Abstract
Puberty is characterized by mood swings and anxiety, often produced by stress. Here, we show that THP (allopregnanolone), a steroid released by stress, increases anxiety in pubertal female mice, a reversal of its well-known anxiety-reducing effect in adults. Anxiety is regulated by GABAergic inhibition in limbic circuits. Although this inhibition is increased by THP before puberty and in adults, THP reduced tonic inhibition of CA1 hippocampal pyramidal cells at puberty, leading to increased excitability. This paradoxical effect of THP was due to inhibition of α4βδ GABAA receptors. These receptors are normally expressed at very low levels, but at puberty, their expression was increased in CA1 hippocampus where they generated outward currents. THP also decreased outward current at recombinant α4β2δ receptors, an effect dependent on arginine 353 in the α4 subunit, a putative Cl− modulatory site. Thus, inhibition of α4β2δ GABAA receptors by THP provides a mechanism for anxiety at puberty.
The onset of puberty is associated with increases in emotional reactivity and anxiety1,2. Responses to stressful events are amplified3, and anxiety and panic disorder first emerge at this time2, twice as likely to occur in girls than in boys2. Few studies have addressed the biological basis of this important issue, although suicide risk increases in adolescence, despite the use of adult-based medical strategies2.
The GABAA receptor plays a pivotal role in the generation of anxiety4. This receptor is the target for endogenous steroids such as THP (3α-OH-5α[β]-pregnan-20-one or [allo]pregnanolone), which increase GABA-gated currents at physiological concentrations5 of the steroid. THP is a metabolite of the ovarian/adrenal steroid progesterone, but is also formed in the brain as a compensatory response to stress6. In adults, THP potently reduces anxiety in humans7, an effect seen in animal models with direct administration into the dorsal CA1 hippocampus8, part of the limbic system that regulates emotion. It is generally accepted that the GABA-enhancing action of THP underlies its well-known anxiety-reducing effect in adults, which is similar to other GABA-enhancing drugs such as the benzodiazepines.
GABAA receptors are pentamers formed predominantly of 2α, 2β and 1γ subunits9 which gate a Cl− current and produce most fast synaptic inhibition in the brain. Substitution of the δ subunit for γ2 yields a receptor with the highest sensitivity to steroids such as THP10-12. These highly sensitive δ-GABAA receptors are extrasynaptic13, and mediate tonic rather than synaptic inhibition in areas such as dentate gyrus14. Thus, THP and related steroids enhance inhibition here by selectively increasing the tonic current14 at physiological concentrations (< 40 nM)15.
Expression of α4βδ GABAA receptors is normally very low in other areas of the brain, such as the CA1 hippocampus16, one area that regulates anxiety8. However, fluctuating levels of THP can increase expression of α4 and δ subunits in this region, an effect which is paradoxically correlated with anxiety-producing effects of THP in female rodents17-19 . Because the onset of puberty is a naturally occurring hormonal transition state associated with increases in anxiety, we tested whether pubertal development was associated with increased expression of α4βδ GABAA receptors in CA1 hippocampus.
In addition to altered expression of α4βδ receptors, other factors determine the level of inhibition in CNS circuits. In particular, the direction of Cl− current varies across CNS regions: In limbic regions of the brain which normally express α4βδ GABAA receptors, such as the dentate gyrus, the Cl− current is inward (i.e., outward Cl− flux)20. However, in CA1 hippocampal pyramidal cells, both dendritic and somatic GABAergic currents are normally outward in response to low concentrations of GABA21-23, as would be found extrasynaptically24. Therefore, in this study, we also determined if the effect of THP on α4β2δ receptors depended on the direction of Cl− current using patch clamp recording techniques with recombinant receptors expressed in human embryonic kidney (HEK)-293 cells as well as in hippocampal slices. These studies were designed to determine whether the anxiety response to stress at puberty in females involves a change in the response of GABAA receptors to a stress steroid.
Results
Effects of THP on α4β2δ GABAA receptors
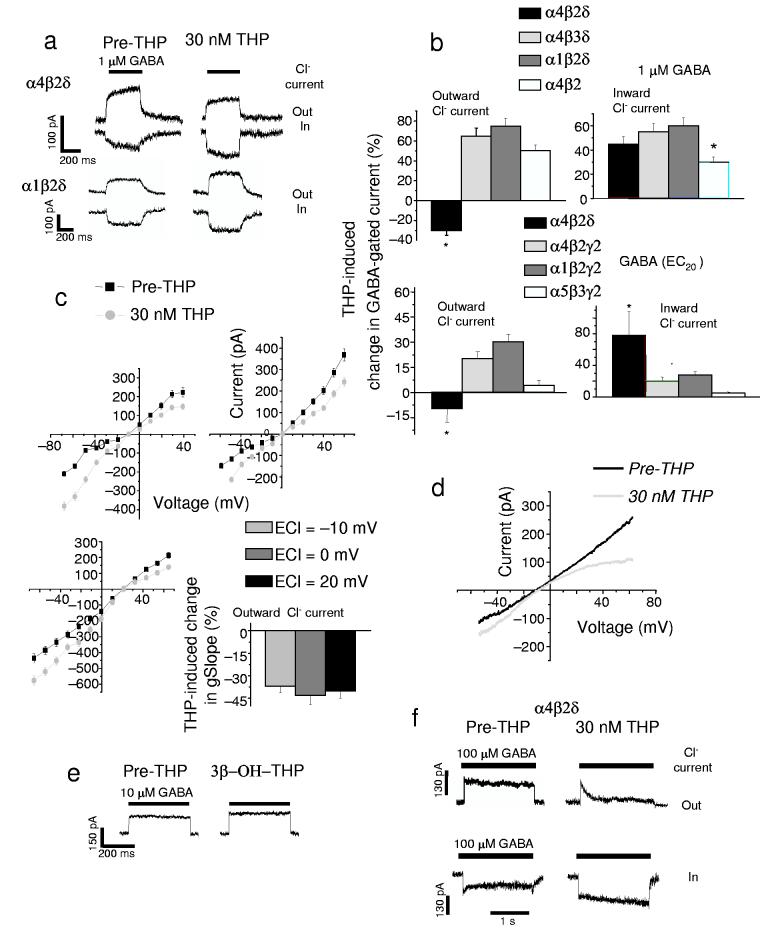
In contrast to its effect at other receptor subtypes, 30 nM THP decreased the outward GABA(1 μM)-gated Cl− current through recombinant α4β2δ receptors expressed in HEK-293 cells by 28 ± 3% (mean ± SEM, P < 0.05, Fig. 1a,b, Supplementary Fig. 1a,b, Supplementary Table 1), recorded at –50 mV with whole cell patch clamp techniques. When assessed across a range of voltage steps (Fig. 1c, Supplementary Fig. 2), THP significantly decreased the conductance of the outward current by 36 – 43%. This action of the steroid was not directly influenced by the membrane potential (Fig. 1c). Thus, in experiments where we varied the reversal potential for Cl− by altering internal Cl− concentration, THP produced equivalent decreases in outward current at a similar Cl− driving force when assessed at different membrane potentials. However, THP application did not itself alter the Cl− reversal potential (Fig. 1c,d, Supplementary Fig. 2) suggesting that it does not alter non-GABA-gated conductances. Similar decreases in outward current were produced by THP assessed using a voltage ramp (Fig. 1d). In contrast, THP robustly increased inward currents through these receptors (Fig. 1a,b). The concentration of GABA used here (1 μM) is an EC75 for α4β2δ GABAA receptors (Supplementary Fig. 1), and represents the GABA concentration to which extrasynaptic GABAA receptors, such as α4βδ, would be exposed24. In contrast, 30 nM THP applied without GABA had no effect (data not shown). We also studied various receptor subtypes using an EC20 concentration of GABA (5 –10 μM for most receptors, Fig. 1b). In contrast to its effects at α4β2δ, THP either increased or had no effect on the outward current at α1β2δ, α4β3δ, α1β2γ2, α4β2γ2 and α5β2/3γ2 receptors (Fig. 1a,b). Thus, the inhibitory effect of THP is dependent on the presence of α4, β2 and δ subunits, and was selective for the active 3α-OH isomer, but not the inactive 3β-OH isomer5, of THP (Fig. 1e).
