Abstract
OBJECTIVE
Cranial base malignancies involving the infratemporal fossa have been considered unresectable. Advanced operative techniques have made tumor resection feasible in an en bloc fashion with negative histological margins, but there are limited data regarding outcome analysis in patients who have undergone resection of malignant tumors in this area.
METHODS
At Memorial Sloan-Kettering Cancer Center, 25 patients underwent anterolateral cranial base resections for tumors that involved the infratemporal fossa during a 7-year period. The most common tumors were sarcoma (n = 9), squamous cell carcinoma (n = 6), and adenoid cystic carcinoma (n = 3). The median size of the tumors was 6 cm, and 12 tumors involved the anterior cranial base and/or orbit. Tumor resections were divided into three types. Twelve patients underwent Type 1 dissection for tumors involving only the infratemporal fossa and maxillary sinus; 2 patients underwent Type 2 dissections involving the infratemporal fossa and anterior cranial base; and 11 patients underwent Type 3 dissection, which included the infratemporal fossa, anterior cranial base, and orbit. All patients required free flap reconstruction, 22 of which were rectus abdominis free flaps.
RESULTS
Complications occurred in seven patients, including a single mortality resulting from a myocardial infarction. The 2-, 3-, and 5-year survival rates were 69, 63, and 56%, respectively. The relapse-free survival rates were 47% at 2 and 3 years and 41% at 5 years. Recurrences were local in nine patients and distant in four patients.
CONCLUSION
Despite the extensive nature of many infratemporal fossa tumors, they can be resected with acceptable morbidity. Survival rates approach those of anterior cranial base malignancies without infratemporal fossa involvement.
Keywords: Cranial base tumors, Infratemporal fossa
Advanced surgical resection and reconstructive techniques have improved survival rates and decreased morbidity for patients undergoing resection of anterior cranial base malignancies; however, malignant tumors involving the infratemporal fossa have generally been considered unresectable. Tumor resection was avoided in this region because of the perceived biological aggressiveness of these tumors, difficulty in achieving negative histological margins, and the associated surgical morbidity and mortality. Although several articles have provided outcome data for resection of anterior cranial base tumors (1–7, 10, 13, 14), very few authors have addressed cranial base malignancies specifically involving the infratemporal fossa (2, 9).
The infratemporal fossa includes the medial and lateral pterygoid muscles, the pterygoid plates, and cranial base margins that include numerous structures with functional significance. The anterior margin is the maxillary sinus, and the posterior margin comprises the parotid gland and medial pterygoid fascia. The lateral border is the ascending ramus of the mandible, and the superior margin comprises the zygomatic arch and the greater wing of the sphenoid, including the foramina rotundum, ovale, and spinosum and associated traversing nerves and arteries. The internal carotid artery is located at the posteromedial aspect of this space.
Several approaches have been described to resect tumors transgressing the infratemporal fossa, including the Fisch C and subtemporal-preauricular infratemporal fossa approaches (8, 15). These are most useful in patients with tumors that involve the petrous apex and carotid artery. The tumors discussed herein predominantly involved the anterior segment of the infratemporal fossa, often extending to the anterior cranial base and orbit. In this article, we propose a scheme for the systematic evaluation and resection of malignant infratemporal fossa tumors. Outcome analysis is presented on a consecutive series of 25 patients.
PATIENTS AND METHODS
Between 1996 and 2002, 25 patients underwent craniofacial resection of tumors involving the infratemporal fossa at Memorial Sloan-Kettering Cancer Center. The median age was 59 years (range, 29–82 yr); 19 patients were male, and 17 tumors were left sided. All patients underwent preoperative biopsy to confirm the histological characteristics of the tumor. The most common histological tumor types were sarcoma (n = 9), squamous cell carcinoma (n = 6), and adenoid cystic carcinoma (n = 3) (Table 1). Eighteen patients were newly diagnosed, and 7 patients had recurrent disease. Three patients had undergone prior anterior craniofacial resection and radiation therapy. Presenting symptoms most commonly included facial swelling, trismus, proptosis, and maxillary division trigeminal nerve pain or anesthesia. The epicenter of the tumor, as judged radiographically, was the maxillary sinus (n = 15), infratemporal fossa (n = 5), orbit (n = 3), and mandible (n = 2). Computed tomographic and magnetic imaging scans were obtained in all patients. The median tumoral diameter, as judged via preoperative x-rays, was 6 cm (range, 2–12 cm).
