Allogeneic hematopoietic stem-cell transplantation (HSCT) is the most reliable and effective cell-based biotherapy currently available to pediatric and adult patients with hematological malignancies. The central role of donor-derived lymphocytes in mediating an effective anti-tumor effect, preventing and controlling opportunistic infection, and causing graft-versus-host-disease (GVHD) is well-documented in both animal experiments and in human trials. The profound lymphopenia after conditioning regimens, coupled with molecular tools to distinguish host versus donor cells provides investigators a window into immune recovery after allogeneic HSCT. Serial analyses of T-cell subsets, linking immunophenotype with function, have revealed the kinetics of donor-derived T-cell recovery after allo-grafting and provided insights into ways the immune system can be manipulated to augment the graft-versus-tumor (GVT)-effect without inducing GVHD. As this session will demonstrate, investigators are not limited to being passive observers of this immune reconstitution; rather we have an opportunity to shape the allo-grafted T cells to selectively augment immune function.
Immune-based therapies to supplement allogeneic HSCT -- an example of combination immunotherapy
Recipients of allogeneic HSCT benefit from a GVT-effect in general, and a graft-versus-leukemia (GVL)-effect in particular, due to donor-derived T cells targeting minor histocompatibility antigens (mHAgs) selectively expressed by cells or cell-subsets of the recipient’s hematopoietic system.1 This observation supports the rationale of infusing mHAg-specific T cells after allogeneic HSCT to augment the GVL-effect. If the tissue distribution of the mHAg is confined to the malignant cells then this adoptive immunotherapy should not cause acute GVHD. However, the development of cellular immunotherapy with effector cells of defined specificity and function has proven challenging and is not widely available. In contrast, antibody and cytokine therapy have already been successfully developed and incorporated into treatment regimens for a range of human malignancies.
Indeed, it is routine practice for patients with hypogammaglobulinemia to receive intravenous immunoglobulin after allogeneic HSCT. As antigen-specific monoclonal antibody (mAb) therapy has been incorporated into chemotherapy regimens, so too have oncologists incorporated these passive immunotherapy approaches into transplant regimens. For example, rituximab is employed in conditioning regimens not just to cytoreduce malignant B-cell burden,2 but to deplete normal B cells with an associated reduction in the risk for GVHD.3 Building upon the potential of CD20-directed mAb therapy to selectively cytoreduce patients prior to HSCT, investigators have successfully used 131I-tositumomab and 90Y-ibritumomab in myeloablative doses in blood and marrow transplantation protocols for high-risk patients.4,5,6,7,8
Cytokine therapy has also been used widely to support allogeneic HSCT. For example, supra-physiological dosing of granulocyte-colony stimulating factor (G-CSF) is used to mobilize donor hematopoietic stem cells (HSCs) and as prophylaxis in an attempt to improve myeloid engraftment, although the latter may significantly add to the economic burden of HSCT.9,10 The infusion of low doses of recombinant human interleukin-2 (IL-2) is tolerated after successful allo-grafting, but has a checkered history when used as an immunomodulator as it has not been associated with an improvement in the rates of relapse.11,12 However, there is a resurgence of the use of this cytokine at low-doses. Il-2 is being used in vitro to help propagate clinical-grade T cells and NK cells for adoptive immunotherapy, and in vivo as a surrogate for a deficient endogenous T-helper response to help sustain proliferation of adoptively transferred CD8+ T cells and to improve the survival of infused NK cells.
Day 0 – not just one day
Allogeneic HSCT is evolving from a field where all the cell therapy was provided on “day zero” (the day of infusion of HSCs) to that where a continuum of therapies is applied to meet patient needs and minimize attendant toxicities. The period of time after completion of the conditioning therapy to engraftment of functional lymphocytes is a unique opportunity for immunotherapists. Thus, while day 0 and infusion of the allograft is a signature event, there will be additional day zeroes signifying infusion of immune cell-based products, such as antigen-experienced lymphocytes, NK cells, and antigen-presenting cells (APCs).
T-cell depletion – are we throwing out the good with the bad?
