Abstract
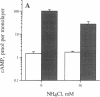
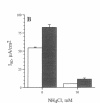
The colon, unlike most organs, is normally exposed to high concentrations of ammonia, a weak base which exerts profound and diverse biological effects on mammalian cells. The impact of ammonia on intestinal cell function is largely unknown despite its concentration of 4-70 mM in the colonic lumen. The human intestinal epithelial cell line T84 was used to model electrogenic Cl- secretion, the transport event which hydrates mucosal surfaces and accounts for secretory diarrhea. Transepithelial transport and isotopic flux analysis indicated that physiologically-relevant concentrations of ammonia (as NH4Cl) markedly inhibit cyclic nucleotide-regulated Cl- secretion but not the response to the Ca2+ agonist carbachol. Inhibition by ammonia was 25-fold more potent with basolateral compared to apical exposure. Ion substitution indicated that the effect of NH4Cl was not due to altered cation composition or membrane potential. The site of action of ammonia is distal to cAMP generation and is not due simply to cytoplasmic alkalization. The results support a novel role for ammonia as an inhibitory modulator of intestinal epithelial Cl- secretion. Secretory responsiveness may be dampened in pathological conditions associated with increased mucosal permeability due to enhanced access of lumenal ammonia to the basolateral epithelial compartment.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Brunkhorst B., Niederman R. Ammonium decreases human polymorphonuclear leukocyte cytoskeletal actin. Infect Immun. 1991 Apr;59(4):1378–1386. doi: 10.1128/iai.59.4.1378-1386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. M., Stephenson R. L., Feldman G. M. Bicarbonate secretion modulates ammonium absorption in rat distal colon in vivo. Am J Physiol. 1988 May;254(5 Pt 2):F657–F667. doi: 10.1152/ajprenal.1988.254.5.F657. [DOI] [PubMed] [Google Scholar]
- Davies L., Satre M., Martin J. B., Gross J. D. The target of ammonia action in dictyostelium. Cell. 1993 Oct 22;75(2):321–327. doi: 10.1016/0092-8674(93)80073-n. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Madara J. L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Mandel K. G., Masui H., McRoberts J. A. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl- cotransport system. J Clin Invest. 1985 Feb;75(2):462–471. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Pandol S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986 Feb;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (2) N Engl J Med. 1989 Sep 28;321(13):879–883. doi: 10.1056/NEJM198909283211307. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Corbin J. D. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 1994;56:237–272. doi: 10.1146/annurev.ph.56.030194.001321. [DOI] [PubMed] [Google Scholar]
- Good D. W. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- Haas M. The Na-K-Cl cotransporters. Am J Physiol. 1994 Oct;267(4 Pt 1):C869–C885. doi: 10.1152/ajpcell.1994.267.4.C869. [DOI] [PubMed] [Google Scholar]
- Khachatrian L., Howlett A., Klein C. Ammonium sulfate modifies adenylate cyclase and the chemotactic receptor of Dictyostelium discoideum. Evidence for a G protein effect. J Biol Chem. 1987 Jun 15;262(17):8071–8076. [PubMed] [Google Scholar]
- Kikeri D., Sun A., Zeidel M. L., Hebert S. C. Cell membranes impermeable to NH3. Nature. 1989 Jun 8;339(6224):478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Visek W. J. Colon mucosal cell damage by ammonia in rats. J Nutr. 1991 Jun;121(6):887–893. doi: 10.1093/jn/121.6.887. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Visek W. J. Large intestinal pH and ammonia in rats: dietary fat and protein interactions. J Nutr. 1991 Jun;121(6):832–843. doi: 10.1093/jn/121.6.832. [DOI] [PubMed] [Google Scholar]
- Liskowsky D. R., Norenberg L. O., Norenberg M. D. Effect of ammonia on cyclic AMP production in primary astrocyte cultures. Brain Res. 1986 Oct 29;386(1-2):386–388. doi: 10.1016/0006-8993(86)90176-9. [DOI] [PubMed] [Google Scholar]
- Lupton J. R., Marchant L. J. Independent effects of fiber and protein on colonic luminal ammonia concentration. J Nutr. 1989 Feb;119(2):235–241. doi: 10.1093/jn/119.2.235. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Awtrey C. S., Madara J. L. Microfilament-dependent activation of Na+/K+/2Cl- cotransport by cAMP in intestinal epithelial monolayers. J Clin Invest. 1992 Oct;90(4):1608–1613. doi: 10.1172/JCI116030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. B., Awtrey C. S., Tally K. J., Smith J. A. Dynamic role of microfilaments in intestinal chloride secretion. Am J Surg. 1994 Jan;167(1):21–26. doi: 10.1016/0002-9610(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Awtrey C. S., Thompson R., Hung T., Tally K. J., Madara J. L. Na(+)-K(+)-2Cl- cotransport and Cl- secretion evoked by heat-stable enterotoxin is microfilament dependent in T84 cells. Am J Physiol. 1993 Aug;265(2 Pt 1):G370–G378. doi: 10.1152/ajpgi.1993.265.2.G370. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Smith J. A., Tally K. J., Awtrey C. S., Nguyen H., Rich J., Madara J. L. Na-K-2Cl cotransport in intestinal epithelial cells. Influence of chloride efflux and F-actin on regulation of cotransporter activity and bumetanide binding. J Biol Chem. 1994 Jun 3;269(22):15703–15709. [PubMed] [Google Scholar]
