Abstract
Our laboratory has previously characterized age-dependent changes in nociception upon acute morphine withdrawal. This study characterizes changes in mechanical and thermal nociception following acute, intermittent, or continuous morphine administration in infant (postnatal day 5–8) and young (postnatal day 19–21). Morphine was given as a single acute administration (AM), intermittently twice a day for 3 days (IM), or continuously for 72 hours via a subcutaneous pump implanted (CM). AM did not produce long-term changes in mechanical or thermal nociception in either infant or young rats. CM produced changes in mechanical nociception that included the development of tolerance, opioid-induced mechanical allodynia and withdrawal-associated mechanical allodynia in young rats, but only tolerance and a prolonged withdrawal-associated mechanical allodynia in infant rats. IM produced withdrawal-associated mechanical allodynia in both infant and young rats. Measuring paw withdrawal responses to thermal stimuli, infant and young rats showed tolerance without opioid-induced thermal hyperalgesia or withdrawal-associated thermal hyperalgesia following CM. In contrast to CM, withdrawal-associated thermal hyperalgesia was seen in both ages following IM. In conclusion, CM versus IM differentially modified mechanical and thermal nociception, suggesting that opioid-dependent thermal hyperalgesia and mechanical allodynia can be dissociated from each other in infant and young rats. Furthermore, tolerance, opioid-induced hypersensitivity, and withdrawal-associated hypersensitivity are age-specific and may be mediated by distinct mechanisms.
Keywords: morphine withdrawal, spinal cord, allodynia, hyperalgesia, neonatal rat
It has long been recognized that morphine exerts two paradoxical actions on the adult nervous system: the inhibition of pain processing manifested as analgesia and the facilitation of nociceptive sensitivity manifested as allodynia and hyperalgesia (Bederson et al., 1990, Kim et al., 1990, Kaplan and Fields, 1991). This facilitation of nociceptive sensitivity can be observed in as little as 20 min post-morphine administration by precipitating withdrawal with an opioid antagonist (Bederson et al., 1990, Kim et al., 1990, Kaplan and Fields, 1991). It has been proposed that allodynia and hyperalgesia upon withdrawal is an unmasking of pronociceptive opioid actions and, hence, is more prominent during withdrawal due to the absence of an opposing analgesic opioid effect (Mao et al., 1994, Vanderah et al., 2001, Mao, 2002). This phenomenon has been termed latent sensitization or latent hyperalgesia (Fry et al., 1980a, Fry et al., 1980b, Basbaum, 1995). Furthermore, the need to escalate opioid dose to maintain analgesia (tolerance) may not be a result of decreased efficacy of opioids but rather reflect the need to overcome increasing opioid-induced facilitation of pronociceptive pathways (Colpaert, 1996, Celerier et al., 1999, Laulin et al., 1999, Vanderah et al., 2000).
The neonatal nervous system is extremely plastic. Dramatic structural and functional reorganization of spinal sensory systems occur during the neonatal period (Alvares and Fitzgerald, 1999, Fitzgerald and Beggs, 2001). These processes are activity-dependent and thus, abnormal or excessive activity such as that generated by injury may alter normal synaptic development producing changes in somatosensory processing and neurobehavioral sequelae that would not occur in similarly exposed adults (Reynolds and Fitzgerald, 1995, Anand et al., 1999, Ruda et al., 2000). In fact, early exposure to painful stimuli has long-term consequences in both humans and rats (Taddio et al., 1997, Oberlander et al., 2000, Walker et al., 2003). These findings have helped increase the recognition of the need to adequately assess and treat pain in children and infants (Howard et al., 2001). This has led to increasing use of opioids in pediatric patients. Currently, human infants are routinely treated with opioids for pain relief and for the purposes of sedation to permit mechanical ventilation (van Dijk et al., 2002, Simons et al., 2003). With the observation of neonatal abstinence syndrome in 48–84% of infants administered intravenous opioids (Norton, 1988, Arnold et al., 1990, French and Nocera, 1994, Franck and Vilardi, 1995, Franck et al., 1998) it is imperative to assess whether tolerance, opioid-induced pain, and withdrawal pain are present in the pediatric population and whether opioid exposure alters normal synaptic development and produces long-term somatosensory changes (Thornton and Smith, 1998, Thornton et al., 2000).
In previous studies we reported an in vitro and in vivo model of age-specific acute morphine withdrawal-associated hypersensitivity (Sweitzer et al., 2004a, Sweitzer et al., 2004b). The present study addressed the effect of acute, continuous, and intermittent morphine administration on mechanical thresholds and thermal latencies in P5-7 (infant) and P18-21 (young) rats that approximately correspond in neurological developmental to newborn human infants and young children (Dobbing, 1981). The first part of the study examined mechanical and thermal responses following a single acute administration of morphine (AM). The second part characterized mechanical and thermal responses following repeated intermittent administration of morphine (IM). The third part examined mechanical and thermal responses following continuous infusion of morphine (CM) from a subcutaneously implanted mini osmotic pump or morphine pellet.
MATERIALS AND METHODS
Animals
P5-12 (infant) and P18-24 (young) Sprague-Dawley rats (Charles River Laboratories) of both sexes were housed in litters culled at 10 pups/dam in a 12/12 hour light/dark cycle (lights on at 7 am) with food and water available ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committees at Stanford University and the University of South Carolina. Efforts were made throughout the experiment to minimize animal discomfort and to reduce the number of animals used. For all behavioral experiments rats were maintained at nesting temperature with overhead heat lamps whenever the pups were separated from the dams. Animal weights were measured daily.
Maternal Care
There are numerous reports that maternal care can alter offspring behavior (Barron and Riley, 1985, Fleming et al., 1999, Champagne and Meaney, 2001, Huot et al., 2001, Meaney, 2001). There is evidence that experimental perturbation of a litter leads to the whole litter receiving similar disrupted care and that maternal care is driven by the pups and not by the dams making it difficult to control for individual pup-dam interactions (Huot et al., 2001, Marino et al., 2002). We can control for overall maternal care at the litter level (for example how much time the dam spends in different nursing postures). For this study, treatments were randomized within each litter so that pups from each treatment were exposed to the same dam. Thus, multiple dams are represented within each treatment group to control for the effect of maternally driven disrupted care on offspring behaviors (Huot et al., 2001, Marino et al., 2002). This design does not preclude pup-driven differential treatment of individual pups by the dam (e.g. amount of anal-genital licking for each pup).
Experimental Design
Acute Morphine (AM)
Following measurement of baseline mechanical threshold and thermal latency a single subcutaneous injection of morphine (0.5, 1, 3, 6, or 12 mg/kg) or saline was given on P7 or P20. Mechanical threshold and thermal latency were measured at 15 and 30 minutes post-morphine, respectively and then daily for 4 days. The experimental design is shown schematically in Figure 1A.
Figure 1.
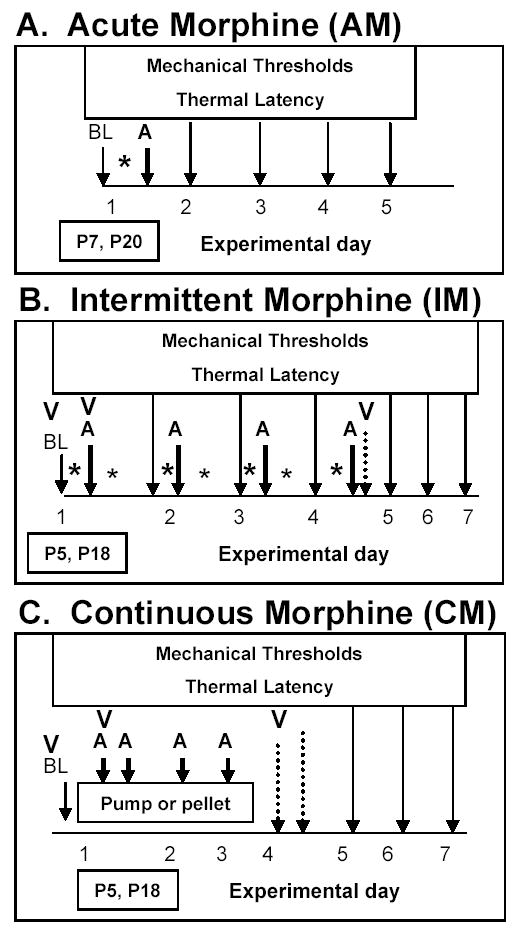
Experimental design for acute morphine administration (A), repeated intermittent morphine (B), and continuous morphine exposure using a subcutaneous mini osmotic pump or morphine pellet (C) in P5-P7 or P18-P20 rats. BL represents collection of baseline mechanical and thermal responses and baseline activity measures. Morphine administration is indicated by an astrick (*). For acute morphine experiments this occurred once on postnatal day 7 or 20. Intermittent morphine studies began on postnatal day 5 or 18 with bid administration for 3 days followed by a single administration on experimental day 4. Morphine pumps or pellets were implanted on postnatal day 5 or 18 for continuous morphine delivery for 72 hours. The A arrow represents time points at which post-morphine analgesia (mechanical and thermal) was assessed. The long arrows indicate mechanical and thermal measures during the withdrawal and abstinence periods. The dashed arrows indicate time points at which acute withdrawal were being assessed. The time points at which animals were video-taped are indicated by a V.
Repeated Intermittent Morphine (IM)
In P5-7 and P18-21 rats, morphine (0.5, 1, or 3 mg/kg for a total daily dose of 1, 2, or 6 mg/kg/day) or saline (subcutaneous in 50 μl volume) was administered twice a day for 3 days (10 am and 5pm) with a final administration on day 4 (10am). Mechanical threshold and thermal latency were measured immediately prior to the first morphine exposure each morning as well as at 1 hour post-exposure. On day 4 mechanical threshold and thermal latency were measured prior to morphine or saline administration as well as at 1 and 4 hours post-morphine or saline and then daily to P11 or P24. The experimental design is shown schematically in Figure 1B.
Continuous Morphine (CM). Mini Pumps
Baseline mechanical threshold and thermal latency were collected on P5 or P18. Mini pumps were implanted as previously described (Thornton and Smith, 1997, Thornton et al., 1997). Briefly, Alzet mini osmotic pumps (Model 1003D) were filled with morphine (0.044, 0.175, 0.7, 1.4 mg/kg/h for a total daily dose of 1.0, 4.2, 16.8, and 33.6 mg/kg/day) or saline vehicle and primed in a heated water bath for 6 hours prior to pump implantation. These pumps provide 72 hours of drug delivery. For pump implantation animals were anesthetized with isoflurane (4–5% induction, 3–4% maintenance). Animals were maintained on a heat pad at nest temperature throughout the surgery. The area above the tail on the lower back was shaved (P18 rats) and cleaned with betadine. A 1 cm incision was made and a small subcutaneous pocket was made using a small pair of hemostats to spread apart the subcutaneous connective tissue. The pump was inserted perpendicular to the incision with the pump inlet facing away from the incision. Vetbond tissue adhesive was used to close the incision. P5 and P18 rats were administered intraperitoneal Penicillin (25,000 or 60,000 units, respectively) and subcutaneous Ringer’s solution (150 or 300 μl, respectively) to prevent infection and dehydration. Immediately following recovery rats were returned to their dam (total time separated from the dam was less than 15 minutes). Animal groups included 1) incision without pump 2) pump with saline vehicle and 3) pump with various concentrations of morphine.
