Abstract
Background
We recently identified MUC1 as a target driving selection for 1q21 amplification and validated it as an independent marker of aggressive behavior in thyroid cancer (TC). The aims of this study were to determine if TC cell lines retain MUC1 expression patterns seen in primary tumors, assess the role of MUC1 in tumor maintenance and develop a virally delivered anti-MUC1 RNAi that is effective in decreasing MUC1 expression in vitro.
Methods
Fifteen TC cell lines were screened for MUC1 protein expression. Cell lines with varying MUC1 protein levels were treated with anti-MUC1 monoclonal antibody to assess cell viability. A recombinant retroviral short hairpin RNAi (shRNA) delivery system against MUC1 was developed. Efficacy and optimal dosing of shRNA against MUC1 was determined.
Results
MUC1 expression patterns in TC cell lines were found to be similar to that seen in primary tumors. Treatment with anti-MUC1 antibody resulted in a significant decrease in cell viability in MUC1 over-expressing cell lines. MUC1-779 RNAi construct showed excellent infection efficiency and reproducible silencing.
Conclusions
These data offer functional evidence implicating MUC1 over-expression as a key molecular event in the pathogenesis of aggressive TC. Retrovirally delivered anti-MUC1 RNAi is effective in silencing MUC1 and merits further investigation to establish therapeutic efficacy and safety in anticipation of potential clinical application.
Introduction
Papillary thyroid carcinoma (PTC) accounts for over 80% of all thyroid malignancies and is usually associated with an excellent prognosis (1). However, 10-15% of cases can display aggressive behavior, hallmarked by early metastasis and increased mortality (2). As a result, the optimal management of thyroid cancer is dependent on the assessment of the malignant potential of the individual tumor at presentation. In this regard, clinicopathological variables are not universally predictive of tumor behavior. Caldas et al. suggest that the first step in rationally treating a disease is to correctly classify the disease, intimating a role for molecular markers in disease classification (3).
Clinical and genetic evidence suggest that PTC represents a continuum of disease, progressing from indolent classical (cPTC) to aggressive tall cell variant (TCV) to poorly differentiated thyroid carcinoma (PDTC) and finally anaplastic thyroid carcinoma (ATC). In this regard, it has been observed that PDTC or ATC can develop as a recurrence months or years after the treatment of cPTC. This has reinforced the progression hypothesis by providing a clinicopathologic bridge between cPTC and ATC (4). Understanding the genetic mechanisms controlling thyroid tumor progression can provide valuable prognostic markers and potential therapeutic targets.
We have previously demonstrated that increasing genomic complexity underlies the histopathological progression of cPTC to ATC (5). More specifically, a comparison of genomic differences between cPTC and TCV revealed a recurrent amplification at 1q21 that was unique to aggressive variants. We identified MUC1 as a gene driving selection for 1q21 amplification based on cDNA microarray screening. This association was validated by real-time PCR and immunohistochemical analyses in independent patient cohorts. Moreover, MUC1 amplification and over-expression in PTC was found to be an independent prognostic factor even after controlling for confounding variables by multivariate analysis (6).
MUC1 is a transmembrane epithelial cell surface glycoprotein. It belongs to the family of mucin proteins which are expressed by various epithelial cell types. Mucins are multifaceted glycoproteins that provide lubrication of epithelial cell surfaces, prevent tissue dehydration, protect cells from proteolytic degradation and constitute a barrier against infection (7). They have a central role in maintaining homeostasis and promoting cell survival. Cell surface associated mucins, such as MUC1, are bound to cells by an integral transmembrane domain and have relatively short cytoplasmic tails that associate with cytoskeletal elements, cytosolic adaptor proteins and/or participate in signal transduction. They may serve as cell surface receptors and sensors and conduct signals in response to external stimuli that lead to coordinated cellular responses that include proliferation, differentiation, apoptosis or secretion of specialized cellular products (8). Cancer cells might use mucins in much the same way as normal epithelia, for protection from adverse growth conditions and to control the local microenvironment during invasion and metastasis.
MUC1 is over-expressed and aberrantly glycosylated in almost all human adenocarcinomas, including >90% of breast, ovarian, pancreatic, colorectal, lung, prostate and gastric carcinomas and has been implicated in their pathogenesis (9-18). MUC1 expression has also been demonstrated in nonepithelial cancer cell lines, such as astrocytoma, melanoma and neuroblastoma, as well as hematological malignancies such as multiple myeloma and some B-cell non-Hodgkin lymphomas (19-22). Interestingly this comprises >50% of all cancers in humans. MUC1 is ubiquitously expressed over the entire cell membrane in cancer cells, while it is restricted to only the apical surface in normal epithelial cells (23).
