Abstract
The longest part of the sperm flagellum, the principal piece, contains the fibrous sheath, a cytoskeletal element unique to spermiogenesis. We performed mass spectrometry proteomics on isolated human fibrous sheaths identifying a unique ADP/ATP carrier protein, SFEC [AAC4], seven glycolytic enzymes previously unreported in the human sperm fibrous sheath, and sorbitol dehydrogenase. SFEC, pyruvate kinase and aldolase were co-localized by immunofluorescence to the principal piece. A homology model constructed for SFEC predicted unique residues at the entrance to the nucleotide binding pocket of SFEC that are absent in other human ADP/ATP carriers, suggesting opportunities for selective drug targeting. This study provides the first evidence of a role for an ADP/ATP carrier family member in glycolysis. The co-localization of SFEC and glycolytic enzymes in the fibrous sheath supports a growing literature that the principal piece of the flagellum is capable of generating and regulating ATP independently from mitochondrial oxidation in the mid-piece. A model is proposed that the fibrous sheath represents a highly ordered complex, analogous to the electron transport chain, in which adjacent enzymes in the glycolytic pathway are assembled to permit efficient flux of energy substrates and products with SFEC serving to mediate energy generating and energy consuming processes in the distal flagellum, possibly as a nucleotide shuttle between flagellar glycolysis, protein phosphorylation and mechanisms of motility.
Introduction
Mitochondrial ADP/ATP carriers (AAC’s a.k.a. adenine nucleotide translocases, ANTs) function as antiporters that exchange cytosolic ADP for matrix ATP in mitochondria (Klingenberg, 1981). These proteins, typically contain six membrane spanning domains that span the inner mitochondrial membrane and exchange ADP for ATP in a 1:1 ratio (Duyckaerts et al., 1980). The genomes of most eukaryotes, including yeast, plants and mammals, contain multiple genes encoding ATP/ADP carriers. These proteins have developed several nomenclatures and abbreviations, the most common being AAC (ADP/ATP carrier) or ANT (adenine nucleotide translocase). The AACs are considered the principal link between the energy generating process of oxidative phosphorylation and energy consuming processes of cell metabolism.
In humans, four AAC genes are now known. Patterns of AAC1-3 expression have been noted to vary in different stages of cell division, in cancers, and in cells exposed to various growth conditions and inhibitors. Among normal tissues, AAC1 is thought to be specific to heart and skeletal muscle (Stepien et al., 1992) and AAC1 deficiency has been related to mitochondrial myopathy and cardiomyopathy (Palmieri et al., 2005; Graham et al., 1997). AAC2 is present in proliferating cells, while AAC3 is ubiquitous (Stepien et al., 1992). AAC4 was only recently identified through a genome scan and shown to function as an active ADP/ATP carrier in the C14ADP/ATP liposome assay and to catalyse an electrophoretic exchange between ADP3− and ATP-4− (Dolce et al., 2005). GFP-fused AAC4 co-localized to mitochondria in CHO cells leading to the conclusion that AAC4 has properties of a classical mitochondrial adenine nucleotide translocase (Dolce et al., 2005).
The fibrous sheath, a unique cytoskeletal structure specific to the sperm, is located only in the principal piece, a region devoid of mitochondria. The FS has been proposed to function as a protective girdle for the axoneme (Fawcett, 1975; Lindemann et al., 1992) and as a scaffold for enzymes involved in signal transduction, including protein kinase A by anchoring to AKAP3 (Vijayaraghavan et al., 1999; Mandal et al., 1999) or AKAP4 (Fulcher et al., 1995, Turner et al., 1998), the Rho signaling pathway through ropporin (Fujita et al., 2000) and rhophilin (Nakamura et al., 1999), as well as calcium signaling via CABYR (Naaby-Hansen et al., 2002; Kim et al., 2005). Previously, two glycolytic enzymes, glyceraldehyde 3-phosphate dehydrogenase-2 (GAPDH-2, Westhoff and Kamp, 1997; Welch et al., 2000) and hexokinase 1 (HK1, Travis et al., 1998; Mori et al., 1998) have been localized to the human fibrous sheath. Recently, the A isoform of aldolase 1 (ALDOA) and lactate dehydrogenase A (LDHA) have been identified in isolated mouse fibrous sheath (Krisfalusi et al, 2006). Such observations led us posit whether glycolysis and signal transduction indeed occur in the distal flagella of human sperm and if evidence for additional enzymes within these pathways as well as energy intermediates might be found in the human fibrous sheath.