Mechanical threshold and thermal latency were measured at 6, 12, 24, and 48 hours after pump-implantation. Theoretically the last administration of morphine occurred at 72 hours after pump-implantation and animals would begin to go into withdrawal following that time point. Withdrawal time points were measured at 6 and 12 hours after 72 hours, as well as daily until postnatal day 11 or 24. The experimental design is shown schematically in Figure 1C.
Morphine Pellet
Morphine pellets (75 mg) were implanted identically to pump implantation procedures detailed above. Half of each morphine pellet was coated in clear nail polish to slow absorption of the pellet. Similar to the pump studies, mechanical threshold and thermal latency were measured at 6, 12, 24, and 48 hours post-pellet implant as well as at 72 hour immediately before a second surgery to remove the remaining pellet. Mechanical threshold and thermal latency were measured at 4, 24, 48, and 72 hours post-pellet removal.
Behavioral testing: Mechanical and Thermal
Mechanical thresholds were determined by placing P5-11 and P18-24 rats on an elevated wire mesh (2 mm openings). Von Frey hairs (Stoelting Co., WoodDale, Illinois, USA) were used to elicit a cutaneous flexion withdrawal response as described by Fitzgerald et. al. (Fitzgerald et al., 1988). Von Frey hairs of increasing size (the force required to bend the filament in grams) were applied three times to the plantar surface of the left hind paw until the animal withdrew the paw. The smallest size von Frey hair required to produce a withdrawal reflex was recorded as the response threshold to a low-intensity mechanical stimulus.
Thermal latency was measured using the Ugo Basile Plantar Testing apparatus (Stoelting Co., WoodDale, Illinois, USA). P7 and P21 rats were placed on top of a heat-controlled glass plate and the plantar surface of the left hind paw was heated from below with the IR intensity of the lamp set at 40 (108 mW/cm). Thermal latency was the average of two measures with a cut-off of 15 (infant rats) or 20 (young rats) seconds enlisted to prevent tissue damage.
Spontaneous withdrawal behaviors. Infant Rats
This set of experiments was designed to determine whether different schedules of morphine administration resulted in spontaneous withdrawal behaviors similar to the withdrawal syndrome seen in infants exposed to opioid in utero. P5-8 rats were placed singly into observation boxes maintained at nest temperature by overhead heat lamps and videotaped for 8 minutes at baseline (prior to morphine administration), post-morphine (a time point when morphine should be present in these animals), and during withdrawal (a time point following the cessation of morphine administration). Behaviors during the last 6 out of 8 minutes of video were analyzed by an experimenter blinded to animal treatment. During the assessment period, behaviors were recorded at 15 second intervals on a checklist of age-specific behaviors associated with naloxone precipitated withdrawal following acute or chronic opioid administration in neonatal rats (Jones and Barr, 1995, Windh et al., 1995, Ceger and Kuhn, 2000). The number of occurrences of each behavior was summed across the 6 minute observation period. Age specific withdrawal behaviors which included head movements, paw movements, rolling, wall climbing, walking, and quiet were scored (Jones and Barr, 1995, Windh et al., 1995, Ceger and Kuhn, 2000). A quiet score was given when the animal was not moving. Head and paw movements were grouped together and termed simple movements. While the more complex coordinated movements included rolling, walking, and wall climbing.
Spontaneous withdrawal behaviors. Young Rats
A modified activity box (Bond and Di Giusto, 1977, Sanders, 1980) was used to assess locomotor behaviors in P18-21 rats. Animals were placed in a square box whose bottom was split into 4 quadrants for behavioral analysis. Rats were videotaped in this box for 8 minutes at baseline (prior to morphine administration), post-morphine (a time point when morphine should be present in these animals), and during withdrawal (a time point following the cessation of morphine administration). An experimenter blinded to animal treatment later analyzed the videos and counted the 1) number of quadrants that the animal moved through and 2) the number of times that the animals reared onto their hindpaws while lifting their forepaws off the floor of the box.
Statistics
Behavioral data was analyzed for significance by one-way ANOVA followed by a post-hoc Bonferroni analysis. Mechanical and thermal data was analyzed for significance by repeated measures ANOVA followed by a post-hoc Bonferroni analysis. P-values <0.05 were considered significant. All statistical analysis was done with GraphPad Prism version 4.0 (GraphPad Software, San Diego California USA, www.graphpad.com).
Results
Weight Gain: Infant rats
Both CM and IM dose-dependently reduced animal weight compared to saline control animals (Table 1). A difference in weight at any one point may be attributed to 1) loss of body weight or 2) decrease in normal weight gain. Normal weight gain during this period of development is approximately 2 grams/day. A decrease in weight gain was observed on experimental day 3 and 4 in rats intermittently administered 0.5 and 1 mg/kg morphine, respectively, as compared to saline controls. Reduced weight gain following CM occurred during both morphine administration and the withdrawal period compared to saline or incision controls. A reduction in weight gain occurred within 48 hours of installation of the pump delivering the highest concentrations of CM (experimental day 3). Maximal suppression of weight gain was on the day following drug withdrawal at the highest concentration of morphine (experimental day 5). Normal (~2g/day) weight gain returned by P10 (experimental day 6) in animals who received the highest concentration of morphine. Pumps delivering the lower concentrations of morphine did not change weight.
Table 1.
Continuous morphine dose-dependently decreases the weights of P5-11 rats across the course of the exposure as well as during abstinence (average ± SEM). The days on which animals were exposed to morphine are indicated by the gray shading. The experimental day (ED) is indicated for each of the three morphine dosing schedules.
| Postnatal age | P5 | P6 | P7 | P8 | P9 | P10 | P11 |
|---|---|---|---|---|---|---|---|
| Acute (n=5−6/group) | ED 0 | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | |
| Saline | 14.5 ± 0.4 | 16.3 ± 0.6 | 18.0 ± 0.5 | 19.5 ± 0.6 | 21.1 ± 0.6 | 23.9 ± 0.8 | |
| 0.5 mg/kg morphine | 15.4 ± 0.4 | 17.2 ± 0.3 | 18.9 ± 0.4 | 20.5 ± 0.4 | 22.3 ± 0.6 | 24.7 ± 0.4 | |
| 1 mg/kg morphine | 14.4 ± 0.4 | 16.2 ± 0.5 | 17.9 ± 0.4 | 19.8 ± 0.4 | 21.4 ± 0.4 | 24.1 ± 1.0 | |
| 6 mg/kg morphine | 14.5 ± 0.4 | 15.5 ± 0.3 | 17.6 ± 0.4 | 19.2 ± 0.4 | 20.9 ± 0.4 | 23.1 ± 0.6 | |
| Continuous – pump (n=5−6/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ED 6 | ED 7 |
| Saline | 11.9 ± 0.4 | 15.5 ± 0.5 | 17.4 ± 0.5 | 19.2 ± 0.5 | 21.2 ± 0.5 | 23.5 ± 0.5 | 25.4 ± 0.7 |
| Sham surgery | 12.1 ± 0.3 | 15.5 ± 0.3 | 17.8 ± 0.3 | 19.7 ± 0.3 | 21.7 ± 0.4 | 24.2 ± 0.4 | 26.1 ± 0.4 |
| 0.044 mg/kg/h morphine | 11.9 ± 0.5 | 15.2 ± 0.5 | 17.3 ± 0.6 | 18.4 ± 0.9 | 20.1 ± 1.1 | 22.5 ± 1.1 | 24.6 ± 1.1 |
| 0.175 mg/kg/h morphine | 11.7 ± 0.4 | 14.4 ± 0.5 | 16.4 ± 0.6 | 18.3 ± 0.6 | 20.1 ± 0.6 | 22.2 ± 0.4 | 24.3 ± 0.6 |
| 0.7 mg/kg/h morphine | 11.7 ± 0.5 | 13.9 ± 0.5 | 15.3 ± 0.6* | 17.4 ± 0.4* | 18.6 ± 0.3* | 20.2 ± 0.5* | 22.0 ± 0.6* |
| 1.4 mg/kg/h morphine | 12.3 ± 0.4 | 13.9 ± 0.4 | 14.2 ± 0.4* | 15.0 ± 0.6* | 15.9 ± 0.5* | 17.8 ± 0.3* | 19.6 ± 0.3* |
| Intermittent (n=10−11/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ED 6 | ED 7 |
| Saline | 12.6 ± 0.5 | 14.4 ± 0.3 | 17.3 ± 0.4 | 19.3 ± 0.4 | 21.9 ± 0.5 | 24.1 ± 0.4 | 29.0 ± 0.3 |
| 0.5 mg/kg morphine | 12.2 ± 0.2 | 13.2 ± 0.1 | 15.2 ± 0.4 | 17.0 ± 0.4* | 19.1 ± 0.3* | 21.3 ± 0.3* | 26.3 ± 0.5* |
| 1 mg/kg morphine | 12.5 ± 0.2 | 14.1 ± 0.4 | 15.1 ± 0.5* | 16.5 ± 0.8* | 18.7 ± 1.0* | 20.9 ± 1.2* | 25.1 ± 1.6* |
p<0.05 compared to saline or sham surgical controls
Weight Gain: Young Rats
AM or IM had minimal effect on weight gain across the days of treatment as compared to saline controls (Table 2). A reduction in weight gain was observed on the first day of abstinence (experimental day 5) in animals given intermittent 3 mg/kg morphine. The highest dose of CM reduced weight gain on the last day of infusion (experimental day 4). Following morphine withdrawal a dose-dependent reduction in weight gain was seen in morphine treated animals compared to vehicle or sham controls (Table 2). Normal weight gain (~5g/day) returned by experimental day 6 and 7 (P23 and P24, respectively) in animals that received 0.7 and 1.2 mg/kg/hr, respectively. Within 72 hours of pellet implant (experimental day 4) rat weight gain was significantly lower than incision controls (Table 2). Following withdrawal rats exposed to morphine had a decreased rate of weight gain compared to incision control rats (Table 2). A normal rate of weight gain returned by P23 in the pellet implanted animals.
Table 2.