Allografts from bone marrow (BM) and G-CSF-mobilized peripheral blood (PB) have been ex vivo depleted of T cells to broaden the donor pool and application of allogeneic HSCT. Profound T-cell depletion can be used to safely engraft HSCs from haploidentical donors, but the loss of allogeneic T cells renders the recipients severely immunocompromised and thus, vulnerable to infection and relapse. The relative risk-versus-benefit of T-cell depleted (TCD) versus T cell-replete allogeneic HSCT must be weighed for individual patients (Figure 1).
Figure 1.
Risk and benefit of inducing lymphopenia after allogeneic HSCT
To decrease the threat of T-cell depletion, investigators are experimenting with the timely add-back of donor-derived T cells which have been stripped of potential unwanted allogeneic reactivity.13,14,15,16,17,18 However, there is a new appreciation that inducing transient lymphopenia may be worth the risk, as the TCD environment may provide a special moment in time to manipulate the host’s immune system. This moment comes about due to loss of Treg cells, freeing up of lymphoid “space” and availability of limiting cytokines (e.g., IL-15 and IL-7). In this environment, adoptively transferred T cells and NK cells can undergo proliferation taking advantage of the homeostatic control mechanisms that may restore the peripheral pool of lymphocytes.19,20
This approach has been exploited at the NCI for treatment of melanoma using adoptive transfer of autologous melanoma-specific T cells expressing endogenous or introduced melanoma-specific alpha/beta T-cell receptors. These T cells, which have been numerically expanded ex vivo, have successfully treated melanoma tumor deposits when infused after a lymphocyte-depleting regimen containing cyclophosphamide and fludarabine.21,22 The anti-tumor effect is dependent on: (1) potency – using tumor-specific T cells with a defined alpha/beta TCR; and (2) T cell “area under the curve” – a measure of the number of infused antigen-specific T cells over time. Recent data suggest that the recovery of CD8+ T-cell response in the setting of lymphopenia requires CD4+ T cells, which may be one reason why adoptive transfer of melanoma-specific CD8+ T-cell clones failed to proliferate in vivo.23,24
Extrapolating these data to allogeneic HSCT, we hypothesize that lymphocyte-depleting preparative regimens may facilitate the therapeutic potential of immunotherapies employing adoptive T-cell transfer. Lympho-depletion may also benefit the efficacy for tumor-specific vaccines. This is based on the premise that: (1) dose-intensive chemotherapy can lengthen the period of progression-free survival, thus allowing time for a slow-acting therapy such as vaccination to be effective, (2) that maximally decreasing the recipient’s tumor burden may increase the effectiveness of immunotherapy by mechanisms including decreases in tumor-induced immunosuppressive effects; and (3) that providing tumor antigen exposure after immune depletion in the form of repeated immunizations may take advantage of the antigen-driven peripheral T-cell expansion that characterizes immune reconstitution at early time points. The T-cell repertoire is then biased towards tumor antigens and anti-tumor responses at later time points.25
Investigators have also combined vaccine therapy with adoptive immunotherapy to improve the T-cell effector function. This has particular appeal to augment cellular immunity after autologous HSCT. Pre-chemotherapy T cells, especially if primed, are very effective for adoptive immunotherapy in conjunction with repeated tumor antigen exposure, when administered after lymphocyte-depleting chemotherapy.26
Immune reconstitution after allogeneic HSCT – righting the wrong
Allogeneic HSCT cures many patients with a history of relapsed or high-risk hematological malignancies. To broaden the application of allogeneic HSCT, alternative non-sibling donors are being recruited; but preparing the host and/or allograft from these donors typically causes further disturbances to the recipient’s immune system. Despite advances in conditioning regimens and supportive care, the newly allografted recipient is profoundly immunocompromised. Thus, infection remains an important cause of morbidity and mortality following allogeneic HSCT and delayed T-cell immune recovery is one of the primary risk factors for death due to infection. When GVHD occurs, the risk of death due to infection is further increased, due to both direct effects of GVHD on the recovering immune system and the consequences of immunosuppressive agents.
