doi: 10.1016/0167-4838(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Akaoka I., Fujimori S., Kamatani N., Takeuchi F., Yano E., Nishida Y., Hashimoto A., Horiuchi Y. A gouty family with increased phosphoribosylpyrophosphate synthetase activity: case reports, familial studies, and kinetic studies of the abnormal enzyme. J Rheumatol. 1981 Jul-Aug;8(4):563–574. [PubMed] [Google Scholar]
- Balzarini J., De Clercq E. 5-Phosphoribosyl 1-pyrophosphate synthetase converts the acyclic nucleoside phosphonates 9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and 9-(2-phosphonylmethoxyethyl)adenine directly to their antivirally active diphosphate derivatives. J Biol Chem. 1991 May 15;266(14):8686–8689. [PubMed] [Google Scholar]
- Becker M. A., Kostel P. J., Meyer L. J. Human phosphoribosylpyrophosphate synthetase. Comparison of purified normal and mutant enzymes. J Biol Chem. 1975 Sep 10;250(17):6822–6830. [PubMed] [Google Scholar]
- Becker M. A., Losman M. J., Itkin P., Simkin P. A. Gout with superactive phosphoribosylpyrophosphate synthetase due to increased enzyme catalytic rate. J Lab Clin Med. 1982 Apr;99(4):495–511. [PubMed] [Google Scholar]
- Becker M. A., Losman M. J., Kim M. Mechanisms of accelerated purine nucleotide synthesis in human fibroblasts with superactive phosphoribosylpyrophosphate synthetases. J Biol Chem. 1987 Apr 25;262(12):5596–5602. [PubMed] [Google Scholar]
- Becker M. A., Losman M. J., Rosenberg A. L., Mehlman I., Levinson D. J., Holmes E. W. Phosphoribosylpyrophosphate synthetase superactivity. A study of five patients with catalytic defects in the enzyme. Arthritis Rheum. 1986 Jul;29(7):880–888. doi: 10.1002/art.1780290710. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Losman M. J., Wilson J., Simmonds H. A. Superactivity of human phosphoribosyl pyrophosphate synthetase due to altered regulation by nucleotide inhibitors and inorganic phosphate. Biochim Biophys Acta. 1986 Jun 19;882(2):168–176. doi: 10.1016/0304-4165(86)90151-0. [DOI] [PubMed] [Google Scholar]
- Becker M. A. Patterns of phosphoribosylpyrophosphate and ribose-5-phosphate concentration and generation in fibroblasts from patients with gout and purine overproduction. J Clin Invest. 1976 Feb;57(2):308–318. doi: 10.1172/JCI108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A., Puig J. G., Mateos F. A., Jimenez M. L., Kim M., Simmonds H. A. Inherited superactivity of phosphoribosylpyrophosphate synthetase: association of uric acid overproduction and sensorineural deafness. Am J Med. 1988 Sep;85(3):383–390. doi: 10.1016/0002-9343(88)90591-8. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Raivio K. O., Bakay B., Adams W. B., Nyhan W. L. Variant human phosphoribosylpyrophosphate synthetase altered in regulatory and catalytic functions. J Clin Invest. 1980 Jan;65(1):109–120. doi: 10.1172/JCI109640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A., Raivio K. O., Seegmiller J. E. Synthesis of phosphoribosylpyrophosphate in mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1979;49:281–306. doi: 10.1002/9780470122945.ch7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Tarlé S. A., Van Antwerp M., Gibbs D. A., Watts R. W., Kelley W. N., Palella T. D. Identification of 17 independent mutations responsible for human hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Am J Hum Genet. 1991 May;48(5):951–958. [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971 Sep 25;246(18):5739–5748. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem. 1972 Apr 10;247(7):2126–2131. [PubMed] [Google Scholar]
- Fry D. W., Becker M. A., Switzer R. L. Inhibition of human 5-phosphoribosyl-1-pyrophosphate synthetase by 4-amino-8-(beta-D-ribofuranosylamino)-pyrimido[5,4-d]pyrimidine-5'- monophosphate: evidence for interaction at the ADP allosteric site. Mol Pharmacol. 1995 Apr;47(4):810–815. [PubMed] [Google Scholar]
- Iizasa T., Taira M., Shimada H., Ishijima S., Tatibana M. Molecular cloning and sequencing of human cDNA for phosphoribosyl pyrophosphate synthetase subunit II. FEBS Lett. 1989 Feb 13;244(1):47–50. doi: 10.1016/0014-5793(89)81159-7. [DOI] [PubMed] [Google Scholar]
- Ishijima S., Kita K., Ahmad I., Ishizuka T., Taira M., Tatibana M. Expression of rat phosphoribosylpyrophosphate synthetase subunits I and II in Escherichia coli. Isolation and characterization of the recombinant isoforms. J Biol Chem. 1991 Aug 25;266(24):15693–15697. [PubMed] [Google Scholar]