Figure 1.
The neurosteroid THP decreases outward current gated by α4β2δ GABAA receptors. (a) Representative traces showing the effects of 30 nM THP (right) on current gated by 1 μM GABA (EC75), under conditions of outward Cl− current (inward Cl− flux, upper trace) and inward current (lower trace) for two δ-containing recombinant GABAA receptor subtypes. The direction of Cl−current was reversed by varying internal Cl− (upper trace, ECl = −70; lower trace, ECl = − 30 mV), but using a constant holding potential of −50 mV. (b) Mean effects of THP on outward and inward currents in response to 1 μM GABA (upper panel) or the GABA EC20 (lower panel, α4β2δ, 0.1 μM; α4β2γ2, 5 μM; α1β2γ2, 10 μM; α5β3γ2, 5 μM) from 6 − 7 cells for each group (*P < 0.05 vs. the other receptor subtypes) (c) Current-voltage plots recorded under conditions of varying ECl (− 10, 0, 20 mV) in the presence or absence of 30 nM THP. Mean ± SEM for the slope conductance (gSlope) of the outward current (n = 7 – 8 cells for each group). (d) 30 nM THP effects on current generated by a voltage ramp over 400 ms. (Leak-subtracted current is presented as the average of 3 traces). (e) Effects of the inactive 3β-OH isomer of THP on outward GABA-gated current at α4β2δ GABAA receptors (representative of 5 – 6 cells). (f) THP effects on desensitization of outward (upper trace) and inward (lower trace) current at α4β2δ receptors. This effect is representative of 6 cells for each group.
One potential mechanism for the THP-induced decrease in outward current at α4β2δ GABAA receptors is through acceleration of receptor desensitization11. Therefore, we used rapid application techniques to administer saturating concentrations of GABA (100 μM) for 2 s to HEK-293 cells expressing α4β2δ GABAA receptors. In fact, 30 nM THP increased desensitization of outward currents from 8 ± 2% to 87 ± 5.6% (100 μM GABA, P<0.001, Fig. 1f), with a markedly faster time-course (τ = 230 ± 35 ms versus pre-THP, 1700 ± 200 ms, P < 0.001). Although peak current was unchanged by steroid exposure, the amplitude of the desensitized current < 50 ms after application of GABA was significantly smaller than control. This desensitized state is relevant for tonic current which is equivalent to the steady-state current. Consistent with this, the decrease in outward steady-state current was correlated with GABA concentration, with THP producing a greater decrease in current gated by higher concentrations of GABA where desensitization is more pronounced (Fig. 1a,f, Supplementary Fig. 1,2)
Residues required for THP inhibition of α4β2δ receptors
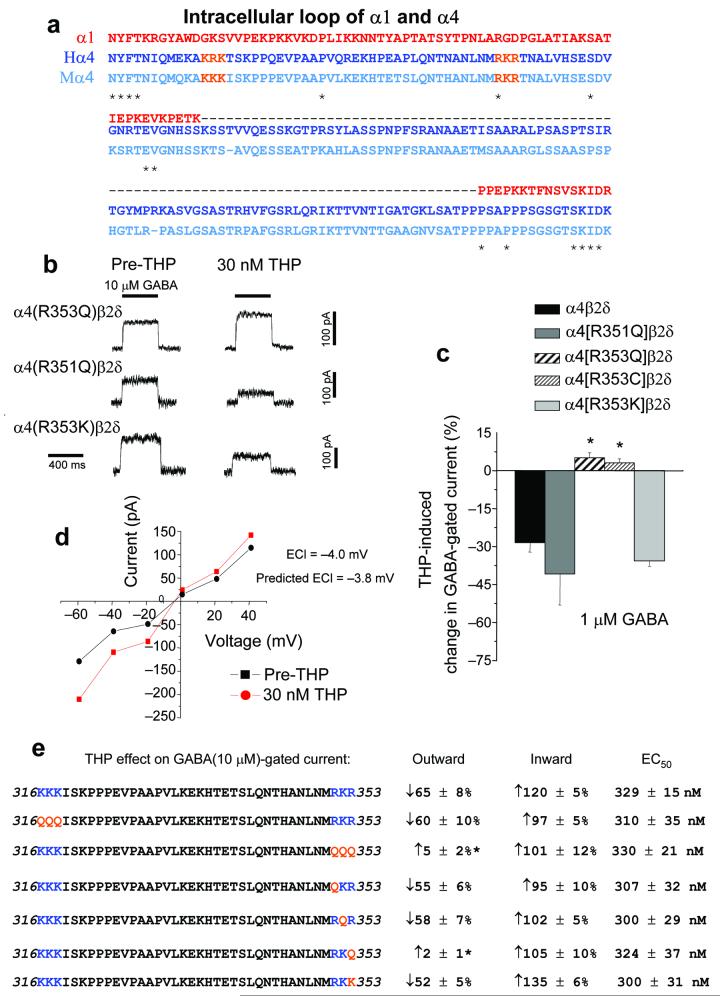
The α1 and α4 subunits have the least homology in the intracellular loop region (Fig. 2a), which may contribute to the permeation pathway in the Cys-loop family of receptors25,26. Because recent studies have reported the existence of charged residues which are ion sensor sites in membrane proteins25-27, we investigated whether positively charged residues within the loop might mediate the Cl− dependent effects of THP seen at α4β2δ receptors. Indeed, mutation of a positively charged arginine (R) at position 353 to a neutral glutamine (Q) or cysteine (C) residue in the α4 subunit prevented the steroid-induced reduction in outward current of α4β2δ GABAA receptors expressed in HEK-293 cells (Fig. 2b-e, Supplementary Tables 2–4), whereas mutation of R353 to another basic residue, lysine (K), did not prevent THP inhibition of outward current (Fig. 2b,c,e).
Figure 2.