Prospective analyses of immune reconstitution parameters reveal a hierarchy of disturbances that are associated with (i) selection of the donor, source of donor’s HSCs, (ii) number of infused donor-derived HSCs, (iii) ex vivo manipulation of the donor-derived HSCs to reduce T cells in general or alloreactive T cells in particular, and (iv) use of immunosuppressive medications such as corticosteroids. For example TCD of the allograft, or use of an umbilical cord blood (UCB), particularly a small UCB unit relative to recipient weight, leads to profound delay in recovery of lymphocyte number and disruption of function after allogeneic HSCT. Some maneuvers may shorten this period of functional and numerical lymphopenia, such as (i) infusion of mega-doses of donor-derived TCD HSC’s, (ii) combining two UCB units, (iii) enhancing thymic function (such as proposed by administration of leuprolide), or (iv) infusion of donor-derived T cells (in some cases expressing a conditional-suicide gene to eliminate infused T cells in the event of severe toxicity).27,28,29
Prolonged and profound lymphopenia renders the recipient at high risk for opportunistic infection. Studies of opportunistic viral infections have improved our understanding of the central role T cells have in control of CMV, EBV, VZV, adenovirus, and BK virus infections. To prevent and treat these viral infections, investigators have harvested virus-specific memory T cells from donor, expanded them ex vivo and infused them after allogeneic HSCT.30,31,32,33,34 As technology has evolved and our understanding has deepened of what sub-populations of T cells might be the most effective in vivo, investigators have used adoptive immunotherapy of viral disease to advance methods to (i) infuse T cells early after day 0 (infusion of allograft), (ii) adoptively transfer CD4+ and CD8+ populations of antigen-specific T cells, as opposed to CD8+ clones, and (iii) to exploit selection methods (e.g., using paramagnetic beads) for rapid acquisition of minimally manipulated antigen-specific T cells (identified by tetramer-binding or Tc1 cytokine secretion). Such lymphocytes include cells that are likely less polarized in their ex vivo differentiation into effector T cells and less likely to undergo replicative senescence, compared with T cells harvested from ex vivo cultures that have undergone multiple repetitive cycles of stimulation to achieve numeric expansion (and possibly selection of an introduced transgene).35,36 These adoptive transfer studies rely on the premise that antigen-specific T cells can be identified in the donor. In the event that the donor is seronegative then vaccination may be undertaken, primary T cells may be genetically manipulated to achieve redirected specificity, or in vitro priming of antigen-inexperienced T cells may be attempted.
T-cell regeneration – it’s not just about numbers
Characterizing the kinetics of the emerging allo-grafted immune system has defined two main pathways of T-cell regeneration. In the absence of thymic function, appreciable populations of competent T cell are generated by peripheral expansion of mature T cells. This peripheral expansion pathway is influenced by antigen, and therefore prone to skewing by vaccination. Recovery of thymic function, the second route of T-cell regeneration, after allo-grafting, may be important for the long-term health of the immune system, as thymopoiesis is sufficient to contribute effectively to reconstitution of lost T-cell populations.
To understand engrafted T-cell function, investigators have employed quantitative tools to show how the T-cell receptor (TCR) repertoire recovers after allogeneic HSCT. Cytokine flow cytometry (CFC) can assess both the number and function of CD4+ and CD8+ T cells at a single-cell level. This approach can demonstrate how the number, function, and maturation status of antigen-specific T cells correlate with protective immunity to individual pathogens capable of causing disease in recipients (e.g., cytomegalovirus, CMV).37,38 Additionally, we have also learned that the thymus, known to be the primary site of lymphopoiesis in childhood, but long assumed to be dormant in adults, still functions after HSCT and contributes to T-cell immune recovery, even in some adult recipients (Figure 2).39,40,41
Figure 2.