In normal tissues MUC1 is heavily glycosylated, thus hiding potential antigenic peptides which could serve as tumor targets. In neoplastic tissue, MUC1 is aberrantly underglycosylated, revealing epitopes and permitting the immune system to access the peptide core of MUC1. This feature can help differentiate tumor cells from normal cells and allow for better targeting strategies (23). Based on this factor, monoclonal antibody therapy seems like the obvious choice, however, it has been fraught with limitations which complicate advancement to human use. The approach has serious practical limitations, such as, poor efficacy and specificity, and a limiting immune response. RNA interference (RNAi) may be free of such limitations. Introduction of siRNA (short interfering RNA) with sequence homology to a target gene can specifically silence its cellular expression at the post-transcriptional level (24, 25). RNAi has been proven effective in mitigating oncogenesis induced by point mutation or translocation, but its efficacy for targeting over-expressed genes has not been previously reported (26, 27). Additionally, it has been suggested that retrovirally delivered RNAi can provide stable, long term silencing of the target gene (26).
The objectives of this study were to determine if thyroid cancer cell lines retain MUC1 expression patterns seen in primary tumors, provide direct experimental evidence to establish MUC1 as a therapeutic target in thyroid cancer and develop a virally delivered anti-MUC1 RNAi that is effective in decreasing MUC1 expression for potential therapeutic application.
Materials and Methods
Cell Culture
Human thyroid carcinoma cell lines ARO, FRO, WRO, and NPA were gifts from Dr. Fagin, University of Cincinnati. These cells were grown in RPMI+non-essential amino acids+10% fetal calf serum (FCS) with 100U/ml penicillin G and 100ug/ml streptomycin sulphate. Cell lines KAT4, KAT5, KAT10 and KAT 18 were kind gifts from Dr. Kenneth Ain, University of Kentucky. These cells were grown in RPMI+non-essential amino acids+1mM sodium pyruvate+10% FCS with 100U/ml penicillin G and 100ug/ml streptomycin sulphate with no phenol red. Cell lines BHP2-7, BHP10-3, BHP7-13, and BHP18-21 were kind gifts from Dr. J. Hershman, UCLA. These cells were grown in RPMI+non-essential amino acids+10% FCS with 100U/ml penicillin G and 100ug/ml streptomycin sulphate. Thyroid cancer cell lines 8305C, 8505C and KHM-5M were purchased from Japan Health Sciences Foundation. 8305C and 8505C were grown in Eagle’s minimal essential medium with 10% FCS with 100U/ml penicillin G and 100ug/ml streptomycin sulphate. KHM-5M was grown in RPMI+10% FCS with 100U/ml penicillin G and 100ug/ml streptomycin sulphate. Cells were maintained in a 5% CO2-95% air humidified incubator at 37°C.
MUC1 protein quantification
MUC1 protein levels were determined by Western blot analysis. Whole cell lysates were prepared following manufacturer’s protocols (Pierce Biotechnology, Rockford, Illinois). A mouse monoclonal antibody (mAb) directed against the APDTR motif of the core peptide region of MUC1 was used (VU4H5, Santa Cruz Biotechnology, Inc.) for Western blots at a concentration of 1:200 following standard protocols. The immunocomplexes were detected with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ). Beta-actin protein levels were measured as internal loading controls.
Cell viability assessment
Three MUC1 expressing (KAT4, KAT5, KAT10) and 1 non-expressing control cell line (KAT18) were chosen for cell viability assessment. 1.5x104 cells were plated in standard 96 well plates and allowed to adhere for 24 hours. The cells were then exposed to increasing concentrations of anti-MUC1 mAb, VU4H5, (1X-0.01ng/μl, 10X-0.1ng/μl, 100X-1.0ng/μl), and were treated for 72 hours. After treatment, the cells were incubated in solution containing MTT (3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) at a concentration of 0.4 mg/ml for 4 hours. MTT values were measured on a MicroELISA reader (Dynatech Laboratories) at OD570. Cell viability was calculated relative to the control group (28).
