Abstract
This paper presents an overview of our recent work on the use of soft lithography and two-phase fluid flow to form arrays of droplets. The crucial issues in the formation of stable arrays of droplets and alternating droplets of two sets of aqueous solutions include the geometry of the microchannels, the capillary number, and the water fraction of the system. Glass capillaries could be coupled to the PDMS microchannels and droplets could be transferred into glass capillaries for long-term storage. The arrays of droplets have been applied to screen the conditions for protein crystallization with microbatch and vapor diffusion techniques.
1. Introduction
Soft lithography uses poly(dimethylsiloxane) (PDMS) “stamps” with patterns on their surface in order to generate features with dimensions from tens of nanometers to hundreds of micrometers.[1] There are several soft-lithographic techniques that can be used to construct microchannels. [2–4] These techniques play an important role in microfluidics because they enable one to construct microchannels rapidly and at low cost.[2] We usually fabricate PDMS-based microfluidic devices by casting PDMS against a silicon wafer containing relief structures complementary to channels. The silicon master is, in turn, patterned by using a rapid prototyping technique.[5] The PDMS “stamp” is then exposed to air plasma and sealed to a piece of flat PDMS or glass in order to generate channels[5] that have dimensions in the range of 10 to 1000 μm.
Various strategies have been demonstrated to fabricate structures in confined spaces via fluid flow. For example, structures as small as 10 μm have been fabricated by laminar flow[6] inside microchannels.[7–10] As a unique type of structure, arrayed droplets can be readily generated by shearing a stream of an aqueous solution into a stream of oil inside microchannels.[11–14] The resulting aqueous droplets are mono-disperse and stable. With soft-lithographic techniques, one can easily fabricate microchannels with various designs for generating and controlling the movement of droplets. The formation and evolution of droplets in confined space have been of interest[13,14] because of the broad range of physical phenomena that can be investigated in such systems. For example, we have applied droplet-based microfluidics to measuring the rate of chemical reactions on the millisecond scale.[15] This method has the advantage of fast mixing and no dispersion compared to a conventional pressure-driven laminar flow.[11] More recently, arrays of droplets in microchannels have also begun to find use as microreactors.[11,15–18]
2. Formation of Arrayed Droplets
Both the choice of material and the method of fabrication are crucial issues involved in the formation of arrayed droplets in microfluidic channels. The low Young’s modulus and transparency of PDMS make it the ideal material for microfluidic devices.[2,3] However, the permeability to water vapor of PDMS is detrimental to long-term storage of aqueous droplets inside PDMS microchannels,[18] therefore it is important to be able to transfer the droplets from PDMS micro-channels into other types of vessels such as glass capillaries and plastic tubing. We have recently fabricated PDMS/glass capillary composite devices (Fig. 1) so that the droplets could be transferred smoothly into a glass capillary after they had been formed.[17] To make a smooth connection between a PDMS microchannel with a square cross-section and a glass capillary with a circular cross-section, the capillary was inserted into the outlet of the PDMS channel. Partially cured, viscous PDMS was then deposited into the junction in order to seal the gap between the capillary and the PDMS channel. The viscous PDMS was drawn into the gap by capillary force while the viscosity prevented excessive flow. After the PDMS had been fully cured, the junction became watertight.
Figure 1.
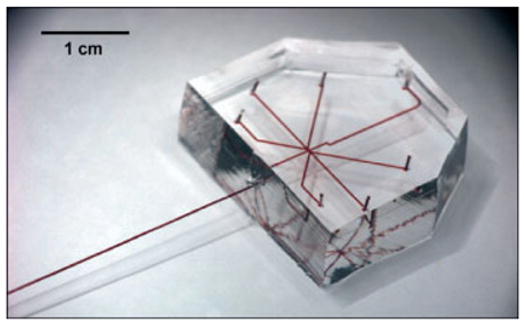
A photograph of a PDMS microfluidic device coupled to a glass capillary. All inlets, channels, and the capillaries were filled with a red aqueous solution for clarity.