TABLE 1.
Tumor histologies
| Tumor Histology | No. of patients |
|---|---|
| Sarcomaa | 9 |
| Squamous cell carcinoma | 6 |
| Adenoid cystic carcinoma | 3 |
| Adenocarcinoma | 2 |
| Neurofibroma | 1 |
| Malignant meningioma | 1 |
| Malignant mixed tumor | 1 |
| Myoepithelial carcinoma | 1 |
| Poorly differentiated carcinoma | 1 |
Sarcoma includes: osteogenic (4), chondrosarcoma (1), hemangiopericytoma (1), leiomyosarcoma (1), spindle cell sarcoma (1), and synovial sarcoma (1).
Two patients underwent preoperative external beam radiation therapy, and 13 received postoperative radiation. One patient underwent intraoperative radiation therapy followed by external beam radiation therapy. The median tumoral dose was 63 Gy (range, 54–70 Gy). All three patients who presented with recurrent disease after tumor resection underwent prior irradiation and were not candidates for further irradiation after craniofacial resection. Six patients did not undergo radiation. All patients had negative histological margins. In addition, six patients received chemotherapy, two preoperatively and four postoperatively.
All tumors involved resection of a portion of the infratemporal fossa to achieve gross tumor resection. All patients underwent total (n = 17) or partial maxillectomy (n = 8). Five patients had dural involvement, and one patient had limited brain involvement. Neck nodes were explored if they were clinically or radiographically suspicious (n = 3). Contraindications to operation included cavernous sinus invasion, carotid artery involvement at foramen lacerum, or bilateral orbital involvement. Patients with radio- and/or chemoresponsive tumors, such as lymphoma, did not undergo operations.
All operations were performed with spinal fluid drainage to limit the degree of brain retraction. An operative classification was devised on the basis of the extent of cranial base resection required to achieve en bloc resection with negative histological margins. Class 1 tumors involved the only infratemporal fossa and maxillary sinus. Class 2 tumors extended to include the anterior cranial base, and Class 3 tumors involved the infratemporal fossa, anterior cranial base, and orbit (Table 2).
TABLE 2.
Tumor classification based on the extent of cranial base resection
| Resection class | No. of patients | Infratemporal fossa | Maxillary sinus | Anterior cranial base | Orbital resection |
|---|---|---|---|---|---|
| 1 | 12 | ● | ● | ||
| 2 | 2 | ● | ● | ● | |
| 3 | 11 | ● | ● | ● | ● |
Operative Descriptions
Type 1 Dissection
Twelve patients underwent Type 1 dissections for tumors that involved the infratemporal fossa and maxillary sinus (Fig. 1). A subtemporal-infratemporal approach has been well described by others (15) but primarily for resection of the petrous apex and midclival lesions rather than infratemporal fossa and maxillary sinus malignancies. In brief, the head is turned 30 degrees, and a standard pterional craniotomy is used. Extradural dissection along the squamous temporal bone and greater wing of the sphenoid is performed in a lateral to medial direction, and the foramen rotundum and maxillary division of the trigeminal nerve (V2) are identified (Fig. 2).
FIGURE 1.

Magnetic resonance imaging scans showing a tumor requiring Type 1 dissection. Sagittal (A) and coronal (B) sections show an adenoid cystic carcinoma extending from the maxillary sinus through the pterygopalatine fossa into the infratemporal and middle cranial fossae. The patient underwent a Type 1 resection, and negative histological margins were achieved.
FIGURE 2.

Illustration showing Type 1 dissection. A, middle cranial floor exposure of the foramina rotundum and ovale. B, osteotomy to expose the infratemporal fossa (dotted lines).
Dissection of the basal dura is then extended posteriorly along the greater wing of the sphenoid to expose the foramen ovale with exposure of the mandibular division of the trigeminal nerve (V3). A perpendicular osteotomy, with the M-8 burr on the Midas Rex drill (Medtronic Midas Rex, Fort Worth, TX), is made from the lateral squamous temporal bone to the foramen ovale (Fig. 2A). The osteotomy is continued anteriorly to the foramen rotundum and V2, which is double ligated with vascular clips. The resulting osteotomy is lateral to the carotid artery, cavernous sinus, and superior orbital fissure (Fig. 2B). The pterygoid muscles are likewise dissected posterior to the tumor.