Generation of recombinant retroviral vectors expressing MUC1 and Null short hairpin RNA
Several custom shRNA constructs, targeting different MUC1 mRNA regions, were developed using standard 3-dimensional computer prediction models for designing short hairpins for RNAi based suppression of gene expression (Oligoengine, Inc.) MUC1-745 and MUC1-779 were the two shRNA oligonucleotides selected for our experiments (Figure 1). Both oligonucleotides maximally conformed to accepted design strategies for shRNA based targeting. The shRNA oligonucleotides were designed to contain a sense strand of 19 nucleotides of MUC1 followed by a short spacer (ttcaagaga), the reverse complement of the sense strand, and five thymidines as an RNA polymerase III transcriptional stop signal. The constructs have a GC richness of 30-50%. The target sequence is at least 75 bases downstream of the start codon. The 19 nucleotides do not contain a stretch of four or more adenines or thymidines. MUC1-745 (5′-gatccccGCACTCCATTCTCAATTCCttcaagagaGGAATTGAGAATGGAGTGCttttta-3′) targets region 745-763 and MUC1-779 targets region 779-797 (5′-gatcccc TACTCCTACCACCCTTGCCttcaagagaGGCAAGGGTGGTAGGAGTAttttta-3′). Forward and reverse oligonucleotides for each target were annealed and ligated into the HindIII-BglII site of pSUPER-retro-neo-GFP. This is a recombinant retroviral vector driving shRNA expression (Oligoengine, Inc.). It is under the control of a RNA-polymerase III promoter and a fused neomycin/eGFP expression cassette driven by a PGK promoter. MUC1-Null, a nonspecific scrambled oligonucleotide (5′-gatccccCGTCTACCTACACTCCCTCttcaagagaGAGGGAGTGTAGGTAGACGttttta-3′) was also designed and ligated into pSUPER-retro-neo-GFP as a control retroviral null vector. The constructs were compared for efficacy in reducing MUC1 protein level.
Fig 1.
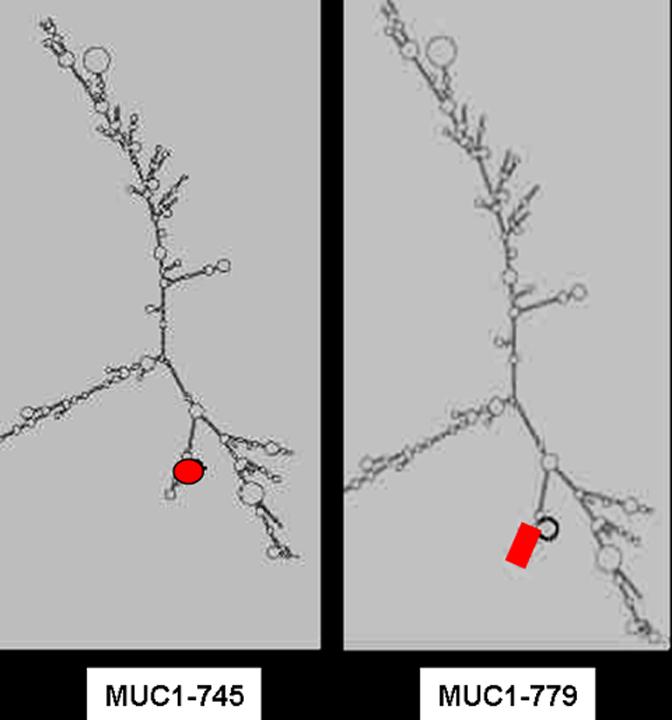
An example of the secondary structure of MUC1 with the target region for MUC1-779 RNAi and MUC1-745, shown in red.
Production of RNAi and Null virus
Virus was produced by transient cotransfection of Phoenix-Ampho, a stable high-titer producing 293T cell sub-line (Nolan lab, Stanford University), with the recombinant pSUPER retroviral constructs (MUC1-745, MUC1-779, MUC1-Null) and the pCL-Ampho packaging vector (29, 30, 31). The transfection cocktail was prepared by bubbling in 2X Hepes buffered saline (pH 7.05) to an equal volume of DNA/CaCl2 solution. Retroviral titer estimation was done by FACS analysis of virus infected NIH-3T3 cells (32). Phoenix-Ampho cells, when plated at 3.0 × 106 cells/10-cm dish, produced the highest viral titer (>105 iu/ml) (33). The amphotropic retroviral supernatant, harboring either the recombinant RNAi construct or the null vector, was collected after 48 hours and used to infect KAT4 (MUC1 over-expressing thyroid cancer cell line). KAT4 cells were triply-infected with 3cc of infective cocktail every 6 hours (MOI 1) for 24 hours. Flow Cytometry (BD Vantage) was used to sterile-sort transduced eGFP-containing cells for expansion in cell culture.