An array of droplets was formed as the stream of an aqueous solution was sheared into the stream of fluorocarbon as oil (Fig. 2). The flow of oil transported the array of droplets into the capillary that was connected to the outlet of the PDMS microchannel. The flow was stopped when the capillary was filled with the array of droplets of the desired composition. If desired, the capillary could be disconnected and sealed with wax for long-term storage. Formation of droplets can be characterized by the capillary number (Ca): Ca = Uμ/γ, where U [m s−1] is the velocity of the fluid flow, γ [N m−1] is the surface tension at the water/oil interface, and μ [kg m−1] is the viscosity of the fluid. When Ca > 1, the diameter of the droplet became smaller than the dimensions of the channel. In the system for generating arrayed droplets, Ca was maintained below one and the length of the droplets was weakly dependent on the total flow rate and Ca.
Figure 2.
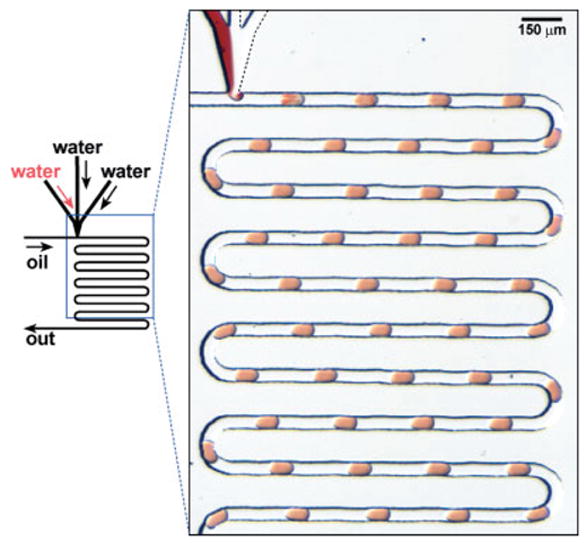
Left: Schematic illustration of the formation of arrays of droplets inside microchannels. Right: A microphotograph showing the arrays of droplets of three aqueous streams formed in a stream of fluorinated oil.
The composition of the droplets was controlled by varying the flow rates for individual aqueous streams. As shown in Figure 3, aqueous streams combined in the junction area and then sheared off to form a droplet. At a constant total flow rate for the aqueous stream, the concentration of solutes in the droplet was proportional to the instantaneous flow rate of the individual aqueous streams.[18] As we varied the flow rate of each aqueous stream, a gradient of concentrations of the solutes was generated along the array of droplets (Fig. 3).
Figure 3.
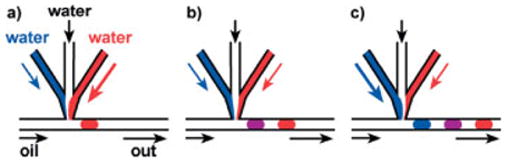
Schematic illustration of the formation of an array of droplets in which the concentration of one solute (blue) in each droplet gradually increases while the concentration of the other solute (red) decreases along the array.
Alternating droplets of two sets of aqueous solutions were formed when we used a microchannel with an arrangement of two head-on aqueous inlets (Fig. 4a). The size of the droplets increased with the water fraction (wf),[19] and the distance between adjacent droplets decreased as the wf increased (Figs. 4b-d). The water fraction was defined as the ratio between the combined flow rate of the aqueous streams and the total flow rate of the oil and aqueous streams. At a given wf, there existed an optimal Ca range for the reliable formation of stable alternating droplets (0.001 < Ca <0.15). When the Ca was lower than 0.001, the aqueous streams reached the inlet junction and coalesced before alternating droplets could be formed. When Ca was too high (Ca > 0.15), shear force dominated and induced laminar flow and the formation of droplets became unstable.[19] This understanding of the physics for the formation of droplets is critical in the use of arrayed droplets in applications such as protein crystallization.
Figure 4.