Continuous morphine dose-dependently decreases the weights of P18-24 rats across the course of the exposure as well as during abstinence (average ± SEM). The days on which animals were exposed to morphine are indicated by the gray shading. The experimental day (ED) is indicated for each of the three morphine dosing schedules.
| Postnatal age | P18 | P19 | P20 | P21 | P22 | P23 | P24 |
|---|---|---|---|---|---|---|---|
| Acute (n=5−6/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ||
| Saline | 56.9 ± 1.3 | 61.0 ± 1.6 | 65.0 ± 1.7 | 68.8 ± 2.4 | 74.3 ± 1.2 | ||
| 1 mg/kg morphine | 53.9 ± 1.6 | 57.6 ± 1.8 | 62.2 ± 1.8 | 65.8 ± 1.5 | 72.8 ± 1.8 | ||
| 3 mg/kg morphine | 56.1 ± 1.5 | 61.9 ± 1.8 | 66.8 ± 1.9 | 69.4 ± 2.2 | 74.6 ± 2.5 | ||
| 12 mg/kg morphine | 50.3 ± 1.0 | 55.4 ± 1.1 | 60.1 ± 0.8 | 63.3 ± 0.9 | 71.5 ± 2.1 | ||
| Continuous - pump (n=5−6/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ED 6 | ED 7 |
| saline | 46.6 ± 1.6 | 49.9 ± 1.0 | 55.7 ± 1.6 | 60.6 ± 2.1 | 65.6 ± 2.5 | 71.6 ± 2.6 | 76.9 ± 2.7 |
| sham | 46.3 ± 1.1 | 49.5 ± 1.6 | 56.4 ± 1.6 | 60.1 ± 1.5 | 66.0 ± 1.5 | 71.6 ± 1.8 | 76.8 ± 1.8 |
| 0.175 mg/kg/h morphine | 47.4 ± 1.4 | 49.4 ± 0.7 | 55.3 ± 1.0 | 58.5 ± 1.2 | 63.2 ± 1.2 | 68.8 ± 0.9 | 73.2 ± 1.1 |
| 0.7 mg/kg/h morphine | 46.0 ± 1.8 | 46.5 ± 1.2 | 52.2 ± 1.9 | 55.7 ± 2.2 | 59.0 ± 2.3* | 64.1 ± 2.0* | 70.3 ± 2.2* |
| 1.4 mg/kg/h morphine | 46.5 ± 0.7 | 47.1 ± 0.6 | 51.9 ± 0.8 | 55.5 ± 1.0* | 57.1 ± 1.4* | 61.3 ± 1.2* | 66.8 ± 1.2* |
| Continuous - pellet (n=5−6/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ED 6 | ED 7 |
| Sham | 45.6 ± 1.1 | 48.1 ± 1.0 | 54.0 ± 0.9 | 61.3 ± 1.6 | 63.9 ± 1.2 | 68.4 ± 0.7 | 74.7 ± 2.6 |
| Pellet | 44.4 ± 1.1 | 44.7 ± 2.0 | 48.8 ± 2.6 | 55.4 ± 2.4* | 51.5 ± 2.6* | 57.9 ± 2.7* | 63.8 ± 2.1* |
| Intermittent (n=5−6/group) | ED 1 | ED 2 | ED 3 | ED 4 | ED 5 | ED 6 | ED 7 |
| saline | 46.3 ± 1.1 | 49.8 ± 1.4 | 56.2 ± 1.5 | 63.2 ± 1.8 | 66.8 ± 2.0 | 72.0 ± 2.0 | 76.6 ± 2.1 |
| 1 mg/kg morphine | 45.3 ± 0.8 | 48.6 ± 0.8 | 53.8 ± 1.0 | 59.9 ± 1.1 | 62.5 ± 1.5 | 68.6 ± 1.7 | 74.4 ± 2.1 |
| 3 mg/kg morphine | 44.4 ± 0.8 | 46.4 ± 0.9 | 51.0 ± 1.1 | 57.7 ± 1.3 | 60.1 ± 1.5* | 65.5 ± 1.5 | 72.1 ± 2.1 |
p<0.05 compared to sham or vehicle control rats at the same postnatal age
Mechanical and Thermal Nociception: Changes across maturation
Mechanical thresholds increase with maturation even within the small windows in which these experiments have been conducted. This is especially evident in the infant rats with baseline mechanical thresholds of around 0.5 grams on postnatal day 5 (experimental day 1) that increase to around 1.5 grams on postnatal day 11 (experimental day 7) (Figures 3&4). The young rats show a much more modest increase from around 1.8 grams on postnatal day 18 (experimental day 1) to around 2.5 grams on postnatal day 24 (experimental day 7) (Figures 3&4).
Figure 3.
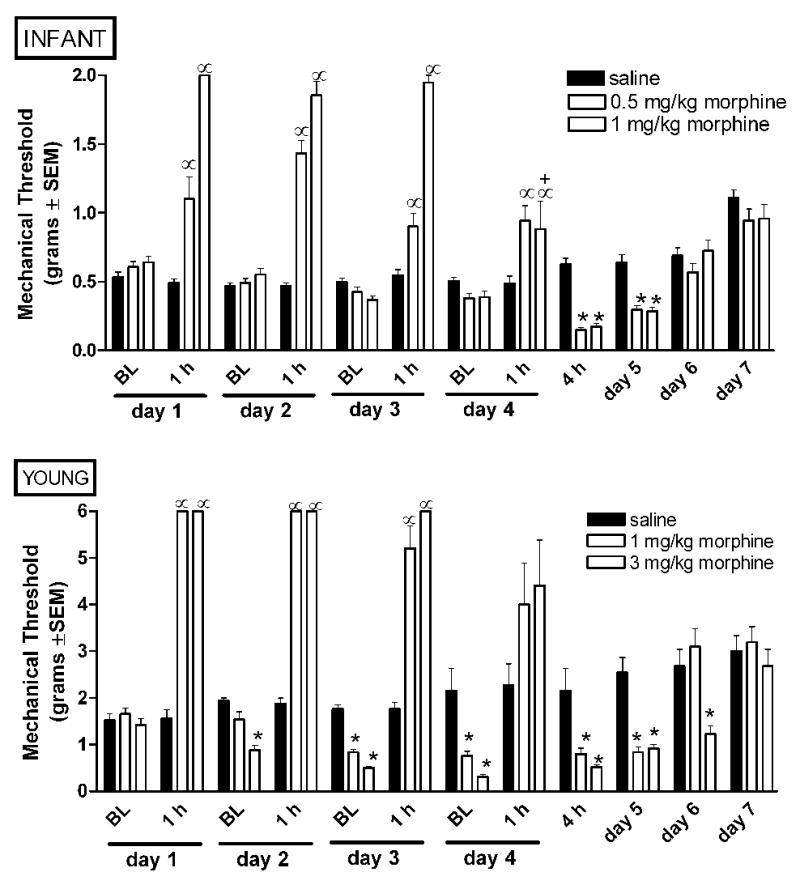
Intermittent morphine age dependently alters mechanical nociception in infant and young rats. Morphine was administered bid from experimental day 1–3, and once on experimental day 4. Baseline mechanical thresholds were collected in the morning immediately prior to the first dose of morphine, and at 1 and 4 hours post-morphine as indicated. Tolerance develops by day 4 only in infant rats administered the higher dose of morphine. A progressive decrease in baseline mechanical thresholds (allodynia) across subsequent days of morphine exposure is only observed in young rats. Withdrawal associated allodynia is seen at both ages. Data is average ± SEM (n=10−11/group in P7 rats, n=5−6/group in P21 rats). α p<0.05 compared to time matched saline injected. *p<0.05 compared to baseline from time matched saline injected animals. +p<0.05 from analgesia observed in the same animals at 1 hours post-morphine on day 1.
Figure 4.
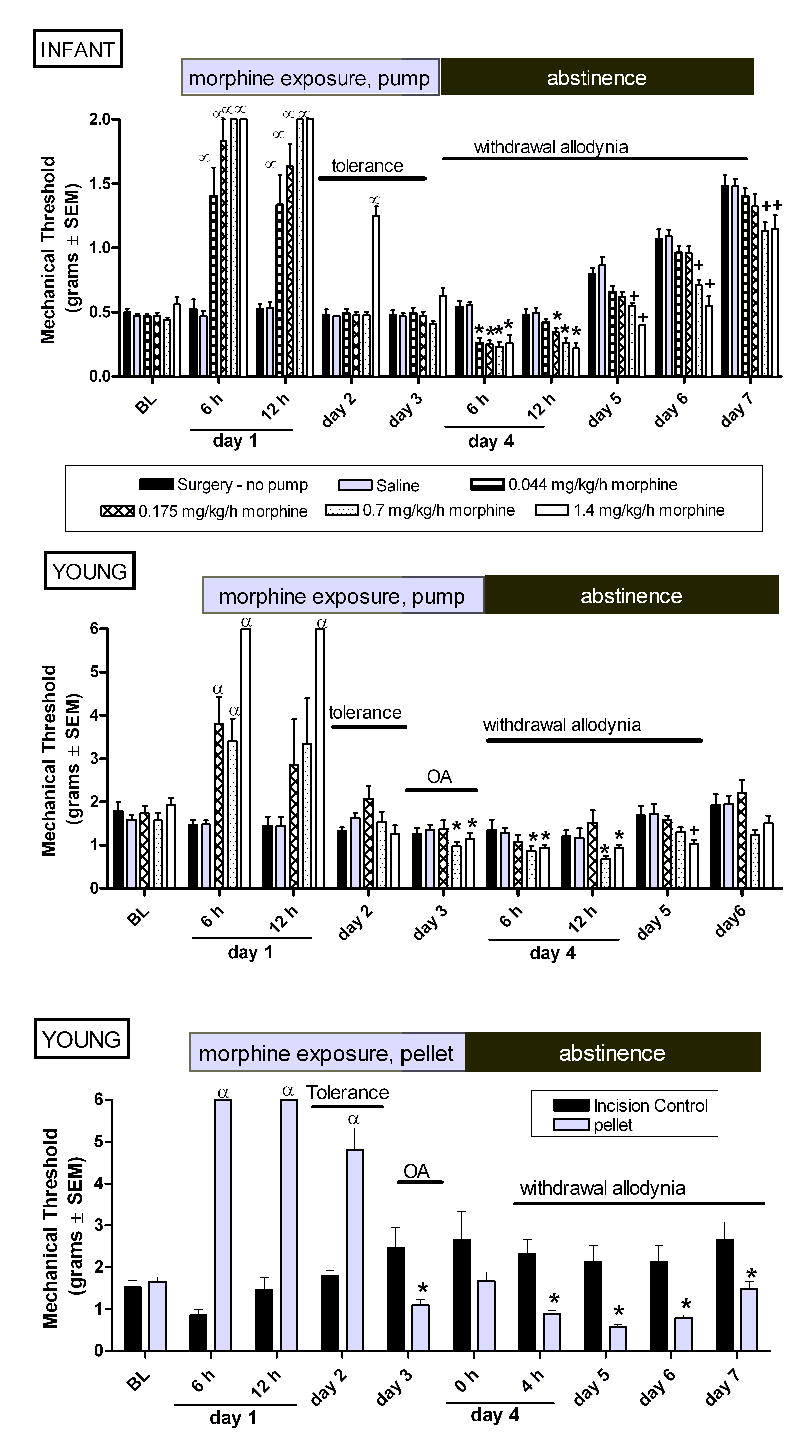
Continuous morphine via a mini osmotic pump age-dependently alters mechanical nociception in infant and young rats. Pumps or morphine pellets were implanted on experimental day 1 and administered continuous morphine for 72 hours. In young rats similar changes in mechanical threshold are observed using either a mini osmotic pump (B) or a morphine pellet (C). Using the same dose of morphine (1.4 mg/kg/hr) tolerance develops more quickly in young (day 2) compared to infant (day 3) rats. By day 3, mechanical thresholds are below control animal responses in young rats indicating the development of opioid-associated mechanical allodynia (OA). Withdrawal associated decreases in mechanical thresholds are seen in both ages. Data is average ± SEM (n=5−6/group). α p<0.05 compared to time matched saline exposed animals, or time matched incision (no pump) controls. *p<0.05 compared to time matched saline exposed animals, or time matched incision (no pump) controls. +p<0.05 from time matched saline injected and time matched saline injected animals.