Statistical analysis
All analyses were performed using JMP4 statistical software (SAS Institute Inc., Cary, NC). Statistical significance was defined as a two tailed p-value less than or equal to 0.05. Qualitative and quantitative non-parametric comparisons were performed using Fisher’s exact test and the Mann-Whitney U-test respectively.
Results
MUC1 protein expression
Fifteen human thyroid cancer cell lines were screened for MUC1 protein expression: 6 anaplastic (ARO, KAT4, KAT18, 8305C, 8505C, KHM-5M), 4 aggressive papillary (BHP2-7, BHP10-3, BHP7-13, BHP18-21), 3 papillary (KAT5, KAT10, NPA) and two follicular (WRO, FRO) thyroid carcinoma cell lines (Table 1). Similar to expression patterns in primary tumors, increased MUC1 expression was found in aggressive thyroid cancer cell lines but not in other papillary and follicular thyroid cancer cell lines. Increased MUC1 protein levels were found in aggressive thyroid cancer cell lines (8505C, KAT4, ARO, BHP2-7, BHP10-3, BHP7-13, BHP18-21). Low level of MUC1 protein expression was seen in papillary thyroid cancer cell lines KAT5 and KAT10 (Figure 2). MUC1 protein level was not detectable in papillary thyroid cancer cell line NPA and follicular thyroid cancer cell lines WRO, FRO.
Table 1.
MUC1 protein expression levels in thyroid cancer cell lines.
| Thyroid Cancer Cell Lines | MUC1 protein expression |
|---|---|
| ARO | +++ |
| KAT4 | +++ |
| 8505C | ++ |
| 8305C | - |
| KHM-5M | - |
| KAT18 | - |
| BHP2-7 | +++ |
| BHP10-3 | +++ |
| BHP7-13 | +++ |
| BHP18-21 | +++ |
| KAT5 | + |
| KAT10 | ++ |
| NPA | - |
| WRO | - |
| FRO | - |
+++ high MUC1 protein expression
++ moderate MUC1 protein expression
+ low MUC1 protein expression
- no detectable MUC1 protein expression
Fig 2.

Representative protein expression of MUC1 in 4 different thyroid cancer cells.
Effect of anti-MUC1 antibody
To determine the role of MUC1 on the biological behavior of thyroid cancer, we targeted MUC1 expression using an anti-MUC1 monoclonal antibody. TC cell lines with varying levels of MUC1 expression (KAT4, KAT5, KAT10, and KAT18) were treated with anti-MUC1 mAb at increasing concentrations. Cell viability was measured 72 hours after exposure to the antibody using the MTT assay (Figure 3). Antibody treatment resulted in a significant decrease in cell viability in MUC1-expressing cell lines (KAT4-p<0.02, KAT5-p<0.0001, KAT10-p<0.005). Conversely, no significant change in viability was seen in KAT18, a cell line with no detectable MUC1 expression.
Fig 3.
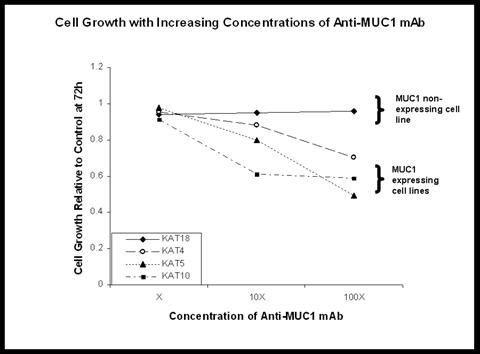
Targeting MUC1 using anti-MUC1 antibody resulted in cell growth inhibition in MUC1 expressing cell lines, suggesting that MUC1 may serve as a potential therapeutic target.