After intracranial exposure, a transfacial approach is used most commonly, with a Weber-Ferguson incision with a subciliary extension. This approach divides the upper lip in the midline and continues along the nasal subunits in the nasolabial crease. The flap is elevated along the lower eyelid and the infraorbital nerve divided. This allows for mobilization of the orbital contents and adequate access for partial or total maxillectomy. In rare instances, a posterior segmental mandibulectomy also will be necessary, and this will require a lower lip-splitting incision to allow elevation of the lower cheek flap. In addition to partial or total maxillectomy, this provides access for resection of a portion of the masseter, temporalis as well as medial, and lateral pterygoid musculature. The pterygoid plates are freed via the transcranial approach from the greater wing of the sphenoid and with the completion of the intracranial osteotomies, allowing en bloc resection of the partial or total maxillectomy and the infratemporal fossa contents. In rare instances in which segmental mandibulectomy is indicated, special attention must be given to the management of part or all of the facial nerve.
Type 2 Dissection
Two patients required Type 2 dissection necessitated by tumor extension involving the infratemporal fossa and maxillary sinus, as well as the ethmoid and/or sphenoid sinuses at the anterior cranial base (Fig. 3). The tumor is resected in en bloc fashion via subtemporal-infratemporal fossa and anterior craniofacial approaches. A bicoronal incision is made, and a galeal-pericranial flap is elevated. A frontotemporal bone flap is turned with the frontal portion extending to the contralateral midorbit. The initial basal dural dissection is similar to a Type 1 dissection with the addition of the dural dissection over the crista galli and fovea ethmoidalis extending to the planum sphenoidale. Dural repair of the anterior basal defect is most commonly performed with a bovine pericardial patch graft secured with running 4-0 Nurolon suture (Ethicon, Inc., Somerville, NJ) to achieve watertight closure. Fibrin glue is used to augment the repair. The base of cranial resection includes Type 1 dissection with additional bone cuts along the fovea ethmoidales and planum sphenoidale, but sparing the orbit.
FIGURE 3.

Magnetic resonance imaging scan (coronal section) showing an adenocarcinoma extending from the infratemporal fossa and maxillary sinus to involve the ethmoid sinuses and anterior cranial base. This tumor presentation requires a Type 2 dissection.
The previously described Weber-Ferguson incision is used with a midline lip split incision. Because of the configuration of these tumors, patients required en bloc resection of the fossa with total maxillectomy, as well as resection of the anterior cranial base secondary to tumor extension to the fovea ethmoidales and cribriform plate. In addition to incorporation of the infratemporal fossa musculature, a unilateral radical maxillectomy is performed. Furthermore, resection of the orbital floor is performed en bloc with the lamina papyracea and the anterior cranial base to facilitate complete tumor removal (Fig. 4).
FIGURE 4.

Illustration showing Type 2 dissection, including the infratemporal fossa, maxillary sinus, orbital floor, and anterior cranial base.
Every effort is made to preserve the orbital roof to prevent pulsatile exopthalamus. Tremendous communication between the neurosurgeon and the head and neck surgeon is required to facilitate en bloc resection of the U-shaped tumor with preservation of the orbital contents and its function. In addition to the infratemporal fossa osteotomies, corresponding osteotomies also are made at the fovea ethmoidalis and cribriform plate.
With complete mobilization via the transfacial approach, the tumor is resected in a monobloc fashion. This creates a considerable cranial base defect and a great challenge for the plastic and reconstructive surgeon. Bone grafts are plated with split calvarial graft to provide orbital support both inferiorly and medially. A rectus abdominus myocutaneous free flap is used to provide the necessary vascularity for support of the bone grafts. As is the case for all of these procedures, medial canthal tendon reconstruction is performed to a fixed bone support, and reconstruction of the lacrimal system is performed with a self-retaining Silastic tube.
Type 3 Dissection
Eleven patients underwent Type 3 dissection. This consists of a Type 2 dissection with the addition of an orbital exenteration (Fig. 5). The incision and dural dissection are similar to those in the Type 2 procedure, with the exception that the orbital dura is dissected to the level of the optic foramen on the involved side in an extradural fashion. The osteotomies of a Type 1 dissection are extended in a coronal plane anterior to the superior orbital fissure. This osteotomy is extended across the lesser wing of the sphenoid anterior to the optic foramen and the posterior planum sphenoidale. The osteotomy then extends anteriorly to include the contralateral ethmoids and cribriform plate. The orbital contents are cut at the optic foramen. Rarely is the optic nerve resected intradurally because of the small risk of producing a nasal field deficit in the remaining eye. The dural defect is reconstructed with a bovine pericardial patch graft, galeopericranial flap, and free flap reconstruction.