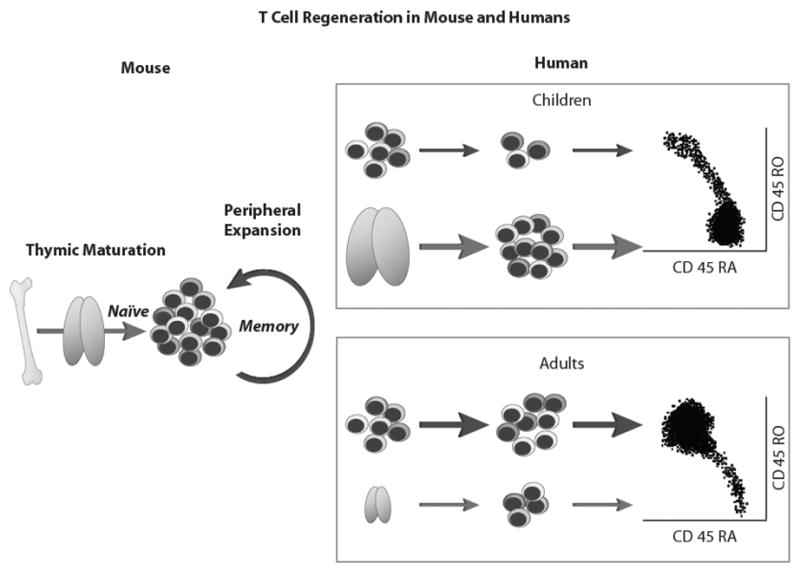
Routes of T-cell recovery in peripheral blood after lymphopenia.
Studies correlating T-cell function (assessed by CFC) with TCR repertoire (assessed by binding of tetramer) have led to an understanding that there are discrepancies in recovering T-cell populations (e.g., after UCB transplantation) between the number of antigen-specific T cells and their function.42 Thymic function plays a central role in regenerating naïve T-cell function and most patients recover thymic function in the weeks to months following allogeneic HSCT. This supports the use of post-thymic interventions (e.g., androgen blockade, administration of desired cytokines such as IL-7) to augment immunity after allogeneic HSCT, to increase both the number and diversity of naïve T cells capable of responding to (vaccine) antigens. For those patients with impaired thymic function, however, e.g., recipients of UCB transplantation, the delay in thymic recovery may be associated with functional “holes” in the T cell repertoire and increased risk of opportunistic infection. This understanding of immune reconstitution has repercussions for vaccination and there are three potential strategies for optimizing or exaggerating T-cell response to tumor-antigens in the setting of allogeneic HSCT: (1) early vaccination post-cessation of lymphodepleting chemotherapy to take advantage of antigen-driven T-cell reconstitution by peripheral expansion, (2) adoptive transfer during a period of lymphopenia of T cells pre-sensitized to specific antigens of choice or rendered specific to a desired antigen, and (3) late vaccination to take advantage of a new, repaired T-cell repertoire that appears with thymic recovery in a subset of adult patients.
Donor-derived NK cells – naturalized killer cells?
It is now accepted that some patients with myeloid malignancies who are the recipients of haplo-disparate grafts enjoy an improved GVL-effect when the donor and recipient "Killer cell Immunoglobulin-like Receptors" (KIR’s) are appropriately mismatched leading to the donor-derived natural killer (NK) cells being released from inhibition.43,44 NK cells are part of the innate immune response and as the first lymphocyte subset to engraft, they have been implicated in contributing to the GVT-effect and suppression of GVHD.45,46 The question for oncologists is now how to (i) reliably predict this NK-mediated effect, (ii) broaden the applicability to malignancies other than acute myelogenous leukemia (AML), (iii) harness the NK effect for HLA-matched transplants, and (iii) improve the NK cell-versus-tumor effect.