RNAi based MUC1 suppression
Retrovirus with shRNA against MUC1 or a scrambled construct was produced at high titers by transfection of Phoenix-Ampho packaging cells with our shRNA plasmid constructs. The derived retrovirus containing our constructs was replication-incompetent, therefore allowing for only one round of infection. Both the MUC1-779 RNAi and MUC1-745 RNAi constructs showed high transfection efficiency into Phoenix-Ampho cells (Figure 4). The virus showed high infective capacity as determined by flow cytometric sorting for eGFP expression of target cells. Of the combinations and dosing tested, three serial infections 6 hours apart with virus carrying the MUC1-779 shRNA uniformly resulted in MUC1 protein suppression as assessed by Western blotting. MUC1-Null infected cells continued to express baseline MUC1 protein levels (Figure 5).
Fig 4.
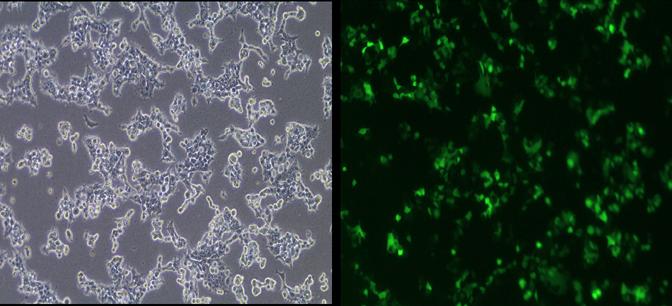
Phoenix-Ampho cells transfected with our MUC1-RNA1 containing plasmid. Green fluourescence represents positively transfected cells.
Fig 5.
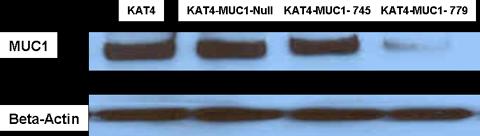
Western blot showing MUC1 protein suppression of high MUC1 expressing KAT4 thyroid cancer cell line. MUC1-779 RNAi construct worked best in stably suppressing MUC1 protein expression.
Discussion
One of the great challenges of cancer genetics is the identification of critical molecular events driving cancer behavior from a background of random events that have little effect on cancer pathogenesis. We have previously demonstrated that MUC1 is a prognostic marker in thyroid cancer, whose expression is increased consequent to gene amplification (6). However, given the degenerate nature of gene amplification, several genes can be co-amplified and even over-expressed in a single amplified region. Accordingly, direct functional evidence is required to establish any gene as an amplification target.
First, we established that MUC1 expression patterns in primary thyroid tumors are maintained in thyroid cancer cell lines. Accordingly, these cell lines were felt to be a representative model for the analysis of the functional role of MUC1 in thyroid cancer. We demonstrated that targeting MUC1 with monoclonal antibody showed selective effect on cell viability that was limited to MUC1 expressing cell lines, suggesting an exaggerated requirement for MUC1 in these lines. Several lines of investigations have shown that critical genetic events maybe an "Achilles heal" and therefore targets for cancer therapy. This concept termed oncogene addiction has been promulgated based on animal, as well as, human studies (34). Combined, our work suggests that MUC1 plays a critical role in thyroid cancer behavior and establishes it as a therapeutic target in thyroid cancer.
Although the precise role of MUC1 in epithelial cell function and cancer pathogenesis is not well defined, it is clear that its function is highly complex. The expression of MUC1 on the cell surface of glandular epithelial cells suggests a role in responding to the luminal environment (35). The extracellular domain of MUC1 has been shown to act as a ligand for intracellular adhesion molecule-1 (ICAM-1) and selectins (36). In malignancy MUC1 looses its polarized expression, redistributes and is expressed on the whole cell surface. This redistribution shields other cell surface molecules from their ligands and interferes with integrin mediated adhesion to the extracellular matrix and with cadherin mediated cell-cell adhesion. Increased MUC1 expression thereby promotes cellular dissociation and oncogenic progression (37, 38, 39). MUC1 up-regulation also protects tumor cells from immune recognition and destruction by the cellular arm of the immune system. Moreover, it has been shown to inhibit human T-cell proliferation, thereby contributing to cancer propagated immunosuppression (40). There is emerging evidence that MUC1 may also contribute to the regulation of differentiation and proliferation of tumor cells. The cytoplasmic tail of MUC1 can bind and signal through β-catenin and the mitogen-activated protein kinase (MAPK) pathways. Recent evidence shows that a fragment of the cytoplasmic tail of MUC1 can be transported to the nucleus in association with β-catenin, raising the possibility that MUC1 might directly influence the transcriptional co-activator status of β-catenin, which in turn has been shown to regulate several genes that are related to cell proliferation and differentiation (41, 42, 43). MUC1 has also been characterized as an oncoprotein, causing cellular transformation resulting in increased anchorage-independent growth and tumor xenograft formation (44). These multiple properties make MUC1 an attractive therapeutic target.