Similarly, thermal latencies increase with maturation within the small windows of these experiments. As with mechanical thresholds, the infant rats show the largest change across the experiment increasing from about 3–5 seconds on postnatal day 5 (experimental day 1) to around 12–14 seconds on postnatal day 11 (experimental day 7) (Figures 6&7). The young rats show very little changes in thermal latency from postnatal day 18–24 (Figures 6&7).
Figure 6.
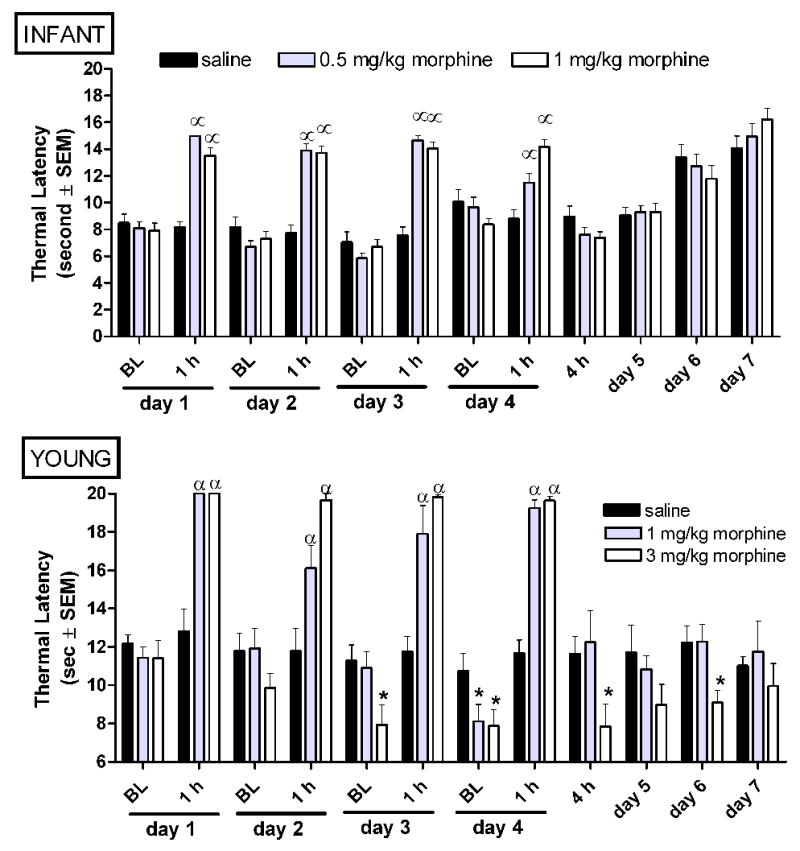
Intermittent morphine age-dependently alters thermal nociception in infant and young rats. Morphine was administered bid from experimental day 1–3, and once on experimental day 4. Baseline thermal latencies were collected in the morning immediately prior to the first dose of morphine, and at 1 and 4 hours post-morphine as indicated. Tolerance to the analgesic effect of morphine does not develop at either age. A progressive decrease in baseline thermal latencies across subsequent days of morphine exposure and withdrawal-associated thermal hypersensitivity are only observed in young rats. Data is average ± SEM (n=10−11/group in P7 rats, n=5−6/group in P21 rats). αp<0.05 compared to time matched saline injected. *p<0.05 compared to time matched saline exposed animals.
Figure 7.
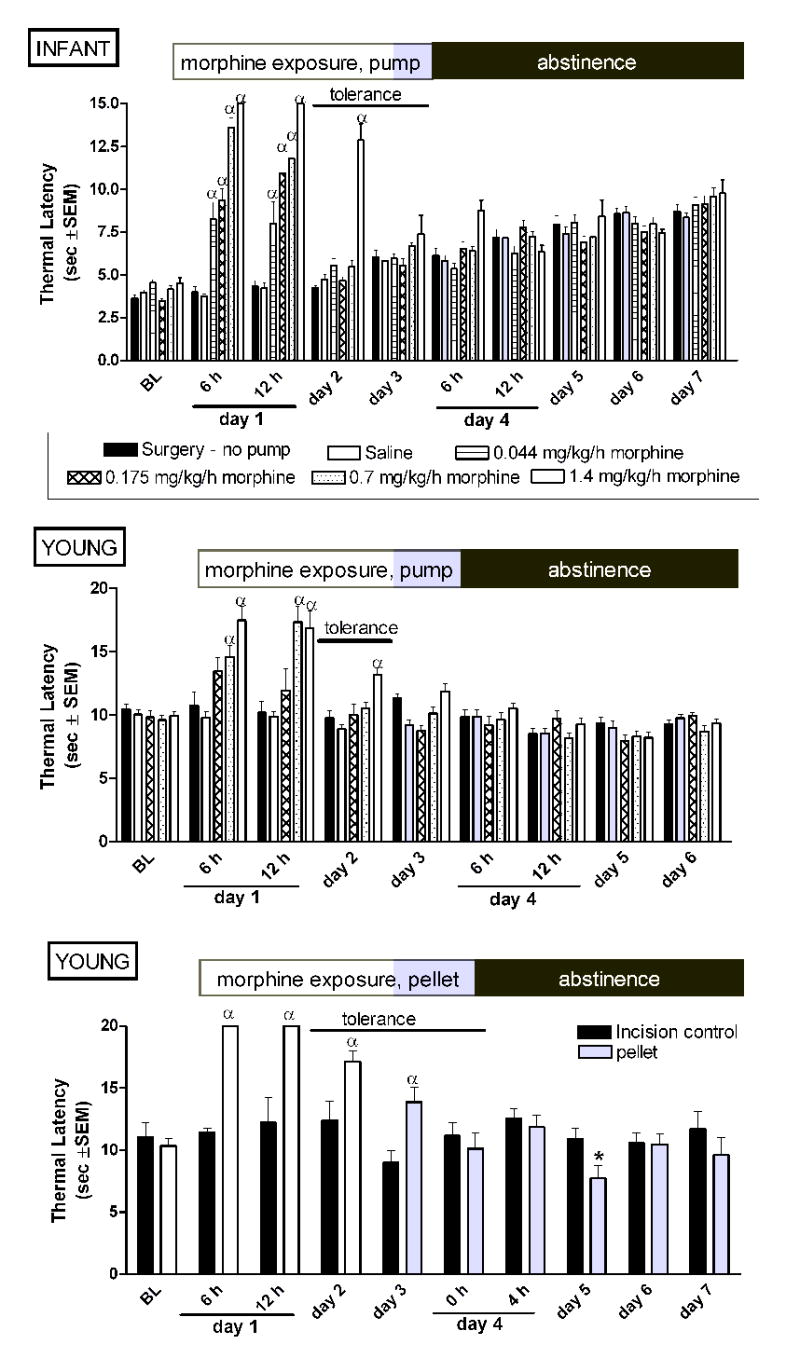
Continuous morphine via a mini osmotic pump age-dependently alters thermal nociception in infant and young rats. Pumps or morphine pellets were implanted on experimental day 1 and administered continuous morphine for 72 hours. Continuous morphine via mini osmotic pump produces tolerance in both infant and young rats. In contrast to mechanical thresholds, discontinuation of morphine does not alter thermal latencies at either age. Similarly, tolerance was observed in young rats implanted with a morphine pellet. A decrease in thermal latency was seen at 24 hours post-pellet removal in the young rats. Data is average ± SEM (n=5−6/group). αp<0.05 compared to time matched saline exposed animals, or time matched incision (no pump) controls. *p<0.05 compared to time matched incision (no pellet) controls.
Mechanical Allodynia: Acute Morphine
In infant rats, AM increased the mechanical force required to elicit paw withdrawal (analgesia) at 15 minutes post-morphine as compared to vehicle saline controls (Figure 2A). We have previously reported an early and transient decrease in mechanical thresholds at 4–5 hours after 1, but not, 0.5 mg/kg AM (Sweitzer et al., 2004b). Similar to previous findings that mechanical threshold returned to baseline levels by 6 hours post-morphine (Sweitzer et al., 2004b), in the present study 0.5 and 1 mg/kg morphine have no effect on mechanical thresholds at 24 hours (experimental day 2). In contrast, a decrease in mechanical threshold was observed at 24 hours (experimental day 2) following 6 mg/kg AM (Figure 2A). This suggests that the duration of withdrawal-associated allodynia may be age-dependent and warrants further investigation.
Figure 2.