The present study provides biochemical and morphological evidence that AAC4 (SFEC) is present in ejaculated human sperm where it associates with the principal piece of the flagellar cytoskeleton and with glycolytic enzymes. The study has been particularly aided by the well recognized ultrastructural compartmentalization in the sperm flagellum which consists of mid, principal and end pieces. Each of these regions contains specific organelles and cytoskeletal elements. Mitochondria are restricted to the mid piece. The principal piece contains the unique circumferential ribs and longitudinal columns of the fibrous sheath. These ribs and columns surround the 9 + 2 array of axonemal microtubules. The end piece contains microtubules, although they no longer form paired “doublets” in this region. At the core of the axoneme, where ATP is utilized to generate flagellar motion, dynein ATPases associate with outer doublet microtubules and span both mid and principal pieces. We purified the human fibrous sheath by mechanical and chemical dissection, and utilized mass spectrometric analysis to identify a novel member of the adenine nucleotide translocase family, SFEC, in association with several glycolytic enzymes, previously unreported in the human sperm principal piece. Localization of SFEC (AAC4) with pyruvate kinase and aldolase in the principle piece of the flagellum confirms a growing literature that glycolysis is compartmentalized within the fibrous sheath, the unique cytoskeletal element of the principal piece, and suggests a role for SFEC as an intermediate between flagellar glycolysis and energy consuming processes such as phosphorylation and motility.
Materials and methods
Isolation of human FS
The fibrous sheath was isolated by a multi-step mechanical and chemical sperm dissection procedure (modified from the previous report by Kim et al., 1997). Isolated human sperm tails were extracted for 30 min in 2% (v/v) Triton X-100 and 5mM DTT with gentle shaking at 4°C. After washing with 50mM Tris-HCl (pH 9.0) containing 0.2mM PMSF, the sperm tails were suspended in 25mM DTT and 4.5M urea and shaken for 2 hours at 4°C. Each step of the procedure was monitored by light and electron microscopy. The purity of the isolated fibrous sheath was confirmed by transmission electron microscopy.
Tandem mass spectroscopic analysis of fibrous sheath
The Coomassie-stained protein bands of the fibrous sheath were cored from 1D SDS-PAGE gels, fragmented into smaller pieces, destained in methanol, reduced in 10 mM dithiothreitol, and alkylated in 50 mM iodoacetamide in 0.1 M ammonium bicarbonate. The gel pieces were then incubated with 12.5 ng/ml trypsin in 50 mM ammonium bicarbonate overnight at 37°C. Peptides were extracted from the gel pieces in 50% acetonitrile and 5% formic acid and microsequenced by tandem mass spectrometry and by Edman degradation at the Biomolecular Research Facility of the University of Virginia.
Northern and dot blot analyses
A human multiple tissue Northern blot containing 2 μg of poly (A)+ RNA from eight selected tissues and a normalized RNA dot-blot containing 76 tissues (Clonetech) were probed with a α-32P-labeled 963-bp cDNA containing the entire open reading frame of SFEC. Probes were prepared by random priming with a DNA labeling kit (Roche, Penzberg, Germany). For the Northern and dot blot analysis, hybridization was performed as previously described (Naaby-Hansen et al., 2002). The blot was exposed to X-ray film for 72 hours at −70 °C.