FIGURE 5.

Magnetic resonance imaging scans (axial sections) showing preoperative (A–D) and postoperative (E–H) views of a malignant meningioma tumor involving the left infratemporal fossa, orbit, and ethmoid sinus. The postoperative resection confirms gross total resection of tumor with rectus abdominis free flap reconstruction.
A slightly different facial incision is used for patients undergoing a Type 3 resection. A midline upper lip split incision is carried into a lateral rhinotomy incision with both an upper and lower medial and lateral subciliary eyelid incision (Fig. 6). We do not attempt to preserve the conjunctiva, although it is often feasible to preserve the eyelid skin. This obviates the need for a separate skin paddle reconstruction of the eyelid defect. These lesions represent massive tumors; therefore, removal of the entire infratemporal fossa contents, including the pterygoid plates; radical maxillectomy; orbital exenteration; and unilateral anterior cranial base resection is planned. Because the orbital contents are sacrificed, there is no contraindication to resection of the orbital roof. Clearance of the unilateral maxillary sinus is performed in a fashion analogous to Type 1 and Type 2 dissections with incorporation of the orbit and anterior and middle fossa contents. Intracranial osteotomy allows for mobilization of the specimen and delivery of a monobloc specimen.
FIGURE 6.

Illustrations showing Type 3 dissection. A, bicoronal incision. B, extended frontotemporal bone plate along the dotted lines. C, bone and orbital content removal (shaded areas).
This procedure creates a large soft tissue defect. However, this is an easier defect to reconstruct than the Type 2 defect. A large rectus abdominus free flap typically is used. Despite the large anterior middle fossa cranial bone defect, bone reconstruction is not indicated. The soft tissue reconstruction provides brain support and an adequate barrier between the neurocranium and the sinonasal cavity. Microvascular reconstruction is typically performed to the facial artery and vein, although on rare occasions, it is possible to use the superficial temporal artery and vein as microvascular donor vessels.
Reconstruction
Because of the large soft tissue defect, nearly all patients underwent free tissue transfer reconstruction (Fig. 7). The most common flap was a rectus abdominis free flap (n = 21), followed by a latissimus free flap (n = 2) and radial forearm free flap (n = 1). Temporalis muscle flaps were used in two patients; one patient underwent a limited Type 1 dissection, and the other had a medical contraindication to a free flap. Free tissue transfer flaps were augmented by galeal-pericranial flaps in 12 of 13 patients, who underwent Type 2 or 3 dissections to reinforce a watertight closure after removal of the cribriform plate and olfactory nerves. Split calvarial grafts were used in six patients for orbital roof and/or floor reconstruction. No patient had bone reconstruction of the anterior or lateral cranial base.
FIGURE 7.

A–C, rectus abdominis reconstruction of a Type 3 dissection.
RESULTS
All patients underwent gross total resection of tumor. Eighteen patients (72%) had negative histological margins, and 7 (28%) had microscopically positive margins. Clinical follow-up was performed in all patients at regular intervals, including radiographic follow-up at 3- to 6-month intervals or as clinically indicated. Significant complications occurred in six patients (24%), including one death. The patient who died had been receiving chronic dialysis, had undergone previous maxillectomy for resection of a low-grade sarcoma, and had presented with local recurrence. He underwent Type 1 resection with free flap closure but died on postoperative Day 3 of an acute myocardial infarction. Three patients developed wound complications requiring revisions. Two patients had prior free flaps, and one developed a cutaneous fistula requiring subsequent free flap closure. No patient experienced a bone plate infection or brain abscess.
Medical problems were limited in most patients, with the exception of one patient who developed pneumonia. One patient had persistent postoperative dysphagia requiring placement of a permanent gastrostomy tube. Because all patients had undergone sectioning of mandibular and maxillary divisions of the trigeminal nerve, sensory loss was not considered a complication. There was no evidence of cerebrospinal fluid leakage or pneumocephalus in any patient. Three patients developed trismus that resolved over time. However, given the nature of the operation, this was considered an expected sequela.