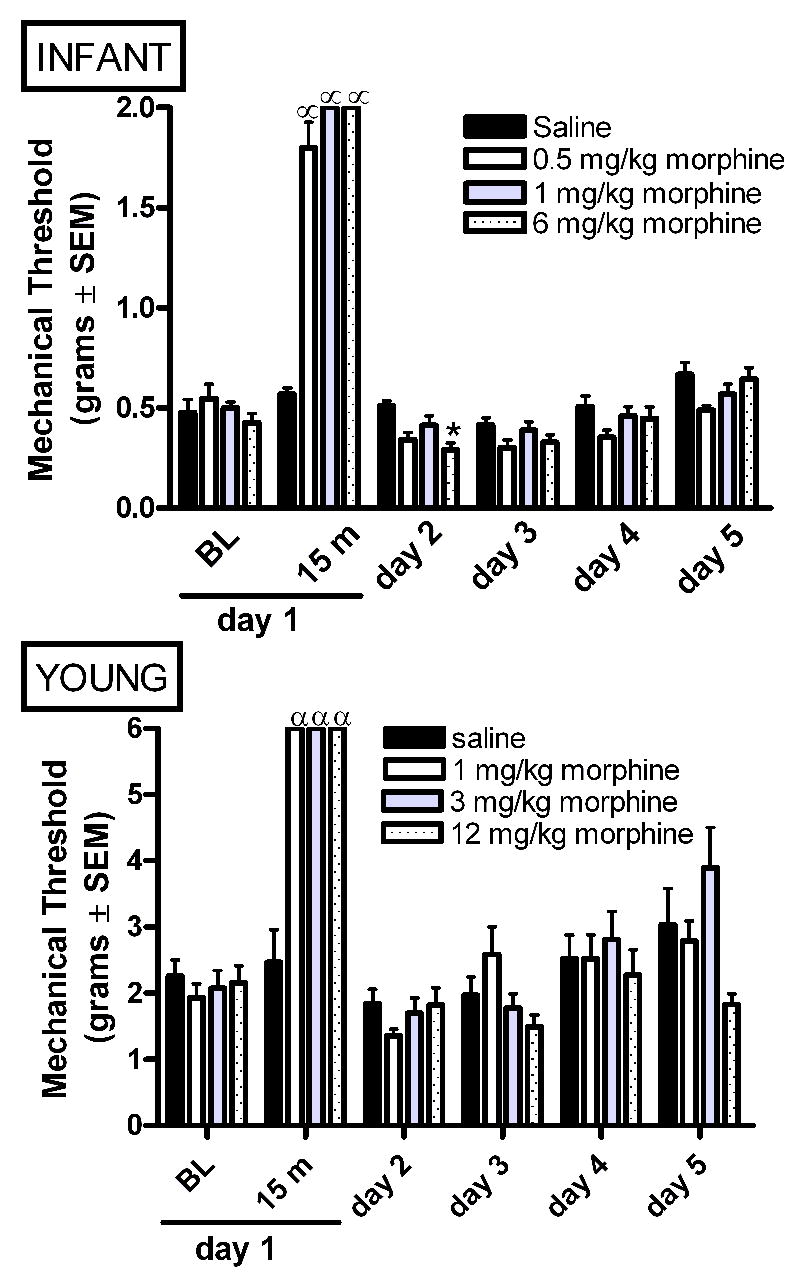
A single acute administration of morphine on experimental day 1 produces analgesia to mechanical stimulation with von Frey hairs in both infant and young rats. The highest dose of morphine produced a transient decrease in mechanical thresholds in infant rats at 24 hours post-morphine (experimental day 2). Data is average ± SEM (n=5−6/group).α p<0.05 compared to time matched saline injected. *p<0.05 compared to baseline from time matched saline injected animals.
In young rats, a single acute administration of morphine produced analgesia at 15 minutes (Figure 2B). We have previously reported an early and transient decrease in mechanical thresholds at 3–5 hours after 1, or 3 mg/kg AM (Sweitzer et al., 2004b). In contrast to the mechanical allodynia present at 24 hours in infant rats, long term changes in mechanical threshold were not observed following AM in the young rats (Figure 2B).
Mechanical Allodynia: Intermittent Morphine
In infant rats IM produced analgesia at 1 hour post-drug administration on all days (Figure 3A). The increase in mechanical threshold produced by 1 mg/kg morphine was significantly less on experimental day 4 as compared to the morphine-induced increase on experimental day 1 suggesting the development of tolerance with the higher dose of morphine. In infant rats, withdrawal-associated allodynia (mechanical thresholds below baseline or vehicle animals following cessation of drug delivery) was observed at 4 hours after the last administration of morphine on experimental day 4 (Figure 3A). Mechanical allodynia persisted 24 hours later on experimental day 5. Mechanical thresholds returned to levels equivalent to saline treated control animals by experimental day 6. This data shows that low dose morphine can result in withdrawal-associated allodynia in the absence of the development of tolerance.
As with infant rats, in young rats IM produced analgesia at 1 hour post-drug on all days examined (Figure 3B). Similarly, the increase in mechanical thresholds produced by both 1 and 3 mg/kg morphine may be less on experimental day 4 as compared to experimental day 1. This decrease in analgesia is preceded by the development of mechanical allodynia at baseline beginning on experimental day 2 (after 2 doses of morphine) with the highest dose of morphine (3 mg/kg), and by day 3 with the low dose of morphine (1 mg/kg). Thus, mechanical allodynia following repeated intermittent morphine administration occurs in the absence of tolerance. Future investigation will be required to differentiate whether the decrease in mechanical thresholds at one hour post-morphine on experimental day 4 are a result of opioid tolerance or rather a consequence of withdrawal-associated mechanical allodynia. The duration of mechanical allodynia was dose-dependent with a return to baseline at 24 and 48 hours in 1 and 3 mg/kg morphine groups, respectively.
Mechanical Allodynia: Continuous Morphine
In infant rats, CM for 72 hours via a subcutaneous mini osmotic pump produced tolerance and withdrawal-associated allodynia at all doses of morphine examined (Figure 4A). Tolerance developed more quickly to the lower doses of morphine. A dose-dependent decrease in mechanical threshold following cessation of morphine persisted for the duration of the experiment.
In young rats, CM via a subcutaneous osmotic pump produced tolerance, opioid-induced allodynia, and withdrawal-associated allodynia that was dependent upon the dose of morphine delivered in the pump (Figure 4B). Tolerance developed more rapidly with lower doses of morphine. The development of mechanical allodynia during continued morphine infusion (opioid-induced allodynia) was only observed at the highest doses of morphine. Despite the age-dependent increase in mechanical threshold, significant and prolonged allodynia was observed in the post-drug infusion rats that had been infused at 0.7 and 1.4 mg/kg/h (Figure 4B). The duration of withdrawal-associated allodynia was dose-dependent with the higher doses producing a more prolonged decrease in mechanical thresholds.
CM via implantation of a morphine pellet produced tolerance, opioid-induced allodynia, and withdrawal-associated allodynia (Figure 4C). Parallel studies using the pellet in P7 rats produced a high rate of mortality from respiratory depression following pellet implantation and precluded parallel studies in the younger rats.
Thermal Hyperalgesia: Acute Morphine
In infant rats, AM increased thermal latencies at 30 minutes post-morphine as compared to vehicle controls (Figure 5A). Thermal latencies were similar in all treatment groups on all days examined.
Figure 5.
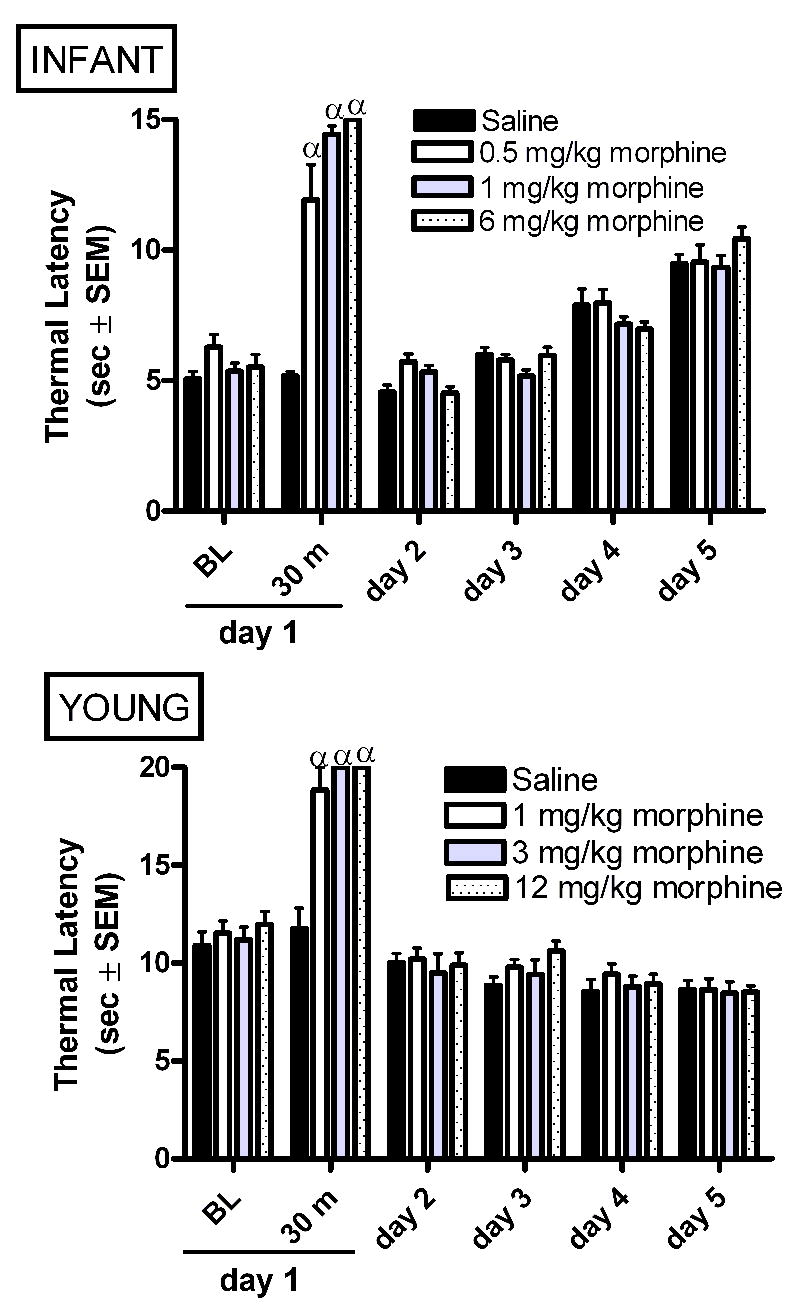
Acute morphine produces analgesia to thermal stimuli in both infant and young rats. Data is average ± SEM (n=5−6/group). α p<0.05 compared to time matched saline injected.
In young rats, a single acute administration of morphine produced analgesia at 30 minutes without producing long term changes in thermal latency (Figure 5B).
Thermal Hyperalgesia: Intermittent Morphine
In infant rats, IM had no effect on morphine analgesia, baseline thermal latencies, or thermal latencies during the abstinence period (Figure 6A).
In contrast, in young rats, IM produced thermal hyperalgesia at baseline on day 3 (Figure 6B). Unlike mechanical thresholds, using thermal stimuli IM does not produce tolerance. Thermal hyperalgesia persisted into the abstinence period on experimental day 5 and 6 (Figure 6B).
Thermal Hyperalgesia: Continuous Morphine
In infant rats, CM via a subcutaneous osmotic pump produced tolerance but not withdrawal-associated hyperalgesia (Figure 7A).
Similarly, in young rats CM via a subcutaneous pump produced tolerance but not withdrawal-associated hyperalgesia (Figure 7B). Tolerance was also observed following implantation of a morphine pellet (Figure 7C). Withdrawal-associated hyperalgesia was present only at 24 hr post-pellet removal. These findings suggest that a higher morphine exposure is required to get withdrawal-associated thermal hyperalgesia.