Expression, purification of SFEC recombinant protein
A truncated construct of human SFEC (aa 4–120) was expressed in bacteria in order to raise a polyclonal antibody. Previous efforts to express the entire SFEC open reading frame were not successful in bacteria presumably because of the existence of putative transmembrane domains in the C-terminus. Gene specific primers were designed to create an Nco1 site at the 5′ end and a Not1 site at the 3′ end of the polymerase chain reaction (PCR) product according to the human SFEC cDNA sequences. Primers (Forward primer: 5′-CATGCCATGGAGCCTGCGAAAAAGAAGGCAGAAAAG-3′: Reverse primer: 5′-ATAGTTTAGCGGCCGCCTGTTTTTCTTTATTAACTCCAGA-3′) were obtained from GIBCO BRL (Life Technologies, CA). PCR was performed with 10 ng of human SFEC cDNAs as a template to obtain the truncated SFEC cDNA using a program of one 2 min cycle at 94 °C followed by 35 cycles of denaturation, annealing and elongation at 94°C for 30 second, 50 °C for 1 min and 68 °C for 2 min. A product of 351 bp, which begins at bp129 and ends at bp 479 of the human SFEC nucleotide sequence, were separated on a 1% NuSieve (FMC BioProducts, Rockland, ME) agarose gel and sequenced in both direction using vector-derived and insert-specific primers to confirm the sequences. The cDNA corresponding to the N-terminal 117 amino acids was cloned into the bacterial expression vector pET28b and transformed into Escherichia coli strain BLR (DE3) (Novagen, Madison, WI). A single colony was picked from a transformation plate to inoculate 2 liters of LB medium containing 50μg/ml of Kanamycin and grown at 37°C until the A600 reached 0.5. Recombinant protein expression was induced at 37°C for 3 hour with 1mM IPTG (isopropyl-1-thio-β-D-galactopyranoside). The cells were centrifuged at 5,000 g for 15 min and suspended in BugBuster Protein Extraction reagent (Novagen, Madison, WI) containing rLysozyme (1KU/ml) and Benzonase (25 units/ml) for the gentle disruption of the cell wall and degradation of DNA and RNA of the E.coli. Recombinant SFEC containing six residues of histidine on the C-terminus of the protein was confirmed using anti-histidine antibody. The recombinant SFEC protein was purified as previously described (Kim et al., 2005). The purity of the isolated recombinant protein was confirmed by Coomassie and SYPRO Ruby stain (Bio-Rad).
Generation of anti-SFEC antibody
Approximately 100 μg of purified recSFEC protein in PBS emulsified with an equal volume of Freund’s complete adjuvant was subcutaneously and intramuscularly injected into each female Sprague Dawley rat. Animals were boosted two times at intervals of 21 days with 50 μg of recombinant protein in incomplete Freund’s adjuvant and serum was collected 7 days after the second boost. Rats were sacrificed after confirmation of antibody production by Western blot analysis of the recombinant SFEC, human sperm and isolated fibrous sheath proteins.
Electrophoresis and Western analyses
The reaction of SFEC antibody was tested by Western blots on recombinant SFEC, human sperm, isolated FS proteins and mitochondrial proteins isolated from human heart. After isolation of a motile fraction by the swim up procedure, sperm proteins were extracted at 4°C for 2 hours by 1% Nonidet P40 and 0.5% sodium deoxycholate containing protease inhibitors: 2mM PMSF, 5mM iodoacetamide, 5mM EDTA, 3 mg/ml L-1-chlor-3-(4-tosylamido)-7-amino-2-heptanon-hydrochloride, 1.46 mM pepstatin A, and 2.1 mM leupeptin. Insoluble material was removed by centrifugation at 10,000 x g for 5 min, and the supernatant containing solubilized human sperm protein was applied to one dimensional SDS PAGE. Isolated human fibrous sheath and human mitochondrial protein (obtained from Molecular Probes) were solubilized with sample buffer for one dimensional SDS-PAGE.