Arginine 353 in the α4 subunit is necessary for the direction-sensitive inhibition of α4β2δ GABAA receptors by THP. (a) Alignment of the intracellular loop of α1 and α4 (H, human; M, mouse) subunits reveals limited identity (< 10%). *identical residues for all three. (The sequences for human and mouse α1 are identical.) Orange, residues to be mutated. (b) Representative traces showing the effect of 30 nM THP on GABA(10 μM)-gated current at the indicated mutated α4β2δ GABAA receptors. Basic arginine (R351 or R353) residues in the α4 subunit were mutated to a neutral glutamine (Q) and/or a basic lysine (K). (c) Averaged data, Effects of 30 nM THP at α4β2δ receptors containing wild-type or mutant α4 subunits on outward GABA(1 μM)-gated current. (n = 4 – 5 cells for each group, *P < 0.05 versus wild-type α4β2δ). (d) Current-voltage plot recorded from α4[R353Q]β2δ GABAA receptors before and after THP; ECl = −4.0 mV; predicted ECl = −3.8 mV, averaged from 5 cells for each point. (e) Summary diagram. Left, amino acid sequences 316-353 within the mouse α4 loop (basic residues, blue; mutated residues, orange). The two regions of the α4 loop with consecutive basic residues (316 – 318 and 351 – 353, in blue) were mutated as a group or singly to a neutral glutamine (Q in red) or to a basic lysine (K). Right, Effects of the indicated mutation on outward and inward GABA(10 μM)-gated current are indicated, as is the GABA EC50 (Mean ± SEM). All mutations produced current of similar magnitude (100 – 200 pA; n = 5 – 6 cells for each group).
Mutations at nearby arginine or lysine residues R351Q, K352Q, K316Q, R317Q and K318Q had no effect (Fig. 2b,d,e) suggesting that residue 353 was uniquely involved in steroid inhibition of the outward current. In contrast, THP increased inward current through α4[R353Q]β2δ GABAA receptors, and this mutation did not alter sensitivity to GABA or the ECl, determined before and after THP administration (Fig. 2d,e). These results suggest that a basic residue at position 353, a putative Cl− modulatory site, is necessary and sufficient for Cl−dependent THP inhibition of α4β2δ GABAA receptors.
Localization of α4 and δ subunits in CA1 hippocampus
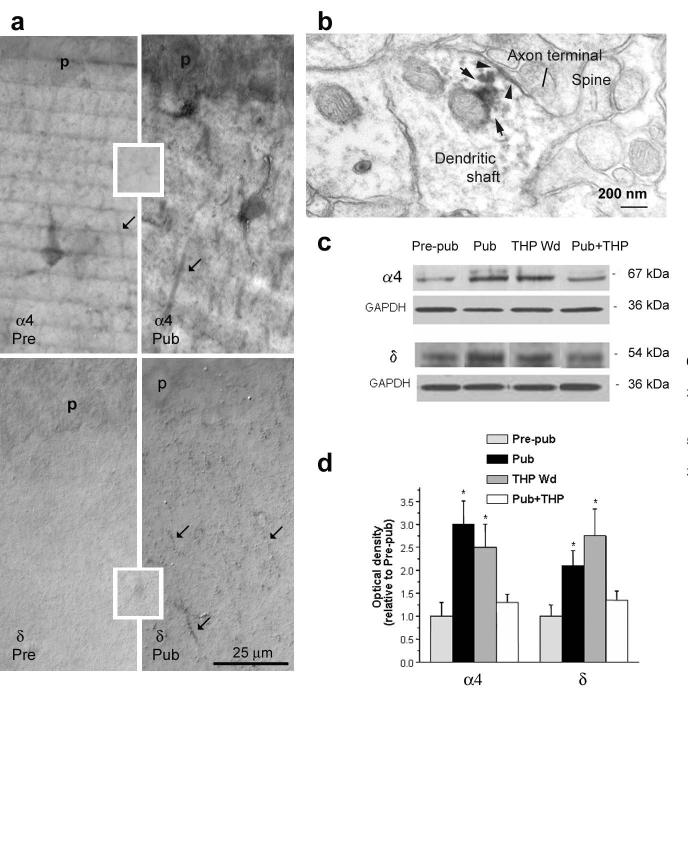
α4βδ receptors are normally expressed at very low levels in CA1 hippocampal pyramidal cells16. Given the novel effects of THP at these receptors, we hypothesized that their expression may be altered during puberty when the anxiety response to stress is increased3. Initially, we localized α4 and δ subunits in CA1 hippocampus using immunohistochemical techniques at the onset of puberty in female mice, defined as the first metestrus stage after vaginal opening. Markedly increased expression of α4 and δ was observed along the pyramidal cell dendrites in the stratum radiatum of CA1 hippocampus at puberty (Fig. 3a,b) from almost undetectable levels before puberty, as reported in the adult16. In fact, expression of both α4 and δ subunits was increased by up to two-fold (P < 0.05) at the onset of puberty (Fig. 3c,d, Supplementary Table 5), quantified using Western blot techniques.
Figure 3.
Increased expression of α4 and δ subunits on pyramidal cell dendrites of CA1 hippocampus at the onset of puberty. (a) Immunocytochemistry of α4 (upper panel) and δ (lower panel) GABAA receptor subunits in stratum radiatum of CA1 hippocampus (40X magnification). Arrows point to immunolabeling along distal portions of dendrites. Pre-pubertal; Pre Pubertal, Pub. Calibration bar applies to all four panels. The insets show background labeling taken from the ventromedial hypothalamus, a region without detectable expression of these subunits16. Representative of results from 5 – 6 mice for each group. (b)Electron micrograph of δ staining along the plasma membrane of the dendritic shaft (arrowhead) that is post-synaptic to an axon terminal as well as intracellularly (arrows). The long arrow points to an axon terminal that is likely to be GABAergic, based on the absence of postsynaptic density. (Representative of results from 5 pubertal mice.) (c) Western blot showing hippocampal expression of α4 and δ subunits after puberty and THP Wd compared to the pre-pubertal state. In one group, the decline in THP levels at puberty was prevented with 48 h administration of THP (10 mg kg−1), Pub+THP. (d) Optical densities from Western blot results averaged from 6 hippocampi for each group normalized to the GAPDH control. *P < 0.05 versus Pre-pub for all graphs. (n = 3 – 4 animals for each group, performed in triplicate).
Puberty and hippocampal THP levels
In addition to upregulation at the onset of puberty, expression of α4 and δ subunits increases in adult hippocampus when circulating levels of THP decrease (i.e., “THP withdrawal”)17,19. Thus, we determined whether endogenous THP levels decrease across pubertal development. In fact, hippocampal THP levels declined by 56 ± 12% (P < 0.05, n = 8) at the onset of puberty, as has been shown previously for humans when fluctuating levels of THP follow prolonged elevations of the steroid prior to puberty onset28.
The decline in THP levels we observe in mouse hippocampus was similar to that produced by administration of a 5α-reductase blocker (58 ± 10%) which prevents formation of THP18. Accordingly, increases in α4 and δ expression were also seen following THP withdrawal (Fig. 3c,d). Because increased expression of α4 and δ subunits at the onset of puberty was prevented by replacement THP (10 mg kg−1 day−1 for 3 days, Fig. 3c,d), these results suggest that declining levels of THP at puberty trigger expression of α4 and δ subunits. In contrast to α4, expression of the α5 subunit, which underlies most tonic inhibition in the CA1 hippocampus29, was unchanged by puberty (data not shown).