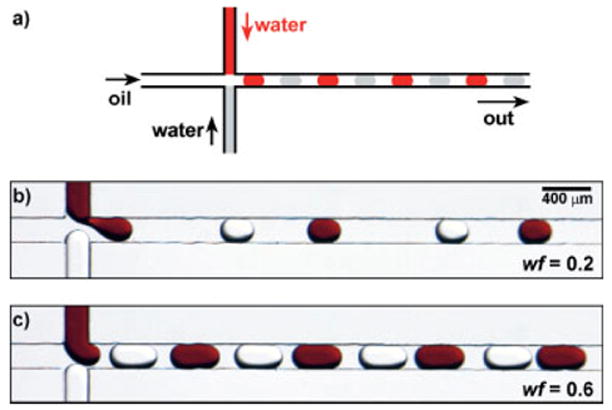
a) Schematic illustration of the formation of alternating droplets. b,c) A series of microphotographs showing the formation of alternating droplets at water fraction wf of 0.2 and 0.6, respectively (water fraction is defined in the text). Both the size of droplets and the distance between adjacent droplets changed when the value of wf was varied.
3. Applications of Arrayed Droplets in Protein Crystallization
Growing high-quality protein crystals is important to structural biology. The conventional methods for protein crystallization consume at least 100 nL of protein solution per trial. In order to reduce the consumption of sample, microfluidics has been used in screening conditions for protein crystallization. For example, Hansen et al. fabricated nanoliter-sized reaction chambers in order to confine the solutions of protein and precipitant and to implement a free-interface diffusion method for crystallization.[20] Here we use droplets to confine the mixtures of protein and precipitant solutions.[17,18]
We found that arrays of droplets were easy to fabricate and were capable of reproducing the results of conventional techniques with a much smaller amount of protein solution. Arrays of droplets are analogues of the conventional micro-batch plates, containing arrays of microwells for the mixtures of protein and precipitant solutions. As the flow rate of aqueous streams was changed during the generation of arrays of droplet (Fig. 2), each droplet that was formed contained a different concentration of protein and precipitant, therefore an array of droplets would represent a series of trials of crystallization conditions. The small volume (~ 10 nL) of these droplets allows us to perform hundreds of trials in order to screen conditions for protein crystallization with microliter amounts of protein solution.[18]
Within the small volume of each droplet, the nucleation rate is significantly reduced. One approach to accelerating the nucleation process is to concentrate the droplet gradually so that the concentration of both protein and precipitant would increase, thus increasing the supersaturation of the solution and the rate of nucleation. The gradual increase of concentration of solutions, commonly used in the vapor-diffusion method of crystallization, was achieved by employing alternating droplets inside capillaries. We successfully generated arrays in which droplets of protein solutions alternated with droplets of concentrated salt solutions. The oil was permeable to water, and diffusion of water from droplets of protein solution to their adjacent droplets of salt solution was caused by the difference in osmotic pressure of the two solutions.[17] This diffusion drove the crystallization of proteins. At the optimal experimental conditions we were able to obtain high quality protein crystals (Fig. 5). X-ray diffraction patterns that had a resolution better than 2.0 A were obtained from the crystals directly on chip, in which crystals grew inside the capillary.[17]
Figure 5.

Polarized-light microphotograph of a segment of an array of droplets in a glass capillary. Three crystals of protein thaumatin were grown under microbatch condition in these droplets.
4. Conclusions and Perspective
The simplicity and versatility of soft lithography enabled us to perform many exploratory experiments, followed by both fundamental studies and applications. Using the devices fabricated by soft lithography, we have demonstrated the formation and transport of the array of droplets under a two-phase fluid flow. We have also illustrated the use of the array of droplets for screening conditions in protein crystallization. We resolved the problem caused by the permeability of PDMS to water by coupling the PDMS device to glass capillaries or plastic tubing. The liquids used in the system (including fluorocarbon and water solutions) are compatible with PDMS. In addition to screening of conditions for crystallization, the array of droplets could find other applications. Droplets of polymers that have been patterned by soft lithography have been used as microlenses,[21,22] and the periodic arrays of droplets in microchannels may also be of interest in optical applications. This is because the period between droplets and the refractive index of the liquid within droplets in the array could be easily varied. Elastomeric properties of the materials used in soft lithography may be utilized to change the shape of droplets.[6,23] Patterning inside confined spaces can be achieved using arrays of droplets—either by chemical reactions which occur inside the droplets, or by removal of the solvent from the droplets.[24] These applications will require that the material properties of microchannels—for example, compatibility with organic solvents,[25,26] permeability to gases and liquids, and mechanical properties—can be controlled. We now understand the basic physics describing the formation of arrays of droplets in terms of the capillary number. However, the more complicated systems that involve non-Newtonian fluids, such as solutions of polymer and concentrated suspensions, remain to be investigated. As this knowledge and the new materials for soft lithography become available, it is anticipated that arrays of droplets will find an even broader range of applications.