Survival analysis was performed with the Kaplan-Meier product limit method. The overall survival rates at 2, 3, and 5 years were 69, 63, and 56%, respectively (Fig. 8A). The relapse-free survival rates were 47% at 2 and 3 years and 41% at 5 years (Fig. 8B). Of the 13 patients who had recurrent tumor, 9 (40%) developed local recurrences, and 4 (12%) developed distant metastases. No patient developed regional failure.
FIGURE 8.
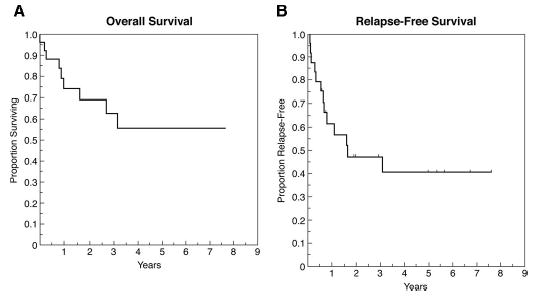
Graphs showing results of Kaplan-Meier analysis of overall survival (A) and relapse-free survival (B).
DISCUSSION
As reported by others, infratemporal fossa tumors are often large at presentation. It has been suggested that 2.5 cm is the smallest size at which a tumor may become symptomatic (16). Guinto et al. (9) reported a series in which the median tumoral diameter was 8 cm at presentation. Some of most common tumors operated in the infratemporal fossa are benign angiofibromas, sphenoid-wing meningiomas, and trigeminal schwannomas, all of which may be cured with gross total resection (8, 9, 12). The referral pattern to our institution typically consists of more aggressive tumor biology. One patient in our series had a benign V2 schwannoma, but the remaining patients had malignant tumors with potentially high rates of local recurrence and metastatic potential.
Many series in the literature have reported tumors of the infratemporal fossa that involve the lateral cranial base, posterior fossa, and clivus (8, 9, 15). The patients in our series with infratemporal fossa lesions were more anteriorly based with involvement of the maxillary sinus, anterior cranial base complex, and orbit. The approach described in our article for exposure and resection of infratemporal fossa is a modification of those described previously (8, 15). The exposure includes an epidural dissection of the greater wing of the sphenoid, the medial extent of which extends from foramen rotundum to foramen ovale. Type 1 dissection is performed through a frontotemporal flap without zygomatic osteotomy (8, 9, 12, 15) or resection of the external ear canal (8), as described by others. Mandibulectomy was only required in selected cases for tumor resection but was not necessary for exposure. As no tumor involved the carotid artery, the posterior osteotomy can be achieved safely from the lateral cranial base anterior to the glenoid fossa extending to foramen ovale. The carotid artery traverses the petrous bone posterior and medial to this osteotomy. The bone cut than extends anteriorly to the foramen rotundum lateral to the cavernous sinus and superior orbital fissure. For the anteriorly placed tumors in our series, this provided excellent exposure when combined with a transfacial approach, with resection of the medial and lateral pterygoid muscles and plates to achieve gross total resection.
Outcome analyses for patients with malignant tumors have been reported in several series, but the numbers are relatively small. Sekhar et al. (15) used a subtemporal-preauricular in-fratemporal fossa approach to large tumors involving the lateral and posterior cranial base in 22 patients. This included 10 patients with a variety of benign neoplasms, 3 patients with malignant cartilaginous neoplasms, and 9 patients with malignant neoplasms. In the 9 patients with malignant tumors, total excision was achieved in 7 patients, subtotal resection was achieved in 1 patient, and partial excision was performed in 1 patient. Of the 8 surviving patients, one developed local recurrence at the margin of previous tumor resection and another developed neck node metastasis without local recurrence. Both patients underwent further treatment for tumor removal. The single patient with partial tumor resection died of disease. Complications included postoperative death from carotid rupture, infection, and stroke with a modest recovery. Wound complications were observed in 8 patients and included cerebrospinal fluid leakage, wound dehiscence, and free-flap failure. Several cranial neuropathies were observed on the basis of the extent of surgery and nerve resection.