THP and tonic current
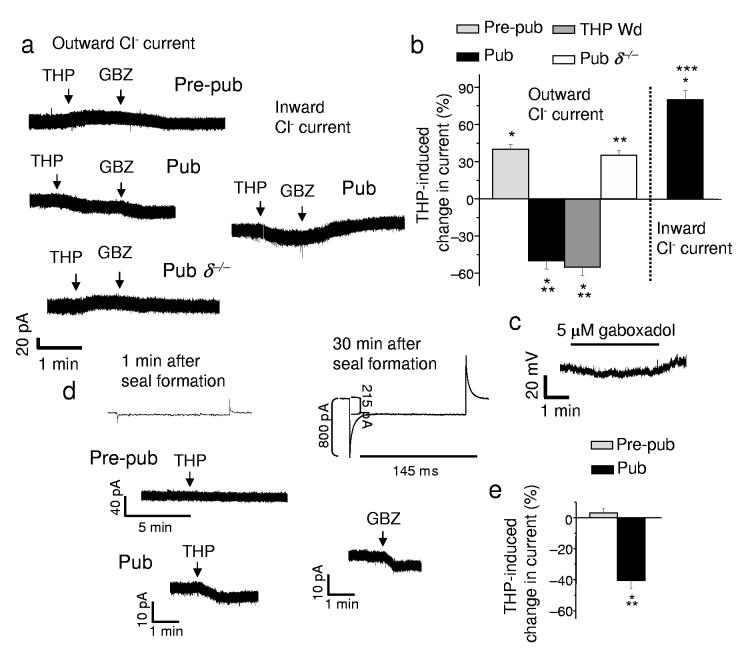
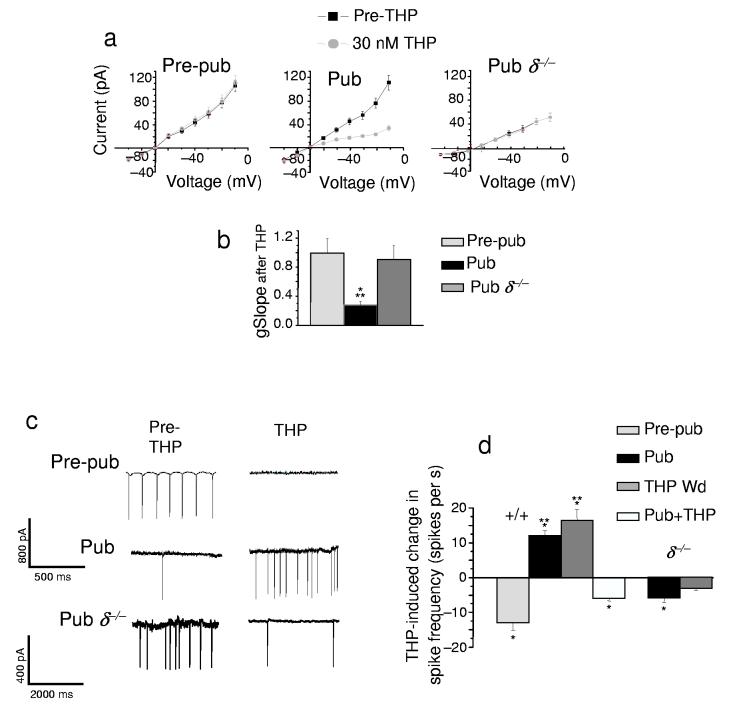
GABAA receptors containing α4 and δ subunits are localized to extrasynaptic sites13 where they generate a tonic current responsive to low concentrations of steroid14. Therefore, we reasoned that THP would reduce the outward tonic GABAergic current after puberty, when α4βδ receptors are expressed at high levels, an effect verified through selective pharmacological tests (Supplementary Fig. 3, Table 6) in addition to immunocytochemical and Western blot detection (Fig. 3). Indeed, in hippocampal slices from pubertal mice, 30 nM THP reduced the tonic current (Fig. 4a,b, Supplementary Table 7) by 48 ± 6%, recorded with whole cell patch clamp techniques from CA1 pyramidal cells using low internal Cl− to achieve outward current. Based on our findings with recombinant receptors, we also predicted that the inhibitory effect of THP on tonic GABAergic currents would be prevented if the direction of the Cl− current were reversed. Indeed, THP increased the tonic GABAergic current when the cell was loaded with Cl− to produce inward current (Fig. 4a inset, 4b). In these recordings, the synaptic current was selectively blocked with a low concentration of gabazine (200 nM), a GABAA receptor antagonist30, to visualize the tonic current.
Figure 4.
THP inhibits tonic GABAergic current recorded from the hippocampal slice at puberty. (a) Outward current recorded from CA1 hippocampal pyramidal cells in the slice by whole-cell patch clamp (ECl = −70 mV, −50 holding potential, pipet solution, K-gluconate; bath, 200 nM gabazine to block synaptic current and 2 mM kynurenic acid to block excitatory current). Pre-pubertal, Pre-pub; pubertal, Pub. Inset, THP effects on the inward tonic current at puberty. (b) THP-evoked change in outward and inward tonic current, Averaged data. THP withdrawal, THP Wd. (mean ± SEM, n = 8 – 12 cells for each group). (c) Tight-seal cell-attached current-clamp recording31 of the holding potential during dendritic application of the GABA agonist gaboxadol (5 μM) to the stratum radiatum. (Representative of cells from 5 pubertal mice). (d) Perforated patch voltage-clamp recordings from the soma of a CA1 pyramidal cell of the post-synaptic response to bath applied THP. Inset, the change in access resistance determined from the current response to a 10 mV step before and after perforation. (Bath, 1 μM TTX and 1 μM GABA; also 200 nM gabazine and L-65,708, to block synaptic current and α5-GABAA receptors, respectively; 10 μM CGP 55845, 5 mM TEA and 50 μM kynurenic acid to block GABAB receptors, K+ channels and excitatory amino acid receptors, respectively.) (e) Averaged data, n = 5 cells for each group. *P < 0.01 versus pre-THP, **P < 0.001 versus Pre-pub.
In contrast, THP increased the outward tonic current before puberty and in the δ−/− mouse after puberty (Fig. 4a,b), both conditions where α4βδ GABAA receptors have low levels of expression. Interestingly, THP produced similar decreases in outward current after THP withdrawal (Fig. 4b) suggesting that the decline in THP at puberty results in this paradoxical inhibitory effect of the steroid on outward tonic current. In contrast to the steroid-induced decrease in tonic current, baseline levels of tonic current were increased at puberty (Fig. 4a), however, compared to levels in pre-pubertal slices.
Cell-attached and perforated-patch recordings
To determine whether THP inhibition of GABAergic current at puberty was a physiological phenomenon, we initially verified that GABA-gated currents were outward in CA1 hippocampal pyramidal cell dendrites at the onset of puberty. To this end, we recorded the change in membrane potential produced by local application of the GABA agonist gaboxadol (4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol, THIP) to the apical dendrite in the stratum radiatum using the hippocampal slice preparation. The voltage change was recorded in current clamp mode from the soma using tight-seal cell attached techniques31, and verified that GABAergic dendritic current was indeed hyperpolarizing (Fig. 4c), as suggested by other reports22,23.