Correlating immunogenetics with NK cell function in vitro and in vivo has improved our understanding of the receptor-ligand barriers that serve to keep NK-cell function quiescent. The relative activation state of NK cells is governed as an amalgamation of activating and inhibitory signals which are currently being elucidated. The NK cell inhibitory signals include the inhibitory killer immunoglobulin-like receptors (KIRs) which have specificities for HLA class I molecules. Thus, cells are susceptible to lysis by activated NK cells if the targets lack expression of classical HLA class I molecules. Furthermore, if the target cells in the recipient lack an HLA class I allele present in the donor HLA genotype and the recipient’s HLA class I is not a ligand for the KIR on donor NK cells, then the allogeneic donor NK cells can be activated for cytolysis and mediate an anti-leukemic effect, especially if the target cells are AML blasts. The activation of NK cells due to the missing KIR ligand effect is possible since the KIR and HLA genes segregate independently of each other which results in the phenotype of persons who lack KIR receptors for their respective HLA ligands and persons who lack HLA ligands for their respective KIR receptors. The predictive models of allogeneic NK-cell behavior based on immunogenetics has led to the pre-selection of donors for haploidentical HSCT based on both KIR and HLA genotyping. However, these models of donor-recipient genetics (KIR and HLA typing) and phenotype (NK-mediated killing) remain incomplete and this limits the application of NK-cell therapy to other malignancies and reliably extrapolating the NK effects outside of the haploidentical HSCT setting. 47,48
Because animal models of allogeneic NK-cell immunobiology and anti-tumor effect are generally of uncertain clinical significance, investigators have piloted Phase I/II clinical studies to directly assess the role of infused allogeneic NK cells. These trials adoptively transferred haplo-identical (CD3-depleted and IL-2 activated) NK cells into lymphopenic patients with hematologic and solid tumors and demonstrated that an anti-tumor effect is dependent on (i) potency – achieving the appropriate mismatch between donor KIR and recipient classical HLA molecules, and (ii) the NK cell “area under the curve” – sustaining the persistence of infused NK cells after lymphodepleting chemotherapy with an associated rise in endogenous IL-15 and accompanied by infusion of exogenous IL-2.49 Oncologists are now bringing together haplo-identical HSCT and adoptive transfer of activated NK cells to infuse NK cells before or after allogeneic HSCT in an effort to limit post-HSCT relapse rates.
The future of combination immunotherapies
The profound lymphopenia after allogeneic HSCT can result in death of the recipient from opportunistic infection or relapse due to an incomplete GVT-effect. It also represents an opportunity, however. Allogeneic HSCT clinicians are familiar with balancing risk/benefit ratios, recognize the power of immunotherapy, and are consequently an ideal group of investigators to advance combination immunotherapies. Investigators are already combining cellular therapy with lymphodepleting chemotherapy and antibody therapy. In the future other combinations will be realized, combining (i) immunotherapy with gene therapy to introduce new cellular functions or remove impediments to function, (ii) adoptive immunotherapy with vaccine therapy to deliver an effector-cell population as well as an immuno-stimulus, (iii) cytokines and antibodies (immunocytokines), (iv) antibodies and T cells, using genetic manipulation to introduce mAb-derived chimeric antigen receptors to redirect the specificity of T cells, and (v) antibodies (or immunocytokines) and Fc receptor-expressing NK cells to achieve tumor-specific NK-cell mediated antibody-dependent cellular cytotoxicity. It is anticipated that these active and passive immune-based therapies will be individualized for each patient based on an understanding of immune recovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riddell SR, Murata M, Bryant S, Warren EH. T-cell therapy of leukemia. Cancer Control. 2002;9:114–122. doi: 10.1177/107327480200900204. [DOI] [PubMed] [Google Scholar]
- 2.Naparstek E. The role of the anti-CD20 antibody rituximab in hematopoietic stem cell transplantation for non-Hodgkin's lymphoma. Curr Hematol Rep. 2005;4:276–283. [PubMed] [Google Scholar]
- 3.Kebriaei P, Saliba RM, Ma C, et al. Allogeneic hematopoietic stem cell transplantation after rituximab-containing myeloablative preparative regimen for acute lymphoblastic leukemia. Bone Marrow Transplant. 2006;38:203–209. doi: 10.1038/sj.bmt.1705425. [DOI] [PubMed] [Google Scholar]
- 4.Cilley J, Winter JN. Radioimmunotherapy and autologous stem cell transplantation for the treatment of B-cell lymphomas. Haematologica. 2006;91:114–120. [PubMed] [Google Scholar]