The proteins resolved by one dimensional SDS-PAGE (4–20% gradient gel) were transferred onto nitrocellulose membrane and detected by the anti-SFEC antibody. The excess protein-binding sites on the membrane were blocked with PBS containing 5% (w/v) non-fat milk powder and 0.2 % (w/v) Tween 20 (Merck-Schuchardt, Hohenbrunn, Germany) for 1 h. The membrane was probed overnight at 4 °C with a rat polyclonal antiserum raised against SFEC protein. Anti-SFEC antibody was diluted 1:2000 with blocking solution. Preimmue sera were used at an identical dilution for control experiments. The membrane was then incubated for 45 min with an anti rat immunoglobulin IgG-secondary antibodies linked to horseradish peroxidase (Jackson ImmunoResearch lab., West Grove, PA. USA), diluted 1:5000 in blocking solution. The blot was developed with a chemiluminescent substrate (Pierce, Rockford, IL) or 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (Kirkegaard and Perry Lab, Gaithersburg, MD. USA).
Indirect immunofluorescence localization of SFEC, pyruvate kinase and aldolase on human sperm
Swim-up human sperm were labeled with 25nM Mito-tracker Red CMX Ros (Molecular Probe, Eugene, OR) for 10 min at room temperature. Sperm were washed with PBS two times, diluted to a concentration of 1 x 106 sperm/ml, and then spotted on glass slides. Before air-drying, the sperm were fixed with 4% formaldehyde for 10 min at room temperature. After washing 3 times in PBS, the samples were permeablized with cold methanol for 15 min, then blocked in 10% normal goat serum in PBS overnight at 4°C. The sperm were then incubated with a 1:50 dilution of the rat anti recombinant human SFEC antibody or pre-immune serum in blocking solution for 2h at room temperature. For an additional control, the primary antibody was pre-absorbed with SFEC recombinant proteins. The slides were then washed 3 times x 5 min in PBS, and the secondary antibody, goat anti-rat IgG FITC conjugated (Jackson ImmunoResearch), was applied at a 1:200 dilution in 10% normal goat serum in PBS for 1 hour at room temperature. The slides were washed 3x5 min in PBS, and a Slow Fade-Light Antifade Kit (Molecular Probes, Inc.) was used to reduce the fading rate of the fluorescein. Indirect immunofluorescence microscopy using goat anti-pyruvate kinase and anti-aldolase antibodies (Novus Biologicals, Inc. Littleton, CO) were performed as described above except using different blocking serum and secondary antibody. Sperm were blocked by 10% normal donkey serum in PBS and donkey anti-goat IgG FITC conjugated secondary antibody (Jackson ImmunoResearch) was used.
Results
Isolation of Human Fibrous Sheath Ribs and Columns
Fibrous sheaths were isolated by mechanical and chemical methods (modified from a previous report by Kim et al., 1997). Each step of the isolation was monitored by light and electron microscopy. The purity of the human fibrous sheath preparation was verified by electron microscopy after studying multiple sections and fields. This final fibrous sheath fraction consisted of longitudinal columns and ribs without other contaminating sperm tail components such as the axonemal complex, outer dense fibers or mitochondrial sheaths (Figure 1A). Although these fine structural observations confirm the structural homogeneity of the preparation with respect to fibrous sheath elements, the possible co-purification of molecules from other cytoplasmic compartments can not be excluded by fine structural assessment alone.
Figure 1.