One complication of whole-cell patch clamp recording is that normal ionic gradients are disrupted. Therefore, in order to verify that THP reduced tonic GABAergic currents in intact cells under conditions of unperturbed internal Cl−, we directly recorded pharmacologically isolated tonic GABAergic current from the soma using perforated-patch voltage-clamp techniques in the hippocampal slice. In order to rule out potential pre-synaptic effects, 1 μM tetrodotoxin (TTX) was used to block activity-driven GABA release, and instead post-synaptic current was generated with the addition of 1 μM GABA added to the bath solution. Under these conditions, 30 nM THP depressed the outward GABAergic current by 40 ± 8% in slices from pubertal animals (Fig. 4d,e). THP also decreased the GABA-gated conductance (Fig. 5a,b), assessed as the slope of the gabazine-sensitive current in response to 10 mV steps (−60 to −40 mV). However, THP did not alter the reversal potential (Fig. 5a), suggesting that it did not alter conductances of other channels. Distinct from its effect at puberty, THP had no effect on the post-synaptic GABAergic tonic current (Fig. 4d,e) before puberty when α4βδ expression is low (Fig. 3).
Figure 5.
THP increases excitability of hippocampal pyramidal cells at the onset of puberty. (a) Current-voltage plots, The difference current recorded before and after bath application of 120 μM gabazine (pipet solution, cesium-methanesulfonate; bath contains 1 μM TTX, 1 μM GABA and 50 μM L-655,708). (b) Averaged slope conductance (gSlope, assessed from −60 to −40 mV; n = 5 – 6 cells for each group). *P < 0.05 versus pre-THP, **P < 0.05 versus Pre-pub. (c) Tight-seal cell-attached voltage-clamp recordings from the soma (−40 mV) of CA1 hippocampal pyramidal cells31. (d) THP effects on spiking, averaged data. (n = 5 cells for each group). *P < 0.05 versus Pre-THP; **P < 0.05 versus Prepub for all graphs.
Interestingly, THP also reduced the tonic current in pre-pubertal thalamic relay neurons by 57 ± 12% (P < 0.05, n = 6, data not shown), which normally have high levels of α4β2δ expression16,32 that underlie a tonic current32, where GABA-gated current is outward33. Thus, α4β2δ GABAA receptor expression and outward Cl− current are necessary and sufficient for the paradoxical effect of THP.
THP and neuronal excitability
We reasoned that the decrease in the tonic dendritic GABAergic conductance produced by THP at puberty would increase input resistance. Indeed, THP significantly increased the input resistance by 38 ± 5%, calculated from the current response to 10 mV steps (−60 to −40) in the hippocampal slice. (Supplementry Fig. 4, Tables 8,9). This effect was not seen in slices from δ−/− mice, and was prevented when 120 μM gabazine was pre-applied, demonstrating that alterations in the GABA-gated conductance underlie the change.
Increases in the input resistance produced by THP would be predicted to increase neuronal excitability at puberty. Indeed, THP significantly (P < 0.001) increased spiking at this time, assessed in cell-attached mode31 where the internal Cl− was undisturbed (Fig. 5c,d, Supplementary Tables 10,11). Baseline levels of neuronal excitability were reduced at puberty, however, as expected for an increase in tonic current.
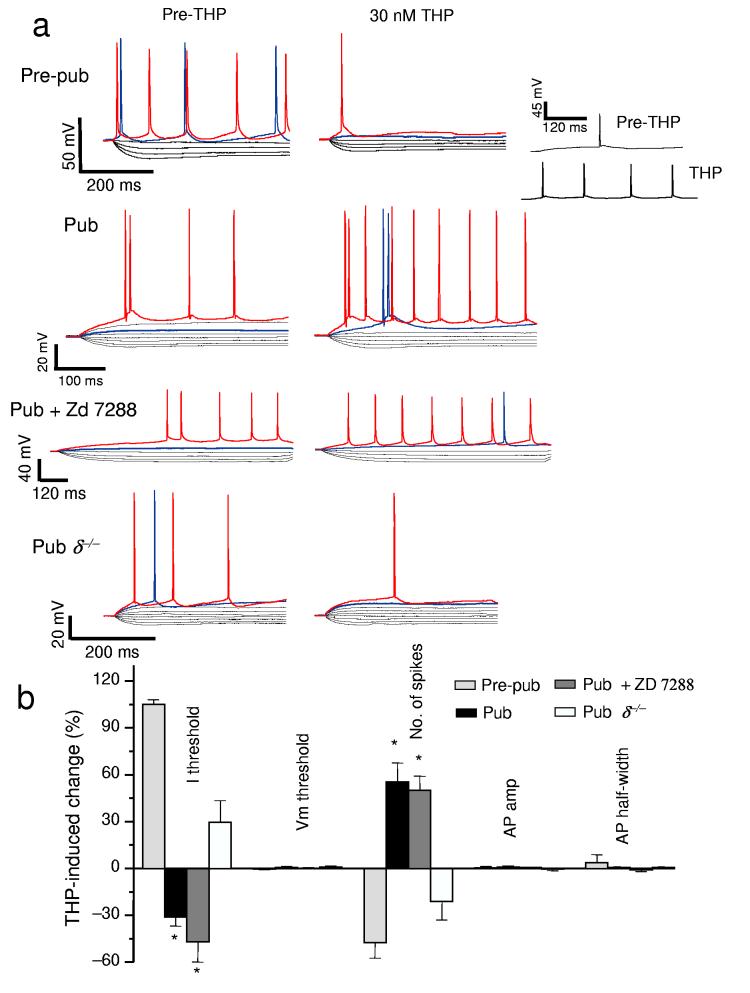
In order to determine the cellular characteristics which might underlie this event, we also conducted whole cell recordings (Fig. 6a,b) in current clamp mode where we monitored spiking of CA1 hippocampal pyramidal cells in response to progressively increasing levels of injected current. Here, THP reduced the amount of current necessary for triggering a spike (Fig. 6a,b) at puberty. THP also increased action potential frequency in these cells (Fig. 6a,b), without changing spiking characteristics or other membrane properties such as voltage threshold, action potential amplitude or action potential half-width (Fig. 6b, Supplementary Tables 12,13). Although the onset of puberty was also associated with a “sag” in the voltage response to hyperpolarizing current injection, suggesting the presence of Ih (hyperpolarizing-induced cation current), selective blockade of this current with 20 μM ZD 7288 did not prevent the excitatory effect of THP on CA1 hippocampal pyramidal cells (Fig. 6a,b). Blockade of Ih altered the after-hyperpolarization to more closely approximate its pre-pubertal level, also ruling out changes in after-hyperpolarization as a potential mechanism for the effect of THP at puberty. This excitatory effect of THP on neuronal firing was not observed in hippocampal slices from δ−/− mice, implicating δ-containing receptors. In contrast, before puberty, THP decreased neuronal excitability (Fig. 5c,d, 6a,b), evidenced by a decrease in the current threshold for spiking and reduced spike frequency at threshold.
Figure 6.