Bigelow et al. (2) used a combination frontotemporal-anterolateral approach for Stage IV malignant disease involving the lateral to mid-cranial base in 25 patients, of which 20 had extension into the infratemporal fossa. Abnormalities included epidermoid carcinoma (n = 7), adenoid cystic carcinoma (n = 6), adenocarcinoma (n = 4), carcinoma expleomorphic adenoma (n = 2), radiation-induced sarcoma (n = 2), other sarcomas (n = 2), and miscellaneous tumor types (n = 2). All patients were thought to have extracranial lesions, although two were determined to have intracranial extension on surgical exploration. All patients underwent complete gross tumor resection, but three patients with adenoid cystic carcinoma had microscopically positive margins. Two patients died postoperatively, as discussed below. Among the surviving 23 patients, 13 received postoperative radiation therapy. Disease-free survival at 2, 3, and 5 years was 57, 50, and 25%, respectively. Patients who had untreated disease had a 31% survival rate at 5 years; those with recurrent disease had a 14% 5-year survival rate. Of the 11 patients who died of their disease, the mean interval from surgery to death was 21 months (range, 2–50 mo).
The complication rate in the series by Bigelow et al. (2) was 64%. These included stroke (n = 4), partial or total flap failure (n = 4), permanent dysphagia requiring gastrostomy (n = 4), pneumonia (n = 3), wound dehiscence (n = 2), cerebrospinal fluid leak (n = 2), major wound infection (n = 2), and fistula (n = 2). The most serious complications resulted from vascular sacrifice of either the carotid or vertebral artery. Despite pre-operative balloon occlusion tests, vascular sacrifice resulted in four cerebrovascular accidents and two deaths. The authors recommend use of vascular bypass grafts to prevent this complication, but whether malignant tumors with major vascular involvement can be cured with resection remains controversial. In our series, no patient required ligation of vascular structures at the cranial base to achieve negative margins, and cavernous sinus invasion was considered a relative contraindication.
In our series, the overall and relapse-free survival rates are similar to those reported by Bigelow et al. (2). Aside from a single mortality from myocardial infarction, the occurrence of significant complications was reduced by excluding patients who required carotid artery resection to achieve negative histological margins. Even with vascular bypass, the risk of a cerebrovascular accident in patients with malignant disease was considered prohibitively high.
Mansour et al. (11) recently published a series of patients with benign and malignant infratemporal fossa malignancies that were operated via a preauricular approach. The zygomatic arch is divided and displaced inferiorly, dividing the malar eminence (zygoma) and displacing it anteriorly and cutting the coronoid process and retracting it superiorly with the attached temporalis muscle. Of the 44 malignant tumors, squamous cell carcinoma and sarcoma were the most common. The anatomy of the tumors was similar to that observed in our series, with 78% involving medial structures (i.e., pterygoid plates and muscles) and 50% involving superior structures (i.e., greater wing of the sphenoid, foramina ovale, and rotundum). Negative microscopic margins were achieved in 67% of patients, similar to the rate of 78% observed in our series. Reconstruction was predominantly local temporalis flaps (17 patients) and 14 free vascularized tissue grafts with either rectus abdominis. Of the 14 patients who survived 2 years, 7 were alive without disease, 4 were alive with disease, and 3 had died of disease (11).
As technical advances continue to be refined and evolve in the treatment of patients with cranial base malignancies, careful evaluation of outcomes must be undertaken to assess the efficacy of these procedures. The justification for operating on patients with infratemporal fossa malignancies may be addressed aptly by comparing these tumors to malignancies involving the anterior cranial base alone. In a recent international collaborative study reported by Patel et al (13), 5-year survival and relapse-free survival rates were 54 and 53%, respectively. Infratemporal fossa extension does not seem to have changed overall survival rates; in our series, the 5-year survival rate was 56%. The morbidity associated with these radical procedures seems justified given the long-term local control rate in many patients. Previously, many patients treated in this series would not have been considered candidates for resection; thus, their disease would have been incurable. With newer chemotherapy regimens and high-dose conformal radiation (e.g., intensity-modulated radiation therapy), surgery seems justified in an attempt to cure patients with these aggressive cranial base malignancies.