Spontaneous withdrawal behaviors: Infant Rats
For the IM and CM studies, baseline activity measures were collected on postnatal day 5 prior to any experimental manipulation. For the IM experiments post-morphine behavioral measures were collected at one hour after the first administration of morphine. For the CM studies, post-morphine measures were collected 6 hours after pump implantation surgery (Table 3). In the IM study, administration of 0.5 and 1 mg/kg morphine increased the frequency of quiet behavior and decreased the frequency of simple behaviors (head and paw movements) compared to saline vehicle and normal naïve rats at 1 hour post-morphine on experimental day 1 (Table 3). Similarly, CM produced a dose-dependent increase in quiet behavior compared to saline infused control animals at 6 hours post-pump implantation (Table 3).
Table 3.
Behaviors at baseline, post-morphine, and during withdrawal in P5-8 rats exposed to intermittent or continuous morphine. Frequency of behaviours were scored for 6 minutes at baseline (prior to drug exposure on experimental day 1), post-morphine (1 hour post-morphine on experimental day 1 for IM study, 6 hours post-pump implantation for CM study), and during withdrawal (4 hours post-morphine on experimental day 4 for IM study, 6 hours post-morphine on experimental day 4 for CM study). All data is the average ± SEM (n=5−6/group)
| Simple Movements | Quiet | Complex Movements | |
|---|---|---|---|
| Normal Naive | |||
| baseline | 5.5 ± 1.0 | 10.8 ± 1.6 | 1.3 ± 0.4 |
| Post-morphine | 5.9 ± 1.1 | 10.5 ± 1.0 | 1.0 ± 0.5 |
| withdrawal | 6.9 ± 0.6 | 9.5 ± 0.7 | 1.5 ± 0.3 |
| Intermittent Saline | |||
| baseline | 7.8 ± 0.7 | 15.3 ± 1.0 | 0.8 ± 0.4 |
| Post-morphine | 10.7 ± 2.7 | 11.3 ± 2.1 | 0.8 ± 0.5 |
| withdrawal | 7.9 ± 1.7 | 9.0 ± 2.4 | 2.6 ± 1.0 |
| 0.5 mg/kg morphine | |||
| baseline | 8.3 ± 1.3 | 14.3 ± 1.1 | 0.7 ± 0.4 |
| Post-morphine | 1.9 ± 0.5ααα,*** | 21.3 ± 0.9ααα,*** | 0.0 ± 0.0 |
| withdrawal | 8.4 ± 2.0 | 5.8 ± 2.4 | 7.9 ± 1.5α,* |
| 1 mg/kg morphine | |||
| baseline | 8.1 ± 1.2 | 13.0 ± 1.4 | 1.2 ± 0.6 |
| Post-morphine | 0.6 ± 0.3 ααα,*** | 23.0 ± 0.5ααα,*** | 0.0 ± 0.0 |
| withdrawal | 16.2 ± 1.8α,* | 3.8 ± 1.6α | 8.1 ± 1.1α,* |
| Incision alone | |||
| Baseline | 7.4 ± 1.4 | 15.5 ± 1.6 | 2.3 ± 0.8 |
| Post-morphine | 6.6 ± 0.7 | 16.1 ± 1.1α | 1.5 ± 0.5 |
| Withdrawal | 9.4 ± 1.2 | 14.0 ± 1.5 | 2.3 ± 0.7 |
| Saline Pump | |||
| Baseline | 7.4 ± 1.1 | 15.7 ± 1.4 | 1.4 ± 0.3 |
| Post-morphine | 7.8 ± 0.5 | 14.7 ± 1.3 | 1.4 ± 0.3 |
| Withdrawal | 12.0 ± 0.3 | 12.8 ± 0.6 | 1.8 ± 0.5 |
| 0.044 mg/kg/h (1.06 mg/kg/day) morphine | |||
| Baseline | 7.1 ± 0.6 | 14.7 ± 1.6 | 2.5 ± 0.7 |
| Post-morphine | 7.8 ± 0.7 | 15.8 ± 0.8α | 1.1 ± 0.3 |
| Withdrawal | 11.3 ± 0.8 | 12.0 ± 2.1 | 3.2 ± 0.5 |
| 0.175 mg/kg/h (4.2 mg/kg/day) morphine | |||
| Baseline | 5.3 ± 0.5 | 16.2 ± 1.9 | 2.3 ± 0.5 |
| Post-morphine | 7.8 ± 0.5 | 15.2 ± 0.7α | 1.6 ± 0.3 |
| Withdrawal | 10.8 ± 0.5 | 9.8 ± 1.3 | 5.3 ± 0.8α |
| 0.7 mg/kg/h (16.8 mg/kg/day) morphine | |||
| Baseline | 7.8 ± 1.1 | 14.7 ± 1.6 | 2.6 ± 0.6 |
| Post-morphine | 4.5 ± 0.7 | 17.7 ± 1.3ααα | 0.5 ±0.2 |
| Withdrawal | 10.3 ± 0.8 | 12.7 ± 0.8 | 0.8 ± 0.2 |
| 1.4 mg/kg/h (33.6 mg/kg/day) morphine | |||
| Baseline | 10.6 ± 2.6 | 13.8 ± 3.3 | 2.8 ± 1.5 |
| Post-morphine | 3.3 ± 0.5 | 20.5 ± 0.6ααα,** | 0.8 ± 0.5 |
| Withdrawal | 5.3 ± 0.6 | 12.3 ± 2.1 | 6.6 ± 1.7αα,+,** |
p<0.05, 0.01, 0.001 compared to time matched normal naïve rats.
p<0.05, 0.01, 0.001 compared to time matched saline injected/infused animals.
p<0.05 from time matched incision (no pump) controls.
For IM spontaneous withdrawal was assessed on experimental day 4 (after 3 days of IM). Rats exposed to 0.5 and 1 mg/kg morphine had an increased frequency of complex behaviors (rolling, walking, wall climbing) compared to saline vehicle and normal naïve rats at 4 hours post-morphine administration. Rats exposed to 1 mg/kg morphine had an increased frequency of simple behaviors and decreased frequency of quiet behavior compared to saline vehicle and normal naïve rats (Table 3). This data suggests that spontaneous abstinence-associated behaviors can be observed in infant rats following IM for 3 days.
Withdrawal behaviors were measured at 6 hours after the cessation of CM. Similar to IM studies, CM dose-dependently increased the frequency of complex behaviors compared to saline infused control animals, incision alone controls, and normal naïve age-matched rats (Table 3).
Spontaneous withdrawal behaviors: Young Rats
For the IM and CM studies, baseline activity measures were collected on postnatal day 18 prior to any experimental manipulation. For the IM experiments post-morphine behavioral measures were collected at one hour after the first administration of morphine. Exposure to IM (1 and 3 mg/kg) decreased the number of line crossings and rearings within one hour of the initial morphine exposure compared to saline controls and/or normal naïve rats (Table 4). During spontaneous withdrawal on experimental day 4 (after 3 days of IM), rats exposed to 1 and 3 mg/kg morphine had increased frequency of line crossings compared to saline controls and normal naïve rats. During the withdrawal period, both 1 and 3 mg/kg morphine exposed rats had increased frequency of rearings compared to saline controls (Table 4). This data suggests that increased line crossings in an activity box may characterize spontaneous withdrawal.
Table 4.
Behaviors at baseline, post-morphine, and during withdrawal in P18-21 rats exposed to intermittent or continuous morphine. All data is the average ± SEM (n=4−7/group). Number of line crossing and rearings were counted for 8 minutes at baseline (prior to drug exposure on experimental day 1), post-morphine (1 hour post-morphine on experimental day 1 for IM study, 6 hours post-pump implantation for CM study), and during withdrawal (4 hours post-morphine on experimental day 4 for IM study, 6 hours post-morphine on experimental day 4 for CM study).
| Baseline | Post-Morphine | Withdrawal | |
|---|---|---|---|
| Normal Naive | |||
| Rearing | 7.5 ± 2.4 | 11.8 ± 4.1 | 17.2 ± 2.4 |
| Line Crossing | 15.8 ± 4.6 | 19.3 ± 5.3 | 24.3 ± 1.4 |
|
| |||
| Intermittent Saline | |||
| Rearing | 1.8 ± 0.6 | 3.0 ± 0.3 | 4.0 ± 1.6 |
| Line Crossing | 6.2 ± 2.7 | 11.8 ± 2.0 | 14.0 ± 2.7 |
| 1 mg/kg morphine | |||
| Rearing | 3.0 ± 0.4 | 1.0 ± 0.6 α | 27.8 ± 5.8** |
| Line Crossing | 4.8 ±0.8 | 1.4 ± 0.3 α | 44.3 ± 3.7 αα,*** |
| 3 mg/kg morphine | |||
| Rearing | 3.0 ± 1.0 | 0.0 ± 0.0 α,* | 26.0 ± 4.1** |
| Line Crossing | 4.0 ± 1.7 | 0.0 ± 0.0 α,* | 41.0 ± 4.0 αα,*** |
|
| |||
| Incision alone | |||
| Rearing | 19.0 ± 4.7 | 13.6 ± 5.1 | 27.7 ± 7.4 |
| Line Crossing | 23.3 ± 9.2 | 23.3 ± 8.7 | 41.7 ± 5.8 |
| Saline Pump | |||
| Rearing | 20.5 ± 4.9 | 22.8 ± 4.6 | 34.3 ± 10.5 |
| Line Crossing | 49.3 ± 3.5 | 48.5 ± 7.0 | 42.3 ± 8.8 |
| 0.175 mg/kg/h (4.2 mg/kg/day) morphine | |||
| Rearing | 18.0 ± 2.7 | 39.3 ± 7.2 α,+ | 52.8 ± 14.3 |
| Line Crossing | 47.3 ± 6.9 | 74.5 ± 18.1 αα,++ | 65.8 ±13.1 αα |
| 0.7 mg/kg/h (16.8 mg/kg/day) morphine | |||
| Rearing | 21.5 ± 5.1 | 29.0 ± 8.9 α | 35.5 ± 6.5 |
| Line Crossing | 45.8 ± 5.4 | 65.3 ± 12.6 α | 55.5 ± 5.5 |
| 1.4 mg/kg/h (33.6 mg/kg/day) morphine | |||
| Rearing | 12.5 ± 3.8 | 16.8 ± 6.9 | 37.0 ± 11.0 |
| Line Crossing | 36.8 ± 10.0 | 61.5 ± 6.3 | 55.8 ± 6.9 |
| Pellet | |||
| Rearing | 2.8 ± 1.1 | 0.0 ± 0.0*, α,+ | 7.0 ± 1.5 |
| Line Crossing | 4.4 ±2.5 | 0.0 ± 0.0*,α,+ | 19.8 ± 3.6 |
p<0.05, 0.01 compared to time matched normal naïve rats.
p<0.05,0.01, 0.001 compared to time matched saline injected/infused animals.
p<0.05 from time matched incision (no pump) controls.