THP lowers the current threshold for spiking of pyramidal cells at the onset of puberty. (a)Whole cell current clamp recordings conducted from CA1 hippocampal pyramidal cells. Voltage responses recorded in response to increasing 0.3 nA current injection (−1 nA, initial current) for slices recorded before puberty (Pre-pub), or at puberty in wild-type (Pub) or δ−/− (Pub . δ−/− ) mice. (The THP trace lacks the 800 pA current trace for ease of comparison.) Inset, spiking at threshold, 800 pA, pre-THP; 500 nA THP in a non-spiking pubertal cell. In some cases, Ih was blocked with 20 μM Zd 7288 (Pub + Zd 7288). .Red trace, equivalent current injection, threshold for the less excitable state. Blue trace, equivalent current injection, threshold for the more excitable state.) (b) Mean ± SEM averaged from 7 – 8 cells for each group. Current threshold to spiking, I threshold; voltage threshold to spiking, Vm threshold; spike frequency, No. of spikes; action potential amplitude, AP amplitude; action potential half-width, AP half-width. Spike frequency was assessed at the minimum current required to produce spiking in both pre- and post-THP traces. *P < 0.05 versus Pre-pub.
THP, stress and anxiety behavior
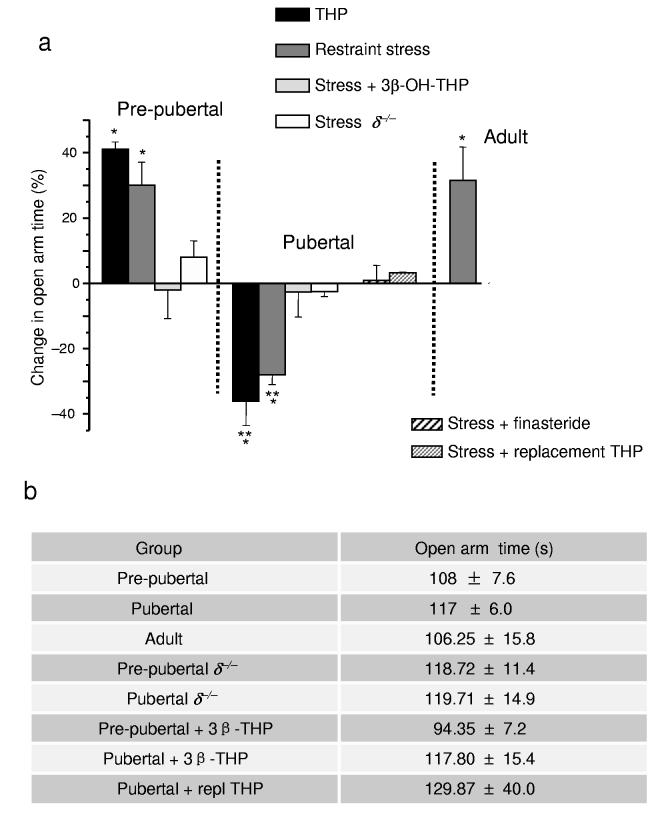
Consistent with the in vitro findings, the onset of puberty reversed the behavioral effect of THP from decreasing anxiety, as normally observed8, to increasing anxiety (Fig. 7a, Supplementary Tables 14,15). To study this, we used an animal model in which the time spent on the open arm of an elevated plus maze reflects a decrease in anxiety18. In fact, following the onset of puberty, acute administration of THP at a physiological dose (10 mg kg−1, intraperitoneally) decreased open arm time by 35 ± 8% on the elevated plus maze, without changing locomotor activity. We have reported similar paradoxical anxiety-producing effects of THP after THP withdrawal18, when α4βδ GABAA receptors are increased. Because endogenous THP is released by stress6,34, we also tested this physiological outcome by assessing anxiety behavior 20 min after restraint stress. As predicted by the anxiogenic effect of THP, restraint stress also significantly increased anxiety in pubertal mice (decreasing open arm time by 27 ± 2.6%, P < 0.05), in contrast to its anxiety-reducing effect in pre-pubertal mice and in adult mice (Fig. 7a).
Figure 7.
THP paradoxically increases anxiety after the onset of puberty. (a) Alterations in anxiety produced by stress or injection of THP (10 mg kg−1, i.p.) are presented as a percentage change in open arm time in the elevated plus maze compared to mean values from a sham control group, identical to the experimental group (age- and genotype-matched) except for the indicated treatment (stress or THP). In order to test the role of THP release in the stress response, in some cases the inactive 3β-OH isomer of THP (stress + 3β-OHTHP) or finasteride were pre-administered. Replacement THP (10 mg kg−1, intraperitoneally, in oil, for three days) was also administered to prevent the decline in THP at puberty. n = 6 – 9 mice for each group, *P < 0.05 versus control, **P < 0.05 versus Pre-pub. (b) Open arm time (Mean ± SEM) for all control groups not subjected to restraint stress.
Stress is also associated with activation of the hypothalamo-pituitary-adrenal axis35. Therefore, we verified that effects of restraint stress were due to THP by pre-administration of the inactive 3β-OH isomer, an antagonist of THP effects at GABAA receptors36 (Fig. 1b), and blockade of endogenous THP formation, which both prevented the stress-induced increase in anxiety (Fig. 7a). Stress-related increases in anxiety after puberty were not observed in δ−/− mice, implicating δ-containing receptors. Replacement THP was also administered at puberty to prevent the decline in THP. Animals tested after this steroid replacement paradigm did not exhibit an anxiety response to stress, suggesting that the decline in THP underlies the anxiety-producing effect of stress. In contrast, anxiety level was not different among the various developmental and treatment groups not exposed to stress, reflected by the open arm time (Fig. 7b), nor was locomotor activity (mean change = 1.6 ± 3%).
Discussion
The results from this study demonstrate that effects of the neurosteroid THP can reverse from its classic effect of enhancing GABA-gated current to inhibiting current at α4β2δ GABAA receptors in a Cl-dependent manner. Expression of these receptors was increased in CA1 hippocampus at the onset of puberty, where they generated an outward current. Under these conditions, THP paradoxically increased anxiety in contrast to its well-known anxiety-reducing effect in pre-pubertal and adult animals8.
The inhibitory effect of THP on outward currents at α4β2δ GABAA receptors was dependent upon arginine 353 in the intracellular loop of α4, a basic residue that may act as a modulatory site for Cl− Recent studies suggest that ion sensor sites can regulate other events such as Cl− activation of HCN subunits which mediate Ih27. In addition, the recent discovery of a cation-triggered phosphorylation event in a novel membrane protein lacking an ion pore37 suggests that ion sensor sites regulate neuronal function beyond ion conductance. Modulatory effects of Cl− have been noted before38, which are necessary for barbiturate and benzodiazepine binding. In addition, the intracellular loop of the Cys-loop family of receptors is ion accessible25,26, while for other membrane receptors this loop functions not only as a permeation pathway, but also as a site necessary for rapid desensitization39. Indeed, the effect of THP was to promote rapid desensitization of the receptor, an effect leading to reduced current amplitude. Direction-sensitive changes in the rate of desensitization have been reported for GABAA receptors, including the homologous α6β3δ11, at which the outward Cl− current desensitizes more then the inward current. Our data are also consistent with the finding that neurosteroids facilitate desensitization of δ-containing GABAA receptors40, but are novel in demonstrating effects of low nanomolar concentrations of THP at an ambient concentration of 1 μM GABA, relevant for the physiological state24.