Our results showed a graded decrease in cell viability in MUC1 expressing thyroid cancer cell lines with exposure to increasing concentrations of anti-MUC1 antibody. Conversely, cells with minimal MUC1 expression, showed no change in their viability. Anti-MUC1 antibodies potentially protect against tumor progression and metastasis by binding to MUC1 expressing tumor cells, capping MUC1 on the cell surface and restoring cell-cell interaction. MUC1 antibodies have also been shown to counteract immunosuppressive effects and result in cell-mediated cytotoxicity (45, 46). We believe that by binding with the MUC1 extracellular domain, the antibody may disrupt the intracellular signaling function of MUC1. The cytoplasmic tail of MUC1 has been shown to promote the malignant phenotype by being transported to the nucleus in association with β-catenin (44, 47). MUC1 has also been shown to activate both the phosphoinositide 3-kinase (PI3K)/Akt and BCL-xL pathways by functioning as a coreceptor for the ErbB family of growth factor receptors. Over-expression of MUC1 could therefore aberrantly stimulate ErbB receptor mediated activation of PI3K and Akt (48). Furthermore, localization of the cytoplasmic domain of MUC1 to the mitochondria is associated with attenuation of the apoptotic response (49). Recent studies have also shown that MUC1 suppresses the p53-dependent apoptotic response (50). This provides a mechanistic basis for an anti-apoptotic phenotype of MUC1 expressing cells. MUC1 can block apoptosis by different mechanisms that are dependent on cell context. Activation of these anti-apoptotic signals may contribute to MUC1 induced transformation. Antibody treatment of the cells expressing MUC1 may block some of the intracellular anti-apoptotic signaling, thus restoring normal cell cycle function and death, however further confirmatory studies still need to be performed.
To better understand the functional role of MUC1 in thyroid cancer progression and to explore novel therapeutic strategies we established a recombinant retroviral RNAi system to stably suppress MUC1 protein expression. We chose a retroviral delivery system for several reasons: simple derivation, genomic flexibility, and most importantly, long-term transgene expression and stable transmission to daughter cells because of host genomic integration (51). Because of these advantages, recombinant retroviruses are presently the most commonly employed viral vectors in human gene therapy trials (52). The clinical applicability of currently available retroviral delivery systems is however hampered by both low attainable viral titers and the replication incompetency of the engineered virions. To rectify these problems, we attempted to boost our RNAi retrovirus titers by utilizing Phoenix-Ampho, a stable high-titer producer 293T sub-line. With three serial infections, followed by eGFP positive cell sorting, we have been able to demonstrate stable MUC1 suppression in cell lines over-expressing MUC1. In vitro and in vivo assays are being applied to study the tumorogenic properties of MUC1 suppressed cells when compared to their unsuppressed parent cell line. This will hopefully provide us with better functional insight of the role of MUC1 in thyroid cancer.
RNAi appears promising as a new category of nucleic acid therapeutics which may be combined with more conventional therapies for treating a variety of cancers including those caused by over-expression of a normal gene. Cioca et al. provided evidence to suggest that RNAi of c-raf and bcl-2 genes may augment the efficacy of chemotherapy in myeloid leukemia (53). Before in vivo cancer gene therapy becomes a reality a number of problems must be overcome. Target specificity for transgene delivery and/or expression is a major concern and the focus of intense investigation and development. The need for improved viral vector systems continues to fuel intense research activity. Replication competent viruses have many advantages over replication defective viruses for cancer therapy applications. With replication competent viruses, the RNAi vector distribution can increase over time and the maximum dose benefit is greater, since progeny virions can infect adjacent cells. With replication incompetent systems, the initial infection is the final therapeutic benefit. Furthermore, replication competent systems allow for a two pronged therapeutic strategy: oncolysis by viral replication and the effect of the RNAi transgene. The combination may reduce the risk of tumor cell resistance (54).