For the CM studies, post-morphine measures were collected 6 hours after pump implantation surgery (Table 4). Surgery or pump implantation alone had effects on behaviors compared to normal naïve rats. Unlike in the infant rat, in young rats CM did not suppress activity, an in fact, the lowest dose (0.175 mg/kg/h) of morphine increased behaviors compared to incision alone and normal naïve rats. Suppression of behaviors was only seen following implantation of the morphine pellet.
Withdrawal behaviors were measured at 6 hours after the cessation of drug delivery or pellet removal. On the day of withdrawal, increased line crossings were only observed in 0.175 mg/kg/h treated rats as compared to normal naïve rats (Table 4). This suggests that animals may not yet have entered into behavioral withdrawal.
DISCUSSION
Acute, intermittent, and continuous opioid administration are used clinically in infants and children (Lynn et al., 2000). The current study shows both modality specific and age-specific alterations in nociception following different morphine schedules. A single acute administration of morphine decreased mechanical thresholds at 24 hours in P7, but not P21 rats. In addition, mechanical and thermal nociception was altered in an age-dependent manner following 72 hours of IM or CM (Table 5). Withdrawal-associated mechanical allodynia were observed in both infant and young rats following IM and CM. In contrast, thermal hyperalgesia and withdrawal-associated hyperalgesia were only observed in young rats exposed to IM. In addition, opioid-induced allodynia was only observed in young rats exposed to CM via mini osmotic pumps. IM produced greater spontaneous withdrawal behaviours in infant and young rats compared to CM via mini osmotic pumps. These findings suggest that both drug schedule and age at exposure are important factors in the development of tolerance, opioid-induced hypersensitivity, withdrawal-associated hypersensitivity and spontaneous withdrawal behaviors.
Table 5.
Summary of experimental findings in P7 and P21 rats exposed to acute morphine, intermittent morphine, or continuous morphine.
| Postnatal day 7 | Postnatal day 21 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mechanical threshold | Thermal latency | WT | Behavior | Mechanical threshold | Thermal latency | WT | Behavior | |
|
Acute
M exposure M w/drawal |
Analgesia |
Analgesia |
o |
Analgesia o |
Analgesia o |
o |
||
|
Intermittent
M exposure M w/drawal |
Tolerance WH ↓ PWT |
o o |
-- |
↑ quiet ↓ behaviors ↑ behaviors |
↓analgesia MA WH ↓ PWT |
o TH WH ↓ PWL |
o |
↓ behaviors; ↑ behaviors; |
|
Pump
M exposure M w/drawal |
Tolerance WH ↓ PWT |
Tolerance |
-- |
↑ quiet ↑ complex behaviors |
Tolerance OA WH ↓ PWT |
Tolerance |
-- |
↑ behaviors no change |
|
Pellet
M exposure M w/drawal |
Tolerance OA WH ↓ PWT |
Tolerance WH ↓ PWL |
-- |
↓ behaviors no change |
||||
WT = weight; WH = withdrawal associated hypersensitivity; OA = opioid-induced allodynia; MA = mechanical allodynia; TH = thermal hyperalgesia. Yellow highlight indicates age-specific responses. Blue highlights indicate modality specific responses. Green highlights indicate age and modality specific responses.
Withdrawal-associated mechanical allodynia
A decrease in mechanical thresholds at 24 hours after AM was observed in infant, but not young rats. This is similar to a previous report that a single acute dose of heroin produces an early allodynia of short duration and a second longer lasting allodynia. The magnitude and duration of this second allodynia is dependent upon the dose of heroin (Laulin et al., 1998). Thus, possibly higher doses of morphine might be required in young rats to produce a second longer lasting allodynia as compared to infant rats.
In contrast, IM altered baseline mechanical thresholds and thermal latencies only in young rats. This is similar to other reports that as few as two low dose injections of heroin or 4 bolus doses of fentanyl can induce mechanical hyperalgesia (Laulin et al., 1999, Celerier et al., 2000). The duration of hyperalgesia has been reported to lengthen with each exposure to heroin (Celerier et al., 2001). This may at least partly explain the prolonged mechanical allodynia in young rats following cessation of IM.
Prolonged mechanical allodynia following withdrawal from CM was observed in both infant and young rats. Infant rats had a longer duration of allodynia compared to young rats. This study did not differentiate whether the prolonged allodynia is a result of 1) a delay in the maturation of paw withdrawal thresholds or 2) the presence of prolonged withdrawal-associated allodynia. Hypersensitivity long into the abstinent period has been previously reported in adult rats following heroin or fentanyl (Laulin et al., 1998, Celerier et al., 2000, Celerier et al., 2001). At the neural level, prolonged excitation persist long after abstinence in dorsal root ganglion cultures exposed to chronic morphine (Crain and Shen, 1995). In addition, methadone maintenance patients are less tolerant to cold pressor pain compared to controls and non-addict siblings (Ho and Dole, 1979, Compton, 1994, Compton et al., 2000, Compton et al., 2001, Doverty et al., 2001).
The current findings suggest that morphine exposure during infancy can change ongoing developmental processes. There is growing evidence that exogenous administration of opioid agonists may have detrimental consequences to the maturation of pain circuitry (Thornton and Smith, 1998, Thornton et al., 2000). In addition, the neccessity of both nociceptive and non-noxious touch in the proper maturation of the sensorimoter circuit has been recently elucidated (Petersson et al., 2003, Schouenborg, 2003, Waldenstrom et al., 2003, Petersson et al., 2004, Schouenborg, 2004).
The development of nociceptive pathways is an activity dependent process (Fitzgerald and Jennings, 1999, Fitzgerald and Beggs, 2001, Beggs et al., 2002) and thus, abnormal activity such as that generated early opioid exposure may alter normal synaptic development producing changes in somatosensory processing and neurobehavioral sequela that would not occur in similarly exposed adults. Infant rats may be more sensitive because 1) mechanical thresholds undergo the greatest amount of maturation during this first to second week of life (Fitzgerald et al., 1988), 2) morphine has a more widespread action in the immature nervous system (Nandi et al., 2004), and 3) there is extensive re-modeling of opioid receptor expression in the first 3 postnatal weeks (Rahman et al., 1998, Rahman and Dickenson, 1999, Beland and Fitzgerald, 2001).
While the infant rats are more sensitive to the long term changes in mechanical thresholds following CM, they are also better able to compensate for changes in mechanical thresholds following IM when compared to young rats. It is possible that short bouts of morphine withdrawal-induced excitation may off-set morphine-induced inhibition in infant, but not young rats and thus, better maintain the balance of activity and in-activity during this crucial developmental phase.
Opioid-induced allodynia
Opioid-induced allodynia was only observed in the young rats. It is possible that a longer exposure window, as is required in adult rats (ie: 7 days), may produce opioid-induced allodynia in infant rats. The relevance of this longer exposure window must be weighed carefully with the clinical evidence. The three day exposure used in the present study represents a prolonged exposure based on the rapid development during the first three weeks of life for a rat. Crucial studies to determine morphine blood and tissue levels in these developmental models are required to determine whether age-specific manifestations of opioid-induced hypersensitivity are due to altered 1) pharmacokinetics and dynamics of opioids, 2) opioid tissue distribution and 3) opioid clearance at different stages of development. Certainly in human infants altered pharmacokinetics and dynamics have been reported as compared to older patients (Morselli et al., 1980)((Koehntop et al., 1986, Lynn et al., 2000).
Thermal hyperalgesia
Thermal hyperalgesia manifests only in young rats with IM exposure. This confirms earlier work in a continuous infusion model of morphine that repetitive perioidic opioid abstinence (both precipitated by antagonist and spontaneous) is required to lower PWLs (Dunbar and Pulai, 1998, Dunbar and Karamian, 2003). Using the tail-flick assay, tolerance was observed within 24 hours of morphine pellet implantation. Over time thermal latencies returned to baseline levels but never went below baseline levels (Gold et al., 1994). These previous findings support our present findings that intermittent withdrawal is necessary for the development of thermal hyperalgesia in young rats. The absence of thermal hyperalgesia in neonatal rats following IM may possibly be a consequence of the maturation of nociceptive pathways during this window of development. The postnatal maturation of primary afferent fibers may act as a compensatory mechanism that prevents behavioral development of thermal hyperalgesia. In summary, these studies suggest that abstinence, especially when repetitive, can produce long-term changes in the CNS which may enhance nociception in an age-specific and modality-specific manner (Table 5).
Spontaneous withdrawal behaviours (abstinence syndrome)
A decrease in quiet time and an increase in rolling have been reported as age-specific behaviors associated with opioid receptor antagonist precipitated withdrawal (Barr, 1992, Jones and Barr, 1995, Windh et al., 1995, Barr et al., 1998, Ceger and Kuhn, 2000). In the present study, similar behaviors reported as markers of precipitated withdrawal, such a decreased time spent quiet and increased head and paw movements and walking, were observed during the period of spontaneous drug withdrawal. More of these behaviors were observed following 3 days of IM as compared to CM when similar total daily intakes are compared.
In the present study, IM produced larger behavioural manifestations of withdrawal compared to CM, and this was age-independent. In animal models, repeated morphine at daily or weekly intervals increases the severity of precipitated withdrawal symptoms (Schulteis et al., 1997) suggesting a progressive development of dependence as one would expect if acute dependence reflects the early stages in the development of a full opioid dependent state. This increase in severity of withdrawal can occur in the absence of naloxone precipitated withdrawal and following exposure to low doses of morphine (Schulteis et al., 2003) suggesting direct neuro-adaptive responses to repeated exposure to morphine itself that may reflect early stages in opioid dependence. Similarly, in humans a single morphine administration the day before acute antagonist precipitated morphine withdrawal produces increased withdrawal symptoms compared to humans that did not see prior opioid exposure. This suggests that antagonist exposure is not required to enhance withdrawal symptoms upon repeated exposure to opioids (Azorlosa, 1994). The present findings suggest that withdrawal-associated allodynia precedes behavioural manifestations of withdrawal or may occur in the absence of behavioural manifestations of spontaneous withdrawal. Alternatively, CM animals may not be in full opioid withdrawal and withdrawal-associated allodynia may precede spontaneous behavioural withdrawal. Future studies need to be undertaken to examine the kinetics of morphine elimination from the body following 72 hours of continuous exposure.