α4 and δ subunits are localized extrasynaptically13 where they co-express with β232. These α4β2δ GABAA receptors have a high sensitivity19 to low concentrations of GABA and a relative lack40 of desensitization making them ideally suited to generate a tonic current. However, by increasing receptor desensitization of α4βδ GABAA receptors at puberty, THP reduced this tonic inhibition of CA1 hippocampal pyramidal cells. This reduction in conductance along the dendrites increased the input resistance of the neuron, similar to effects reported after blockade of dendritic K+ channels41. Increasing the input resistance would allow ongoing excitatory synaptic currents to produce a larger depolarizing effect on the cell body of the neuron, thus increasing the likelihood of triggering an action potential. Alterations in this type of shunting inhibition have been shown to affect both sub-threshold events, as well as drive a higher firing frequency42, consistent with the results shown here. In contrast, action potential characteristics were not altered nor was the voltage threshold for triggering an action potential, suggesting that changes in excitatory transmission were not affected by THP. Other conductances, such as Ih and K+ channel current, were similarly not involved in the excitatory effects of THP, which were solely dependent on the presence of δ-containing GABAA receptors.
Our findings suggest that the effects of THP predominate at the output neurons of the hippocampus at puberty because application of THP reduced tonic inhibition generated either by ambient GABA or following addition of GABA to the slice while blocking interneuron activity with TTX. This effectively led to increases in excitability of CA1 pyramidal cells. Increases in excitability of the major output neurons of the hippocampus produced by THP would impact upon behavioral end-points influenced by this limbic structure8, leading to increased emotional reactivity, which we observed. In fact, recent evidence43 suggests that anxiety-reducing effect of benzodiazepines is due to direct modulation of the tonic current, as has also been shown for the anti-seizure effect of the GABA agonist gaboxadol44.
In contrast to its effect at puberty, THP had no effect on the post-synaptic tonic current recorded from CA1 pyramidal cells before puberty when expression of α4βδ receptors is low16. This is consistent with the finding that the extrasynaptic receptors present at this time, which contain the α5 subunit29, are relatively insensitive to THP12. In contrast, α4β2δ receptors are expressed at high levels on the dendrites of dentate gyrus granule cells16, where the GABAergic current is inward20. Thus, THP and related steroids enhance inhibition of this limbic structure14, consistent with their anxiety-reducing effect before puberty and in the adult8.
The anxiety-promoting effect of THP at the onset of puberty may contribute to the aversive effects of stress which emerge at puberty in humans3. Distinct from effects of corticosterone, which are long-lasting45, release of THP is a relatively short-term response to acute stress, because it is produced directly in the hippocampus46. Its effects last one to two hours and are accompanied by decreases in anxiety6, as demonstrated in rodents47 and humans34. The THDOC metabolite of corticosterone (5α-pregnane-3α, 21-diol-20-one) is also released following stress47, although to a lesser degree, but as a similar neuroactive steroid, would likely contribute to the effect exerted by THP. In contrast, baseline levels of anxiety were not altered by puberty in female mice. Instead, the stress-induced increase in anxiety produced by THP in adolescent females would be evidenced as a transient increase in anxiety, reflected as a “mood swing”.
Emotional changes also occur in males, but these may additionally involve changes in male-specific steroids45 which can also alter mood.
Steroid fluctuations in the adult also result in anxiety-producing effects of THP or its precursor, progesterone: These include premenstrual syndrome48,49 and post-menopausal irritability50. Taken together, these results suggest that a reversal of the normally anxiety-reducing effect of THP via effects at α4β2δ GABAA receptors may represent an adaptive response to steroid fluctuations when increases in emotional reactivity occur.
Materials and Methods (see Supp. Methods for additional details)
Animal subjects
Pre-pubertal and pubertal female C57/BL6 mice (3 ½ - 6 weeks old, +/+ and δ−/−) were housed in a reverse light:dark cycle (12 :12). In some cases, adult (3 months of age) female C57/BL6 mice were also tested. The onset of puberty was determined by vaginal opening, and pubertal mice tested on the day of first metestrus, identified by vaginal morphology. Pre-pubertal mice were tested before the beginning of the pubertal period. Only female mice were used. In some cases, pubertal mice were administered replacement THP (10 mg kg−1, intraperitoneally, in oil, for 3 days) to prevent the decline in THP occurring at this time. Procedures were in accordance with the SUNY Downstate Institutional Animal Care and Use Committee.
Radioimmunoassay for 3α,5α-THP
Hippocampal levels of 3α,5α-THP were assessed by radioimmunoassay (RIA) during the nocturnal surge15, 1 h after dark onset, according to previously published methods (See Supplementary Methods).
Western blot
Procedures were performed on hippocampal membranes at protein concentrations in the linear range (5 – 10 μg), as we have described17 (see Supplementary Methods) using selective antibodies for α417 (67 kDa) and δ (54 kDa, a generous gift from W. Sieghart). Bands were visualized with enhanced chemiluminescence (Pierce Supersignal WestFemto substrate) and quantified using One-Dscan software from the scanned image. Results were normalized to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control, and are expressed as a ratio relative to the pre-pubertal values.
Immunocytochemistry
Immunocytochemical labeling of receptor subunits was performed using the pre-embed DAB (3,3′-diaminobenzidine) procedure (see Supplementary Methods) with antibodies selective for α4 (SC 7355, Santa Cruz Biochemicals) and δ13 (generously supplied by W. Sieghart).
Recombinant receptors
Human embryonic kidney (HEK)-293 cells were transfected with α4β2δ or other indicated subunit combinations (see Supplementary Methods) and co-transfected with enhanced green fluorescent protein for visualization. GABA-gated currents were recorded at room temperature (20 – 22 °C) at a holding potential of − 50 mV17 using a pipet solution containing 120 mM: N-methyl-D-glucamine chloride. Internal [Cl-] or the holding potential were varied to alter the direction of Cl− flow. A piezo-controlled double-barreled theta tube (Sutter Instr., 80 −100 μm dia.) containing GABA (0.001 – 1000 μM) or GABA plus THP (30 nM, Steraloids) delivered drugs to the cell for 400 ms or 2 s exposures (see Supplementary Methods). In some cases, current-voltage curves were constructed using the peak current response to agonist or agonist + THP across a range of holding potentials (−60 to +60) applied as 10 mV steps, or as a voltage ramp generated by ramping the membrane potential from −60 to +60 mV (over 400 ms) in the presence of 1 μM GABA. Ramps are presented as the average of 3 traces after subtraction of the leak current (obtained in the absence of GABA). Currents were recorded using an Axopatch 1D amplifier (Axon Instruments) filtered at 2 kHz (four-pole Bessel filter), detected at 10 kHz and analyzed with pClamp 9.2. Desensitization rate was determined using non-linear curve-fitting routines (Origin, Microcal; see Supplementary Methods).