In summary, MUC1 over-expression has been shown to be a poor prognosticator in papillary thyroid carcinoma. We provide the first functional evidence of the role of MUC1 in thyroid cancer. We developed an RNAi based MUC1 suppression model to help better delineate the role of MUC1 in thyroid cancer progression and potentially serve as a new therapeutic targeting strategy for aggressive thyroid carcinomas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaha AR. Controversies in the management of the thyroid nodule. Laryngoscope. 2000;110:183–193. doi: 10.1097/00005537-200002010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Shaha AR, Shah JP, Loree TR. Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck. 1998;20:26–30. doi: 10.1002/(sici)1097-0347(199801)20:1<26::aid-hed5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Caldas C, Aparicio SA. The molecular outlook. Nature. 2002;415:484–5. doi: 10.1038/415484a. [DOI] [PubMed] [Google Scholar]
- 4.Spires JR, Schwartz MR, Miller RH. Anaplastic thyroid carcinoma: association with differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 1998;114:40–44. doi: 10.1001/archotol.1988.01860130044012. [DOI] [PubMed] [Google Scholar]
- 5.Wreesmann VB, Ghossein RA, Patel SG, et al. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002;161:1549–1556. doi: 10.1016/S0002-9440(10)64433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wreesmann VB, Sieczka EM, Socci ND, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64:3780–3789. doi: 10.1158/0008-5472.CAN-03-1460. [DOI] [PubMed] [Google Scholar]
- 7.von Mensdorff-Pouilly S, Snijdewint FG, Verstraeten AA, et al. Human MUC1 mucin: a multifaceted glycoprotein. Int J Biol Markers. 2000;15:343–56. doi: 10.1177/172460080001500413. [DOI] [PubMed] [Google Scholar]
- 8.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 9.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim Biophys Acta. 1999;1455(23):301–13. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 10.Apostolopoulos V, Pietersz GA, McKenzie IF. MUC1 and breast cancer. Curr Opin Mol Ther. 1999;1:98–103. [PubMed] [Google Scholar]
- 11.Kohlgraf KG, Gawron AJ, Higashi M, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–20. [PubMed] [Google Scholar]
- 12.Ginestier C, Charafe-Jauffret E, Bertucci F, et al. Distinct and complementary information provided by use of tissue and DNA microarrays in the study of breast tumor markers. Am J Pathol. 2002;161:1223–33. doi: 10.1016/S0002-9440(10)64399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- 14.Aoki R, Tanaka S, Haruma K, et al. MUC-1 expression as a predictor of the curative endoscopic treatment of submucosally invasive colorectal carcinoma. Dis Colon Rectum. 1998;41:1262–72. doi: 10.1007/BF02258227. [DOI] [PubMed] [Google Scholar]
- 15.Willsher PC, Xing PX, Clarke CP, et al. Mucin 1 antigens in the serum and bronchial lavage fluid of patients with lung cancer. Cancer. 1993;72:2936–42. doi: 10.1002/1097-0142(19931115)72:10<2936::aid-cncr2820721013>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Maeshima A, Miyagi A, Hirai T, Nakajima T. Mucin-producing adenocarcinoma of the lung, with special reference to goblet cell type adenocarcinoma: immunohistochemical observation and Ki-ras gene mutation. Pathol Int. 1997;47:454–60. doi: 10.1111/j.1440-1827.1997.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Zhang HS, Reuter VE, Slovin SF, et al. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 18.Medina M, Velez D, Asenjo JA, et al. Human colon adenocarcinomas express a MUC1-associated novel carbohydrate epitope on core mucin glycans defined by a monoclonal antibody (A10) raised against murine Ehrlich tumor cells. Cancer Res. 1999;59:1061–70. [PubMed] [Google Scholar]
- 19.Oosterkamp HM, Scheiner L, Stefanova MC, et al. Comparison of MUC-1 mucin expression in epithelial and non-epithelial cancer cell lines and demonstration of a new short variant form (MUC-1/Z) Int J Cancer. 1997;72:87–94. doi: 10.1002/(sici)1097-0215(19970703)72:1<87::aid-ijc13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Treon SP, Mollick JA, Urashima M, et al. MUC-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999;93:1287–98. [PubMed] [Google Scholar]
- 21.Dyomin VG, Palanisamy N, Lloyd KO, et al. MUC1 is activated in a B-cell lymphoma by the t(1;14)(q21;q32) translocation and is rearranged and amplified in B-cell lymphoma subsets. Blood. 2000;95:2666–71. [PubMed] [Google Scholar]
- 22.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–50. [PubMed] [Google Scholar]
- 23.Moore A, Medarova Z, Potthast A, Dai G. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004;64:1821–7. doi: 10.1158/0008-5472.can-03-3230. [DOI] [PubMed] [Google Scholar]