Footnotes
This work was supported in part by the NIH (R01 EB001903) and the Beckman Young Investigator Program, and was performed at the MRSEC microfluidic facility funded by the NSF.
References
- 1.Xia YN, Whitesides GM. Angew Chem Int Ed. 1998;37:551. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 3.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu HK, Schueller OJA, Whitesides GM. Electrophoresis. 2000;21:27. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Science. 2000;288:113. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 5.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70:4974. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 6.Weigl BH, Yager P. Science. 1999;283:346. [Google Scholar]
- 7.Kim E, Xia YN, Whitesides GM. J Am Chem Soc. 1996;118:5722. [Google Scholar]
- 8.Kenis PJA, Ismagilov RF, Whitesides GM. Science. 1999;285:83. doi: 10.1126/science.285.5424.83. [DOI] [PubMed] [Google Scholar]
- 9.Kenis PJA, Ismagilov RF, Takayama S, Whitesides GM, Li SL, White HS. Acc Chem Res. 2000;33:841. doi: 10.1021/ar000062u. [DOI] [PubMed] [Google Scholar]
- 10.Li SL, Macosko CW, White HS. Science. 1993;259:957. [Google Scholar]
- 11.Song H, Tice JD, Ismagilov RF. Angew Chem Int Ed. 2003;42:768. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- 12.Anna SL, Bontoux N, Stone HA. Appl Phys Lett. 2003;82:364. [Google Scholar]
- 13.Link DR, Anna SL, Weitz DA, Stone HA. Phys Rev Lett. 2004;92:054–503. doi: 10.1103/PhysRevLett.92.054503. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen T, Roberts RW, Arnold FH, Quake SR. Phys Rev Lett. 2001;86:4163. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- 15.Song H, Ismagilov RF. J Am Chem Soc. 2003;125:14–613. doi: 10.1021/ja0354566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin K, Henkel T, Baier V, Grodrian A, Schon T, Roth M, Kohler JM, Metze J. Lab Chip. 2003;3:202. doi: 10.1039/b301258c. [DOI] [PubMed] [Google Scholar]
- 17.Zheng B, Tice JD, Roach LS, Ismagilov RF. Angew Chem Int Ed. 2004;43:2508. doi: 10.1002/anie.200453974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B, Roach LS, Ismagilov RF. J Am Chem Soc. 2003;125:11–170. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
- 19.Zheng B, Tice JD, Ismagilov RF. Anal Chem. 2004;76 doi: 10.1021/ac0495743. ASAP article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen CL, Skordalakes E, Berger JM, Quake SR. Proc Natl Acad Sci USA. 2002;99:16–531. doi: 10.1073/pnas.262485199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HK, Odom TW, Whitesides GM. Anal Chem. 2002;74:3267. doi: 10.1021/ac020151f. [DOI] [PubMed] [Google Scholar]
- 22.Wu HK, Odom TW, Whitesides GM. J Am Chem Soc. 2002;124:7288. doi: 10.1021/ja020551k. [DOI] [PubMed] [Google Scholar]
- 23.Ismagilov RF, Rosmarin D, Kenis PJA, Chiu DT, Zhang W, Stone HA, Whitesides GM. Anal Chem. 2001;73:4682. doi: 10.1021/ac010374q. [DOI] [PubMed] [Google Scholar]
- 24.Yi GR, Thorsen T, Manoharan VN, Hwang MJ, Jeon SJ, Pine DJ, Quake SR, Yang SM. Adv Mater. 2003;15:1300. [Google Scholar]
- 25.Lee JN, Park C, Whitesides GM. Anal Chem. 2003;75:6544. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 26.Rolland JP, Van Dam RM, Schorzman DA, Quake SR, DeSimone JM. J Am Chem Soc. 2004;126:2322. doi: 10.1021/ja031657y. [DOI] [PubMed] [Google Scholar]