Conclusions
In summary we have shown that morphine-induced changes in nociception are dependent upon the developmental window in which exposure occurs. IM may lead to sensitization while CM may preferentially produce tolerance. In a study comparing intravenous IM versus CM more post-operative infants had pain scores indicating distress in the intermittent versus continuous exposure group (Lynn et al., 2000). Thus, in the clinic increased opiate doses may be required to alleviate pain in infants and children due to enhancement of pain sensitivity induced by repeated opiate administration. This conclusion would appear to be up-held by the clinical literature in adult opioid addicts and pain patients currently being treated with opioids who report increased pain severity following surgery and use more analgesic agents post-operatively (Paige et al., 1994, Rapp et al., 1995). Importantly, this data suggests that withdrawal-associated allodynia is distinct from withdrawal-associated behaviours and that withdrawal-associated allodynia may be present in the absence of gross behavioural manifestation of withdrawal. This is of important clinical significance with greater than one-half of infants exposed to opioids in the neonatal intensive care unit exhibiting neonatal abstinence syndrome (Norton, 1988, Arnold et al., 1990, French and Nocera, 1994, Franck and Vilardi, 1995, Franck et al., 1998). It remains to be determined the percentage of infants with opioid-induced alterations in nociception and how that influences analgesic efficacy of opioids in this population.
Acknowledgments
Supported by United States National Institutes of Health Grants NS13108 (JJK), NS4472901 (SMS), and the Alejandro and Lida Zaffaroni Innovation Fund for Addiction Research (SMS).
Abbreviations
- P
postnatal day
- AM
acute morphine
- IM
intermittent morphine
- CM
continuous morphine
Footnotes
Section Editor: Dr. Donna Hammond
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvares D, Fitzgerald M. Building blocks of pain: the regulation of key molecules in spinal sensory neurones during development and following peripheral axotomy. Pain Suppl. 1999;6:S71–85. doi: 10.1016/S0304-3959(99)00140-2. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JH, Truog RD, Orav EJ, Scavone JM, Hershenson MB. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990;73:1136–1140. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL. The effect of chronic naltrexone pretreatment on associative vs. non-associative morphine tolerance. Drug Alcohol Depend. 1994;36:65–67. doi: 10.1016/0376-8716(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Barr G, Zmitrovich A, Hamowy A, Liu P, Wang S, Hutchings D. Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacology, Biochemistry & Behavior. 1998;60:97–104. doi: 10.1016/s0091-3057(97)00596-0. [DOI] [PubMed] [Google Scholar]
- Barr GA. Behavioral effects of opiates during development. In: Miller M, editor. Development of the Central Nervous system: Effects of Alcohol and Opiates. Wiley-Liss; New York: 1992. pp. 221–254. [Google Scholar]
- Barron S, Riley EP. Pup-induced maternal behavior in adult and juvenile rats exposed to alcohol prenatally. Alcohol Clin Exp Res. 1985;9:360–365. doi: 10.1111/j.1530-0277.1985.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Basbaum AI. Insights into the development of opioid tolerance. Pain. 1995;61:349–352. doi: 10.1016/0304-3959(95)00009-H. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Beland B, Fitzgerald M. Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain. 2001;90:143–150. doi: 10.1016/s0304-3959(00)00397-3. [DOI] [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. Prenatal alcohol consumption and open-field behaviour in rats: effects of age at time of testing. Psychopharmacology (Berl) 1977;52:311–312. doi: 10.1007/BF00426717. [DOI] [PubMed] [Google Scholar]
- Ceger P, Kuhn CM. Opiate withdrawal in the neonatal rat: relationship to duration of treatment and naloxone dose. Psychopharmacology (Berl) 2000;150:253–259. doi: 10.1007/s002130000413. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847:18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. J Pain Symptom Manage. 1994;9:462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Chronic morphine-treated sensory ganglion neurons remain supersensitive to the excitatory effects of naloxone for months after return to normal culture medium: an in vitro model of 'protracted opioid dependence'. Brain Res. 1995;694:103–110. doi: 10.1016/0006-8993(95)00773-j. [DOI] [PubMed] [Google Scholar]
- Dobbing J. Later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Paediatrics. Vol. 2. William Heinemann; London: 1981. pp. 744–759. [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Dunbar SA, Karamian IG. Periodic abstinence enhances nociception without significantly altering the antinociceptive efficacy of spinal morphine in the rat. Neurosci Lett. 2003;344:145–148. doi: 10.1016/s0304-3940(03)00227-1. [DOI] [PubMed] [Google Scholar]
- Dunbar SA, Pulai IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther. 1998;284:678–686. [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Shaw A, McIntosh N. Postnatal development of the cutaneous flexor reflex: Comparitive study of preterm infants and newborn rat pups. Developmental Medicine and Child Neurology. 1988;30:520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Franck L, Vilardi J. Assessment and management of opioid withdrawal in ill neonates. Neonatal Netw. 1995;14:39–48. [PubMed] [Google Scholar]
- Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7:364–369. [PubMed] [Google Scholar]
- French JP, Nocera M. Drug withdrawal symptoms in children after continuous infusions of fentanyl. J Pediatr Nurs. 1994;9:107–113. [PubMed] [Google Scholar]
- Fry JP, Herz A, Zieglgansberger W. A demonstration of naloxone-precipitated opiate withdrawal on single neurones in the morphine-tolerant/dependent rat brain. Br J Pharmacol. 1980a;68:585–592. doi: 10.1111/j.1476-5381.1980.tb14574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JP, Zieglgansberger W, Herz A. Development of acute opioid tolerance and dependence in rat striatal neurones. Naunyn Schmiedebergs Arch Pharmacol. 1980b;313:145–149. doi: 10.1007/BF00498571. [DOI] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Ho A, Dole VP. Pain perception in drug-free and in methadone-maintained human ex-addicts. Proc Soc Exp Biol Med. 1979;162:392–395. doi: 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- Howard R, Hatch D, Cole T, Fitzgerald M. Inflammatory Pain and Hypersensitivity are selectively reversed by epidural bupivacaine and are developmentally regulated. Anesthesiology. 2001:95. doi: 10.1097/00000542-200108000-00026. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behav Neurosci. 1995;109:1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–1439. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Fields HL, Barbaro NM. Morphine analgesia and acute physical dependence: rapid onset of two opposing, dose-related processes. Brain Res. 1990;516:37–40. doi: 10.1016/0006-8993(90)90894-h. [DOI] [PubMed] [Google Scholar]
- Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65:227–232. [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–785. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Lynn AM, Nespeca MK, Bratton SL, Shen DD. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain. 2000;88:89–95. doi: 10.1016/S0304-3959(00)00313-4. [DOI] [PubMed] [Google Scholar]
- Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Morselli PL, Franco-Morselli R, Bossi L. Clinical pharmacokinetics in newborns and infants. Age-related differences and therapeutic implications. Clin Pharmacokinet. 1980;5:485–527. doi: 10.2165/00003088-198005060-00001. [DOI] [PubMed] [Google Scholar]
- Nandi R, Beacham D, Middleton J, Koltzenburg M, Howard RF, Fitzgerald M. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain. 2004;111:38–50. doi: 10.1016/j.pain.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Norton SJ. Aftereffects of morphine and fentanyl analgesia: a retrospective study. Neonatal Netw. 1988;7:25–28. [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics. 2000;105:e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- Paige D, Preble L, Watrous G, Kaalaas-Setting J, Compton P. PCA use in cocaine using patients: a pilot study. AJPM. 1994;4:101–105. [Google Scholar]
- Petersson P, Granmo M, Schouenborg J. Properties of an adult spinal sensorimotor circuit shaped through early postnatal experience. J Neurophysiol. 2004;92:280–288. doi: 10.1152/jn.00063.2004. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenstrom A, Fahraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Rahman W, Dashwood MR, Fitzgerald M, Aynsley-Green A, Dickenson AH. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108:239–254. doi: 10.1016/s0165-3806(98)00054-6. [DOI] [PubMed] [Google Scholar]
- Rahman W, Dickenson AH. Development of spinal opioid systems. Reg Anesth Pain Med. 1999;24:383–385. doi: 10.1016/s1098-7339(99)90001-9. [DOI] [PubMed] [Google Scholar]
- Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain. 1995;61:195–201. doi: 10.1016/0304-3959(94)00168-E. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. 1995;358:487–498. doi: 10.1002/cne.903580403. [DOI] [PubMed] [Google Scholar]
- Ruda M, Qing-Dong L, Hohmann A, Peng Y, Toshiya T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–630. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Sanders B. Withdrawal-like signs induced by a single administration of ethanol in mice that differ in ethanol sensitivity. Psychopharmacology (Berl) 1980;68:109–113. doi: 10.1007/BF00432126. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Somatosensory imprinting in spinal reflex modules. J Rehabil Med. 2003:73–80. doi: 10.1080/16501960310010188. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Learning in sensorimotor circuits. Curr Opin Neurobiol. 2004;14:693–697. doi: 10.1016/j.conb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology (Berl) 1997;129:56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76:493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. Jama. 2003;290:2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Wong S, Tjolsen A, Allen C, Mochly-Rosen D, Kendig JJ. Exaggerated nociceptive responses on morphine withdrawal: roles of protein kinase C epsilon and gamma. Pain. 2004a;110:281–289. doi: 10.1016/j.pain.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Allen CP, Zissen MH, Kendig JJ. Mechanical allodynia and thermal hyperalgesia upon acute opioid withdrawal in the neonatal rat. Pain. 2004b;110:269–280. doi: 10.1016/j.pain.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- Thornton SR, Lohmann AB, Nicholson RA, Smith FL. Fentanyl self-administration in juvenile rats that were tolerant and dependent to fentanyl as infants. Pharmacol Biochem Behav. 2000;65:563–570. doi: 10.1016/s0091-3057(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Thornton SR, Smith FL. Characterization of neonatal rat fentanyl tolerance and dependence. J Pharmacol Exp Ther. 1997;281:514–521. [PubMed] [Google Scholar]
- Thornton SR, Smith FL. Long-term alterations in opiate antinociception resulting from infant fentanyl tolerance and dependence. Eur J Pharmacol. 1998;363:113–119. doi: 10.1016/s0014-2999(98)00783-3. [DOI] [PubMed] [Google Scholar]
- Thornton SR, Wang AF, Smith FL. Characterization of neonatal rat morphine tolerance and dependence. Eur J Pharmacol. 1997;340:161–167. doi: 10.1016/s0014-2999(97)01434-9. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Bouwmeester NJ, Duivenvoorden HJ, Koot HM, Tibboel D, Passchier J, de Boer JB. Efficacy of continuous versus intermittent morphine administration after major surgery in 0–3-year-old infants; a double-blind randomized controlled trial. Pain. 2002;98:305–313. doi: 10.1016/S0304-3959(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Windh RT, Little PJ, Kuhn CM. The ontogeny of mu opiate tolerance and dependence in the rat: antinociceptive and biochemical studies. J Pharmacol Exp Ther. 1995;273:1361–1374. [PubMed] [Google Scholar]