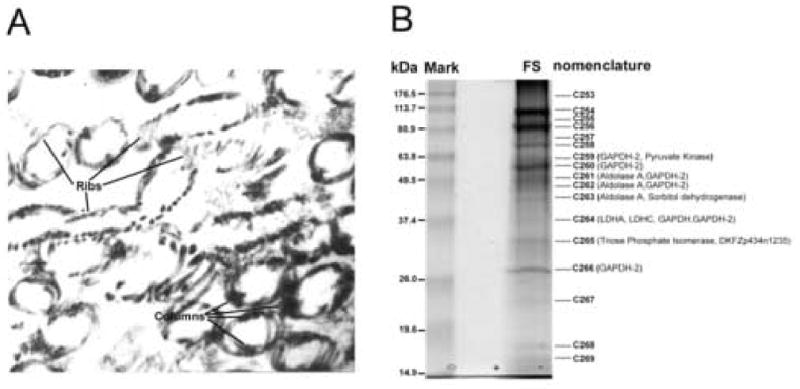
A, Transmission electron micrograph of the longitudinal columns (C) and transverse ribs (R) of isolated human sperm fibrous sheath confirming the purity of the isolated preparation. No other sperm tail components including the outer dense fibers, axoneme and mitochondrial sheaths were observed. Original magnification, x 30,000 B, SDS-PAGE(12.5%) of the human fibrous sheath proteins revealing at least 17 Coomassie blue stain bands. A nomenclature of C253 to C269 was established for each band before coring. Peptides for seven glycolytic enzymes, including somatic and testis specific forms, were recovered including: GAPDH-2, GAPDH, LDHA, LDHC, aldolase A, triosephosphate isomerase, pyruvate kinase as well as sorbitol dehydrogenase. C265 contained peptides for DKFZp434N1235, identified as a unique member of the ADP/ATP carrier protein family (AAC), also known as adenine nucleotide translocases (ANT).
Microsequences of glycolytic enzymes in the isolated fibrous sheath
Protein extracts of the purified fibrous sheath fraction were resolved by one dimensional SDS-PAGE, revealing at least 17 Coomassie protein bands ranging from 15.5 to 140 kDa (Figure 1B). Each protein band was assigned a nomenclature C253–C269, and subsequently cored and microsequenced by tandem mass spectrometry. Analysis of peptide masses derived from isolated human fibrous sheaths confirmed the presence of GAPDH-2, one of two previously reported glycolytic enzymes in the human fibrous sheath (Welch et al., 2000), while another enzyme, hexokinase (HK1, Travis et al. 1988), was not identified in the isolated fibrous sheath preparation perhaps indicating that the HK1 was not bound to the fibrous sheath as tightly as GAPDH2 (Travis et al., 2001). Importantly, seven glycolytic enzymes, previously unreported as components of the human fibrous sheath, were identified in the preparation. These (Table 1) were isoforms of aldolase A, triose phosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and pyruvate kinase known to be present in somatic tissues; the testis specific isoform of lactate dehydrogenase, LDH C, as well as the somatic isoform LDH A; and sorbitol dehydrogenase, from the polyol pathway. Table 2 summarizes each peptide obtained by mass spectrometry microsequencing the human fibrous sheaths and summarizes the percent of the predicted amino acid sequence of each glycolytic enzyme covered by the microsequences.
Table 1.
Eight glycolytic enzymes identified in the present FS proteomics including GAPDH -2 which is previously reported in the FS.
| Name | Nomenclature (Identified Band) | Respective NCBI# and Molecular Weight (kDa) | Gene locus | Expression | Testis EST# | References |
|---|---|---|---|---|---|---|
| Aldolase A | C261, C262, C263 | 4557305, 39.4 | 16q22-q24 | Multiple tissues | BQ433120.1 | Izzo et al., (1988) |
| Triose Phosphate Isomerase (TPI) | C265 | 4507645, 26.7 | 12p13 | Multiple tissues | BQ223970.1 | Maquat et al., (1985) |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | C264 | 7669492, 36.4 | 12p13 | Multiple tissues | BG393651.1 | Ercolani et al., (1988) |
| Glyceraldehyde 3-phosphate dehydrogenase2 (GAPDH-2) | C259, C260, C262, C264, C266 | 7657116, 44.5 | 19q13.1 | Testis specific | BG718767.1 | Welch et al., (2000) |
| Pyruvate Kinase | C259 | 4505839, 57.9 | 15q22 | Multiple tissues | BM478612.1 | Tsutsumi et al., (1988) |
| Lactate dehydrogenase-A (LDH-A) | C264 | 5031857, 36.7 | 11p15.4 | Multiple tissues | BG506200.1 | Chung et al., (1985) |
| Lactate dehydrogenase-C (LDH-C) | C264 | 4504973, 36.2 | 11p15.5-p15.3 | Testis specific | BQ435561.1 | Edwards et al., (1989) |
| Sorbitol Dehydrogenase (SDH) | C263 | 1583520, 38.3 | 15q15.3 | Multiple tissues | BG393670.1 | Iwata and Carper (1995) |
Table 2.