- Molitoris B. A., Nelson W. J. Alterations in the establishment and maintenance of epithelial cell polarity as a basis for disease processes. J Clin Invest. 1990 Jan;85(1):3–9. doi: 10.1172/JCI114427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossberg S. M., Ross G. Ammonia movement in the small intestine: preferential transport by the ileum. J Clin Invest. 1967 Apr;46(4):490–498. doi: 10.1172/JCI105551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Chudzik J., Poole B. The effects of basic substances and acidic ionophores on the digestion of exogenous and endogenous proteins in mouse peritoneal macrophages. J Cell Biol. 1986 Mar;102(3):959–966. doi: 10.1083/jcb.102.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulais M., Turner R. J. Beta-adrenergic upregulation of the Na(+)-K(+)-2Cl- cotransporter in rat parotid acinar cells. J Clin Invest. 1992 Apr;89(4):1142–1147. doi: 10.1172/JCI115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince L. S., Workman R. B., Jr, Marchase R. B. Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5192–5196. doi: 10.1073/pnas.91.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priver N. A., Rabon E. C., Zeidel M. L. Apical membrane of the gastric parietal cell: water, proton, and nonelectrolyte permeabilities. Biochemistry. 1993 Mar 16;32(10):2459–2468. doi: 10.1021/bi00061a002. [DOI] [PubMed] [Google Scholar]
- Raabe W. Effects of hyperammonemia on neuronal function: NH4+, IPSP and Cl(-)-extrusion. Adv Exp Med Biol. 1993;341:71–82. doi: 10.1007/978-1-4615-2484-7_7. [DOI] [PubMed] [Google Scholar]
- SILEN W., HARPER H. A., MAWDSLEY D. L., WEIRICH W. L. Effect of antibacterial agents on ammonia production within the intestine. Proc Soc Exp Biol Med. 1955 Jan;88(1):138–140. doi: 10.3181/00379727-88-21516. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992 Jun;262(6 Pt 1):L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Barasch J., Al-Awqati Q. Plasticity of functional epithelial polarity. 1985 Nov 28-Dec 4Nature. 318(6044):368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- Seagrave J. C., Barker S., Curry M., Martinez J. R. Effects of NH4Cl and dimethylamine on Cl- fluxes in resting and stimulated rat submandibular acinar cells. Am J Physiol. 1992 Oct;263(4 Pt 1):G558–G565. doi: 10.1152/ajpgi.1992.263.4.G558. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Matthews J., Hecht G., Delp C., Madara J. L. Stabilization of F-actin prevents cAMP-elicited Cl- secretion in T84 cells. J Clin Invest. 1991 Jun;87(6):1903–1909. doi: 10.1172/JCI115215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeier G. R., Reppert S. M., Lencer W. I., Madara J. L. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995 Feb 3;270(5):2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- Triebling A. T., Korsten M. A., Dlugosz J. W., Paronetto F., Lieber C. S. Severity of Helicobacter-induced gastric injury correlates with gastric juice ammonia. Dig Dis Sci. 1991 Aug;36(8):1089–1096. doi: 10.1007/BF01297452. [DOI] [PubMed] [Google Scholar]
- Tsuruoka S., Takeda M., Yoshitomi K., Imai M. Cellular heterogeneity of ammonium ion transport across the basolateral membrane of the hamster medullary thick ascending limb of Henle's loop. J Clin Invest. 1993 Oct;92(4):1881–1888. doi: 10.1172/JCI116780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglarik C. J., Bridges R. J., Frizzell R. A. A simple assay for agonist-regulated Cl and K conductances in salt-secreting epithelial cells. Am J Physiol. 1990 Aug;259(2 Pt 1):C358–C364. doi: 10.1152/ajpcell.1990.259.2.C358. [DOI] [PubMed] [Google Scholar]
- Waisbren S. J., Geibel J. P., Modlin I. M., Boron W. F. Unusual permeability properties of gastric gland cells. Nature. 1994 Mar 24;368(6469):332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- Wolpert E., Phillips S. F., Summerskill W. H. Transport of urea and ammonia production in the human colon. Lancet. 1971 Dec 25;2(7739):1387–1390. doi: 10.1016/s0140-6736(71)90667-2. [DOI] [PubMed] [Google Scholar]
- Yanaka A., Muto H., Ito S., Silen W. Effects of ammonium ion and ammonia on function and morphology of in vitro frog gastric mucosa. Am J Physiol. 1993 Aug;265(2 Pt 1):G277–G288. doi: 10.1152/ajpgi.1993.265.2.G277. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]