- 24.Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 25.Borkhardt A. Blocking oncogenes in malignant cells by RNA interference--new hope for a highly specific cancer treatment? Cancer Cell. 2002;2:167–8. doi: 10.1016/s1535-6108(02)00129-0. [DOI] [PubMed] [Google Scholar]
- 26.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–7. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 27.Wilda M, Fuchs U, Wossmann W, et al. Killing of leukemic cells with a bcr/abl fusion gene by RNA interference (RNAi) Oncogene. 2002;21:5716–24. doi: 10.1038/sj.onc.1205653. [DOI] [PubMed] [Google Scholar]
- 28.Singh B, Reddy PG, Goberdhan A, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–93. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuBridge RB, Tang P, Hsia HC, et al. Analysis of mutation in human cells by using an epstein-barr virus shuttle system. Mol Cell Biol. 1987;7:379–87. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pear WS, Nolan GP, Scott ML, et al. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naviaux RK, Costanzi E, Haas M, et al. The pcl vector system: Rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limon A, Briones J, Puig T, et al. High-titer retroviral vectors containing the enhanced green fluorescent protein gene for efficient expression in hematopoietic cells. Blood. 1997;90:3316–21. [PubMed] [Google Scholar]
- 33.Maghami E, Patel KN, Talbot S, et al. RNAi-based gene therapy against squamous cell carcinoma related oncogene (SCCRO) amplified in mucosal squamous cell cancers (MOSCC) (for submission, Cancer Res) [Google Scholar]
- 34.Weinstein IB. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 35.Walsh MD, Luckie SM, Cummings MC, et al. Heterogeneity of MUC1 expression by human breast carcinoma cell lines in vivo and in vitro. Breast Cancer Res Treat. 1999;58:255–66. doi: 10.1023/a:1006345301364. [DOI] [PubMed] [Google Scholar]
- 36.Regimbald LH, Pilarski LM, Longenecker BM, et al. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244–9. [PubMed] [Google Scholar]
- 37.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo K, Kohno N, Yokoyama A, et al. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–9. [PubMed] [Google Scholar]
- 40.Taylor-Papadimitriou J, Burchell J. MUC1 and the immunobiology of cancer. J Mamm Gland Biol and Neo. 2002;7:209–221. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder JA, Adriance MC, Thompson MC, et al. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22(9):1324–32. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 42.Huang L, Ren J, Chen D, et al. MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther. 2003;2:702–6. [PubMed] [Google Scholar]
- 43.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–4. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Liu D, Chen D, et al. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–10. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, et al. Antibody-dependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer. 2001;93:97–106. doi: 10.1002/ijc.1286. [DOI] [PubMed] [Google Scholar]
- 46.Akewanlop C, Wantanabe M, Singh B, et al. Phagocytosis of breast cancer cells mediated by anti-MUC1 monoclonal antibody, DF3, and its bispecific antibody. Cancer Res. 2001;61:4061–65. [PubMed] [Google Scholar]
- 47.Li Y, Chen W, Ren J, et al. DF3/MUC1 signaling in multiple myeloma cells is regulated by interleukin-7. Cancer Biol Ther. 2003;2:187–93. doi: 10.4161/cbt.2.2.282. [DOI] [PubMed] [Google Scholar]
- 48.Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–12. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 49.Ren J, Agata N, Chen D, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–75. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–78. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Barquinero J, Eixarch H, Perez-Melgosa M. Retroviral vectors: new applications for an old tool. Gene Ther. 2004;11(Suppl 1):S3–9. doi: 10.1038/sj.gt.3302363. [DOI] [PubMed] [Google Scholar]
- 52.Wysocki PJ, Grabarczyk P, Mackiewicz A. Recent developments in retroviral gene delivery systems. Expert Opin Biol Ther. 2001;1:911–3. doi: 10.1517/14712598.1.6.911. [DOI] [PubMed] [Google Scholar]
- 53.Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10:125–33. doi: 10.1038/sj.cgt.7700544. [DOI] [PubMed] [Google Scholar]
- 54.Kasuya H, Pawlik TM, Mullen JT, et al. Selectivity of an oncolytic herpes simplex virus for cells expressing the DF3/MUC1 antigen. Cancer Res. 2004;64:2561–7. doi: 10.1158/0008-5472.can-03-3431. [DOI] [PubMed] [Google Scholar]