- 5.Vose JM, Bierman PJ, Enke C, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:461–467. doi: 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 6.Gopal AK, Rajendran JG, Petersdorf SH, et al. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002 May 1;99:3158–3162. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 7.Fietz T, Uharek L, Gentilini C, Muessig A, Rieger K, Marinets O, Sandrock D, Munz DL, Glass B, Thiel E, Blau IW. Allogeneic hematopoietic cell transplantation following conditioning with 90Y-ibritumomab-tiuxetan. Leuk Lymphoma. 2006;47:59–63. doi: 10.1080/10428190500260478. [DOI] [PubMed] [Google Scholar]
- 8.Nademanee A, Forman S, Molina A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106:2896–2902. doi: 10.1182/blood-2005-03-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svahn BM, Alvin O, Ringden O, Gardulf A, Remberger M. Costs of allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82:147–153. doi: 10.1097/01.tp.0000226171.43943.d3. [DOI] [PubMed] [Google Scholar]
- 10.Ringden O, Labopin M, Gorin NC, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 11.Fefer A, Robinson N, Benyunes MC, et al. Interleukin-2 therapy after bone marrow or stem cell transplantation for hematologic malignancies. Cancer J Sci Am. 1997;3 (Suppl 1):S48–53. [PubMed] [Google Scholar]
- 12.Soiffer RJ, Murray C, Cochran K, Cameron C, Wang E, Schow PW, Daley JF, Ritz J. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992;79:517–526. [PubMed] [Google Scholar]
- 13.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon SR, Mielke S, Savani BN, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gress RE, Hodes RJ. Generation of the alloreactive T-cell repertoire: interaction of T-cell genotype and maturation environment. Proc Natl Acad Sci U S A. 1982;79:4728–4732. doi: 10.1073/pnas.79.15.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komanduri KV. Thymic function and allogeneic T-cell responses in stem-cell transplantation. Cytotherapy. 2002;4:333–342. doi: 10.1080/146532402760271118. [DOI] [PubMed] [Google Scholar]
- 17.Reisner Y, Gur H, Reich-Zeliger S, Martelli MF, Bachar-Lustig E. Hematopoietic Stem Cell Transplantation across Major Genetic Barriers: Tolerance Induction by Megadose CD34 Cells and Other Veto Cells. Ann N Y Acad Sci. 2005;1044:70–83. doi: 10.1196/annals.1349.010. [DOI] [PubMed] [Google Scholar]
- 18.Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, Gribben JG. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 19.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci U S A. 2001;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baccala R, Gonzalez-Quintial R, Dummer W, Theofilopoulos AN. Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin Immunopathol. 2005;27:75–85. doi: 10.1007/s00281-004-0196-9. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006 Aug 31; doi: 10.1126/science.1129003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich JR, Yang JC, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakim FT, Gress RE. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology. Eur J Immunol. 2005;35:3099–3102. doi: 10.1002/eji.200535385. [DOI] [PubMed] [Google Scholar]
- 26.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 27.Reisner Y, Martelli MF. Transplantation tolerance induced by "mega dose" CD34+ cell transplants. Exp Hematol. 2000;28:119–127. doi: 10.1016/s0301-472x(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 30.Heslop HE, Ng CYC, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 31.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 32.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 33.Einsele H, Hebart H. CMV-specific immunotherapy. Hum Immunol. 2004;65:558–564. doi: 10.1016/j.humimm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 35.La Rosa C, Wang Z, Lacey SF, et al. In vitro expansion of polyclonal T-cell subsets for adoptive immunotherapy by recombinant modified vaccinia Ankara. Exp Hematol. 2006;34:497–507. doi: 10.1016/j.exphem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Rauser G, Einsele H, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004;103:3565–3572. doi: 10.1182/blood-2003-09-3056. [DOI] [PubMed] [Google Scholar]
- 37.Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 38.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 39.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 41.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, Komanduri KV. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 43.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 44.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 45.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 46.Asai O, Longo DL, Tian Z, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggeri L, Mancusi A, Burchielli E, et al. Natural killer cell recognition of missing self and haploidentical hematopoietic transplantation. Semin Cancer Biol. 2006;16:404–11. doi: 10.1016/j.semcancer.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]