Peptides identified by tandem mass spectrometric analysis of isolated human fibrous sheaths. Except sorbitol dehydrogenase, multiple peptides were obtained for each glycolytic enzyme covering 8.3 to 72.3% of the entire protein. The Sequest algorithm was used to interpolate the spectra.
| Enzymes | MS Peptides | Positions | Protein coverage by residue count |
|---|---|---|---|
| Aldolase A | GILAADESTGSIAK
ADDGRPFPQVIK GVVPLAGTNGETTTQGLDGLSER IGEHTPSALAIMENANVLAR FSHEEIAMATVTALR ALQASALK YTPSGQAGAASESLFVSNHAY |
29–42
88–99 112–134 154–173 244–258 305–312 343–364 |
31.0% |
| Triose phosphate isomerase (TPI) | KFFVGGNWK
KQSLGELIGTLNAAK VPADTEVVCAPPTAYIDFAR IAVAAQNCYK VTNGAFTGEISPGMIK DCGATWVVLGHSER RHVFGESDELIGQK VAHALAEGLGVIACIGEK LDEREAGITEK VVLAYEPVWAIGTGK TATPQQAQEVHEK SNVSDAVAQSTR IIYGGSVTGATCK |
6–14
19–33 34–53 60–69 70–85 86–99 100–113 114–131 132–142 161–175 176–188 195–206 207–219 |
72.3% |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | GALQNIIPASTGAAK
LISWYDNEFGYSNR |
214–228
323–336 |
8.3% |
| Glyceraldehyde-3-phosphate dehydrogenase 2 (GAPDH-2) | AEVEPQPQPEPTPVR
EEIKPPPPPLPPHPATPPPK VVAVNDPFIDPEYMVYMFK NGQLVVDNHEISVYQCK FGIVEGLMTTVHSYTATQK GAHQNIIPASTGAAK LTGMAFRVPTPDVSVVDLTCR LAQPAPYSAIK AGIALNDNFVK LISWYDNEYGYSHR VVDLLR |
34–48
49–68 101–119 135–151 240–258 273–287 300–320 321–331 371–381 382–395 396–401 |
41.2% |
| Pyruvate kinase | NTGIICTIGPASR
TATESFASDPILYRPVAVALDTK GADFLVTEVENGGSLGSK GVNLPGAAVDLPAVSEK |
44–56
93–115 189–206 208–224 |
13.4% |
| Lactate dehydrogenase-A (LDH-A) | DQLIYNLLKEEQTPQNK
DLADELALVDVIEDK LKGEMMDLQHGSLFLR DYNVTANSK LVIITAGAR VIGSGCNLDSAR VTLTSEEEAR SADTLWGIQK |
6–22
43–57 58–73 82–90 91–99 158–169 306–315 319–328 |
29.5% |
| Lactate dehydrogenase-C (LDH-C) | LIEDDENSQCK
ITSGKDYSVSANSR YLIGEK QVIQSAYEIIK INLNSEEEALFK SAETLWNIQK |
12–22
77–90 172–177 233–243 306–317 319–328 |
19.3% |
| Sorbitol dehydrogenase | LENYPIPEPGPNEVLLR | 22–38 | 4.8% |
SFEC (AAC4) was identified in the purified fibrous sheath
In addition to the identification of glycolytic enzymes within the purified fibrous sheaths a hypothetical protein, DKFZp434N1235, was sequenced from band C265 and identified as a member of the ADP/ATP carrier family (AAC). This previously unannotated protein was cloned from human testis cDNA and the amino acid and nucleotide sequences deposited in GenBank under the name SFEC (Genbank accession no, AY550240), sperm flagella energy carrier [Note on nomenclature: the SFEC sequence was deposited by the authors in GenBank on February 17, 2004, whereas the identical protein was deposited as AAC4 on November 15, 2004; By priority date, SFEC terminology takes precedence. However, for purposes of standard nomenclature we suggest the term AAC4 be used for this protein when referring to it in somatic tissues and the term SFEC be employed when referring to sperm].