- Ishizuka T., Iizasa T., Taira M., Ishijima S., Sonoda T., Shimada H., Nagatake N., Tatibana M. Promoter regions of the human X-linked housekeeping genes PRPS1 and PRPS2 encoding phosphoribosylpyrophosphate synthetase subunit I and II isoforms. Biochim Biophys Acta. 1992 Mar 24;1130(2):139–148. doi: 10.1016/0167-4781(92)90521-z. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., LIEBERMAN I., SIMMS E. S. Enzymatic synthesis and properties of 5-phosphoribosylpyrophosphate. J Biol Chem. 1955 Jul;215(1):389–402. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lejeune E., Bouvier M., Mousson B., Llorca G., Baltassat P. Anomalies de la phosphoribosylpyrophosphate synthétase dans deux cas de goutte à début précoce. Rev Rhum Mal Osteoartic. 1979 Jul-Sep;46(7-9):457–465. [PubMed] [Google Scholar]
- Losman M. J., Rimon D., Kim M., Becker M. A. Selective expression of phosphoribosylpyrophosphate synthetase superactivity in human lymphoblast lines. J Clin Invest. 1985 Oct;76(4):1657–1664. doi: 10.1172/JCI112151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Akaoka I., Horiuchi Y. Altered isoelectric property of a superactive 5-phosphoribosyl-1-pyrophosphate (PRPP) synthetase in a patient with clinical gout. Biochem Med. 1981 Dec;26(3):387–394. doi: 10.1016/0006-2944(81)90014-4. [DOI] [PubMed] [Google Scholar]
- Nosal J. M., Switzer R. L., Becker M. A. Overexpression, purification, and characterization of recombinant human 5-phosphoribosyl-1-pyrophosphate synthetase isozymes I and II. J Biol Chem. 1993 May 15;268(14):10168–10175. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roessler B. J., Nosal J. M., Smith P. R., Heidler S. A., Palella T. D., Switzer R. L., Becker M. A. Human X-linked phosphoribosylpyrophosphate synthetase superactivity is associated with distinct point mutations in the PRPS1 gene. J Biol Chem. 1993 Dec 15;268(35):26476–26481. [PubMed] [Google Scholar]
- Rosenberg A. L., Bergstrom L., Troost B. T., Bartholomew B. A. Hyperuricemia and neurologic deficits. A family study. N Engl J Med. 1970 Apr 30;282(18):992–997. doi: 10.1056/NEJM197004302821802. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Webster D. R., Lingam S., Wilson J. An inborn error of purine metabolism, deafness and neurodevelopmental abnormality. Neuropediatrics. 1985 May;16(2):106–108. doi: 10.1055/s-2008-1052552. [DOI] [PubMed] [Google Scholar]
- Sonoda T., Taira M., Ishijima S., Ishizuka T., Iizasa T., Tatibana M. Complete nucleotide sequence of human phosphoribosyl pyrophosphate synthetase subunit I (PRS I) cDNA and a comparison with human and rat PRPS gene families. J Biochem. 1991 Feb;109(2):361–364. [PubMed] [Google Scholar]
- Sperling O., Boer P., Persky-Brosh S., Kanarek E., De Vries A. Altered kinetic property of erythrocyte phosphoribosylpsyrophosphate synthetase in excessive purine production. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):703–706. [PubMed] [Google Scholar]
- Sperling O., Persky-Brosh S., Boer P., De Vries A. Human erythrocyte phosphoribosylpyrophosphate synthetase mutationally altered in regulatory properties. Biochem Med. 1973 Jun;7(3):389–395. doi: 10.1016/0006-2944(73)90059-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Iizasa T., Yamada K., Shimada H., Tatibana M. Tissue-differential expression of two distinct genes for phosphoribosyl pyrophosphate synthetase and existence of the testis-specific transcript. Biochim Biophys Acta. 1989 Mar 1;1007(2):203–208. doi: 10.1016/0167-4781(89)90040-7. [DOI] [PubMed] [Google Scholar]
- Taira M., Ishijima S., Kita K., Yamada K., Iizasa T., Tatibana M. Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J Biol Chem. 1987 Nov 5;262(31):14867–14870. [PubMed] [Google Scholar]
- Taira M., Kudoh J., Minoshima S., Iizasa T., Shimada H., Shimizu Y., Tatibana M., Shimizu N. Localization of human phosphoribosylpyrophosphate synthetase subunit I and II genes (PRPS1 and PRPS2) to different regions of the X chromosome and assignment of two PRPS1-related genes to autosomes. Somat Cell Mol Genet. 1989 Jan;15(1):29–37. doi: 10.1007/BF01534667. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Nord L. D., Fujitaki J. M., Robins R. K. Potent and specific inhibitors of mammalian phosphoribosylpyrophosphate (PRPP) synthetase. Adv Enzyme Regul. 1989;28:167–182. doi: 10.1016/0065-2571(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Yen R. C., Adams W. B., Lazar C., Becker M. A. Evidence for X-linkage of human phosphoribosylpyrophosphate synthetase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):482–485. doi: 10.1073/pnas.75.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoref E., De Vries A., Sperling O. Metabolic cooperation between human fibroblasts with normal and with mutant superactive phosphoribosylpyrophosphate synthetase. Nature. 1976 Apr 29;260(5554):787–788. doi: 10.1038/260786a0. [DOI] [PubMed] [Google Scholar]
- Zoref E., De Vries A., Sperling O. Mutant feedback-resistant phosphoribosylpyrophosphate synthetase associated with purine overproduction and gout. Phosphoribosylpyrophosphate and purine metabolism in cultured fibroblasts. J Clin Invest. 1975 Nov;56(5):1093–1099. doi: 10.1172/JCI108183. [DOI] [PMC free article] [PubMed] [Google Scholar]