Hippocampal slice (see Supplement)
Pyramidal cells in CA1 hippocampal slice (400 μm) or thalamic relay neurons were visualized with DIC-microscopy and recorded at −50 or −60 mV at room temperature (20 – 22 °C) using whole cell patch clamp procedures (Axopatch 200B amplifier, Axon Instruments, 20 kHz sampling frequency, 2 kHz 4-pole Bessel filter) and pClamp 9.2 software. The direction of Cl− current was varied by altering internal Cl− (K-gluconate and KCl, internal solution) or by applying 10 mV voltage steps (−90 to −10 mV, 2 s). Kynurenic acid (2 mM) and TEA (5 mM) were added to the bath solution to isolate the GABAergic current, and 200 nM gabazine to isolate the non-synaptic GABAergic current30. Action potential-driven GABA release was blocked with 1 μM TTX, and 1 μM GABA added to generate post-synaptic GABA-gated current.
Tonic current was recorded as the difference current produced by the selective GABAA receptor antagonist gabazine (120 μM) before and after 30 nM THP14,29, while gramicidin perforated-patch recordings33 were accomplished using 140 mM KCl plus 25 μg ml−1 gramicidin in the pipet solution, recorded when the access resistance dropped to < 60 MΩ after tight seal formation. Estimates of the direction of Cl− current were obtained by recording using tight-seal cell-attached techniques31 (> 1GΩ seal) in current clamp mode. A downward deflection signified outward (i.e., hyperpolarizing) Cl− current.
Effects of THP on cell excitability were tested by monitoring spiking using cell attached patch recordings31 in voltage clamp mode (−40 mV holding potential, 150 mM NaCl intrapipet solution) or assessing the current threshold to spiking and spike frequency in current clamp mode (0.01 – 0.3 nA steps, starting from − 1nA, 1 s duration). (More details in the Supplementary Methods.)
Restraint stress
In order to test the effect stress-induced release of THP6,46,47 on anxiety, mice were restrained in a clear Plexiglas tube-type holder (Harvard Apparatus) for 45 min. and tested 20 min. later on the elevated plus maze (see Suplementary Methods). Open arm time was evaluated for 5 min. on the elevated plus maze. A decrease in open arm time reflects an increase in anxiety18. In all cases, the results from each mouse tested after restraint was expressed relative to the averaged results from the sham controls, which were identical to the stressed animals (age, genotype, sex, drug-injected), except that they were not subjected to restraint stress.
Statistics
All data are presented as mean ± SEM. Complete details on the statistical procedures are provided (Supplementary Methods, Supplementary Tables 1–15). Comparisons of GABA-gated current before and after THP application to the same cell were determined using the paired t-test. Comparisons between > 2 groups were assessed using an analysis of variance (ANOVA) following confirmation that the data followed a normal distribution with the Kolmogorov-Smirnov normality test. Unless otherwise noted, statistical significance was achieved when P < 0.05.
Supplementary Material
Acknowledgements
The authors thank C.A. Frye for performing steroid assays on hippocampal tissue, W. Sieghart for supplying the δ antibody, and G. Homanics for supplying and genotyping the δ−/− mice. We thank K. Perkins and D.H. Smith for helpful discussions. We are also grateful to C. McBain and J. Celentano for a critical reading of the manuscript. The work in this study was supported by grants from the US National Institutes of Health (to S.S.S., C.A. and K.W.) and from the US National Institute of Alcoholism and Alcohol Abuse (to S.S.S.).
Footnotes
The authors declare no competing interests.
Supporting Material
Figures are submitted as separate files.
Supplementary Methods and tables are separate files.
Supplementary Figs. 1 – 4
Reference List
- 1.Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol. Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolescent Health. 2002;30S:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- 3.Modesti PA, et al. Changes in blood pressure reactivity and 24-hour blood pressure profile occurring at puberty. Angiology. 2006;45:443–450. doi: 10.1177/000331979404500605. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph U, et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 5.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 6.Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. Proc. Natl. Acad. Sci. USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Purdy RH, Coutifaris C, Rickels K, Paul SM. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendo. 1993;58:478–484. doi: 10.1159/000126579. [DOI] [PubMed] [Google Scholar]
- 8.Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- 9.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABA-A receptor. J. Neurosci. 1990;16:534–541. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharm. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 12.Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharm. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc. Natl. Acad. Sci. USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpechot C, et al. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- 16.Wisden W, Laurie DJ, Monyer H, Seeburg P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SS, et al. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 18.Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: A possible model of premenstrual dysphoric disorder. Psychopharm. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundstrom-Poromaa I, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat. Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABA-A receptor-mediated postsynaptic conductance. J. Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- 21.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl- and HCO3- transport. J. Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alger BE, Nicoll RA. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J. Physiol. 1982;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert NA, Borroni AM, Grover LM, Teyler TJ. Hyperpolarizing and depolarizing GABA-A receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J. Neurophysiol. 1991;66:1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Wang W, Richerson G. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J. Neurosci. 2001;21:2630–2639. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;424:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 26.Kelley SP, Dunlop JI, Kirkness E, Lambert JJ, Peters JA. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Mitcheson JS, Lin M, Sanguinetti MC. Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. J. Biol. Chem. 2000;275:36465–36471. doi: 10.1074/jbc.M007034200. [DOI] [PubMed] [Google Scholar]
- 28.Fadalti M, et al. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr. Res. 1999;46:323–327. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Caraiscos VB, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belelli D, Peden DR, Rosahl TW, Wafford K, Lambert JJ. Extrasynaptic GABA-A receptors for thalamocortical neurons: A molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrich D, Huguenard JR. Nucleus-specific chloride homeostasis in rat thalamus. J. Neurosci. 1997;17:2348–2354. doi: 10.1523/JNEUROSCI.17-07-02348.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girdler SS, Beth Mechlin M, Light KC, Morrow AL. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. Stressed or stressed out: what is the difference? J. Psychiatr. Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- 36.Lundgren P, Stromberg J, Backstrom T, Wang M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3beta-hydroxy-5alpha-pregnan-20-one (isoallopregnanolone) Brain Res. 2003;982:45–53. doi: 10.1016/s0006-8993(03)02939-1. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen RW, Snowman A. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J. Neurosci. 1982;2:1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner JH, Raymond JR. Interaction of calmodulin with the serotoin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J. Biol. Chem. 2005;280:30741–30750. doi: 10.1074/jbc.M501696200. [DOI] [PubMed] [Google Scholar]
- 40.Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc. Natl. Acad. Sci. USA. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prescott SA, de Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl. Acad. Sci. USA. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai D, et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by y-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharm. 2000;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 44.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 45.Romeo RD. Neuroendocrine and behavioral development during puberty: a tale of two axes. Vitam. Horm. 2005;71:1–25. doi: 10.1016/S0083-6729(05)71001-3. [DOI] [PubMed] [Google Scholar]
- 46.Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mele P, et al. Increased expression of the gene for the Y1 receptor of the neuropeptde Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J. Neurochem. 2004;89:1471–1478. doi: 10.1111/j.1471-4159.2004.02444.x. [DOI] [PubMed] [Google Scholar]
- 48.Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J. Clin. Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. New England J. Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 50.Andreen L, et al. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendo. 2004;30:212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.