Contributor Information
Mark H. Bilsky, Neurosurgical Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, and Department of Neurological Surgery, Weill Medical College, Cornell University, New York, New York
Brandon Bentz, Department of Otolaryngology, Huntsmen Cancer Center, University of Utah, Salt Lake City, Utah
Todd Vitaz, Department of Neurological Surgery, University of Kentucky Neurosurgical Institute, Louisville, Kentucky
Jatin Shah, Head and Neck Service, Memorial Sloan-Kettering Cancer Center, New York, New York
Dennis Kraus, Head and Neck Service, Memorial Sloan-Kettering Cancer Center, New York, New York
References
- 1.Bentz BG, Bilsky MH, Shah JP, Kraus DH. Anterior skull base surgery for malignant tumors: A multivariate analysis of 27 years of experience. Head Neck. 2003;25:515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- 2.Bigelow DC, Smith PG, Leonetti JP, Backer RL, Grubb RL, Kotapka MJ. Treatment of malignant neoplasms of the lateral cranial base with the combined frontotemporal-anterolateral approach: Five-year follow-up. Otolaryngol Head Neck Surg. 1999;120:17–24. doi: 10.1016/S0194-5998(99)70364-5. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky MH, Kraus DH, Strong EW, Harrison LB, Gutin PH, Shah JP. Extended anterior craniofacial resection for intracranial extension of malignant tumors. Am J Surg. 1997;174:565–568. doi: 10.1016/s0002-9610(97)00172-4. [DOI] [PubMed] [Google Scholar]
- 4.Bridger GP, Kwok B, Baldwin M, Williams J, Smee RI. Craniofacial resection for paranasal sinus cancers. Head Neck. 2000;22:772–780. doi: 10.1002/1097-0347(200012)22:8<772::aid-hed5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Cantu G, Solero CL, Mariani L, Mattavelli F, Pizzi N, Licitra L. A new classification for malignant tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1999;125:1252–1257. doi: 10.1001/archotol.125.11.1252. [DOI] [PubMed] [Google Scholar]
- 6.Dias FL, Sa GM, Kligerman J, Nogueira J, Galvao ML, Lima RA. Prognostic factors and outcome in craniofacial surgery for malignant cutaneous tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123:738–742. doi: 10.1001/archotol.1997.01900070082013. [DOI] [PubMed] [Google Scholar]
- 7.Dos Santos LR, Cernea CR, Brandao LG, Siqueira MG, Vellutini EA, Velazco OP, Cruz OL, Morais-Besteiro J, Freitas CA. Results and prognostic factors in skull base surgery. Am J Surg. 1994;168:481–484. doi: 10.1016/s0002-9610(05)80106-0. [DOI] [PubMed] [Google Scholar]
- 8.Fisch U. The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope. 1983;93:36–44. doi: 10.1288/00005537-198301000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Guinto G, Abello J, Molina A, Gallegos F, Oviedo A, Nettel B, Lopez R. Zygomatic-transmandibular approach for giant tumors of the infratemporal fossa and parapharyngeal space. Neurosurgery. 1999;45:1385–1398. doi: 10.1097/00006123-199912000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Kraus DH, Shah JP, Arbit E, Galicich JH, Strong EW. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck. 1994;16:307–312. doi: 10.1002/hed.2880160403. [DOI] [PubMed] [Google Scholar]
- 11.Mansour OI, Carrau RL, Snyderman CH, Kassam AB. Preauricular infra-temporal fossa surgical approach: Modifications of the technique and surgical indications. Skull Base. 2004;14:143–151. doi: 10.1055/s-2004-832256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mickey B, Close L, Schaefer S, Samson D. A combined frontotemporal and lateral infratemporal fossa approach to the skull base. J Neurosurg. 1988;68:678–683. doi: 10.3171/jns.1988.68.5.0678. [DOI] [PubMed] [Google Scholar]
- 13.Patel SG, Singh B, Polluri A, Bridger PG, Cantu G, Cheesman AD, deSa GM, Donald P, Fliss D, Gullane P, Janecka I, Kamata SE, Kowalski LP, Kraus DH, Levine PA, dos Santos LR, Pradhan S, Schramm V, Snyderman C, Wei WI, Shah JP. Craniofacial surgery for malignant skull base tumors: Report of an international collaborative study. Cancer. 2003;98:1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 14.Richtsmeier WJ, Briggs RJ, Koch WM, Eisele DW, Loury MC, Price JC, Mattox DE, Carson BS. Complications and early outcome of anterior craniofacial resection. Arch Otolaryngol Head Neck Surg. 1992;118:913–917. doi: 10.1001/archotol.1992.01880090029010. [DOI] [PubMed] [Google Scholar]
- 15.Sekhar LN, Schramm VL, Jr, Jones NF. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67:488–499. doi: 10.3171/jns.1987.67.4.0488. [DOI] [PubMed] [Google Scholar]
- 16.Som PM, Biller HF, Lawson W, Sacher M, Lanzieri CF. Parapharyngeal space masses: An updated protocol based upon 104 cases. Radiology. 1984;153:149–156. doi: 10.1148/radiology.153.1.6089262. [DOI] [PubMed] [Google Scholar]