Human SFEC contained three repeating motifs (Figure 3) as well as the PX(D/E)XX(K/R) sequence, both characteristics of all known mitochondrial carriers (Walker and Runswick, 1993; Nelson et al., 1998) as well as the hexapeptide signature RRRMMM (Figure 2) which is present in all ADP/ATP carriers but absent from other mitochondrial carriers (Pebay-Peyroula et al., 2003). The orthologous murine gene (RIKEN cDNA 1700034J06) was also identified (Fig. 4) and deposited in GenBank as mSFEC (GenBank accession no, AY550241). Human and mouse SFEC protein sequences share 83% identity and 89% similarity (Figure 4). Comparison with other human AACs (Figure 4) revealed the highest homology (69% identity and 79% similarity) with ADP/ATP carrier 1, (AAC1, ANT1), from human heart/skeletal muscle (Cozens et al., 1989) and a 67% identity and 80% similarity to AAC3 (ANT3) of human liver (Cozens et al., 1989). It also revealed a 67 % identity and 79 % similarity with a human fibroblast isoform, AAC2 (Ku et al., 1990). The SFEC protein was localized to human chromosome 4q28.1, whereas the other known ADP/ATP carrier proteins localize to chromosome 4q35.1 (AAC1, Haraguchi et al., 1993); chromosome Xp22.32 and Yp (AAC3, Slim et al., 1993; Schiebel et al., 1993), and Xq24 (AAC3, Schiebel et al., 1993), respectively. The mouse SFEC protein localized to chromosome 3B, which is syntenic to locus 4q28.2 in the human (Dehal et al., 2001). The mouse AAC1 and AAC2 were localized to chromosome 8 and chromosome X, respectively (Ellison et al., 1996). However, there is presently no known mouse homologue for the human ANT 3 (Levy et al., 2000).
Figure 3.
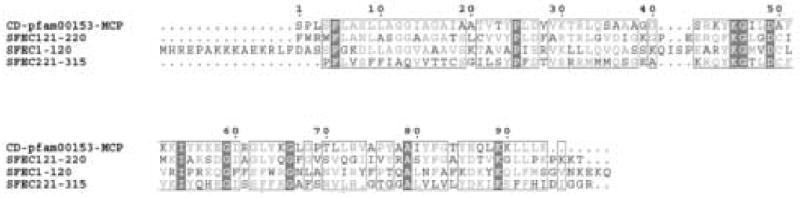
Alignment of SFEC with mitochondrial carrier protein (pfam153) using the conserved domain architecture retrieval tool (CDART, Geer et al., 2002). SFEC contains three repeat motifs characteristic of mitochondrial carrier proteins. In addition PX(D/E)XX(K/R) consensus sequences are located at amino acids 40–45 (PIERVK), 145–150 (PLDFAR) and 242–247 (PFDTVR), another characteristic of all mitochondrial carriers (Walker and Runswick, 1993; Nelson et al., 1998).
Figure 2.
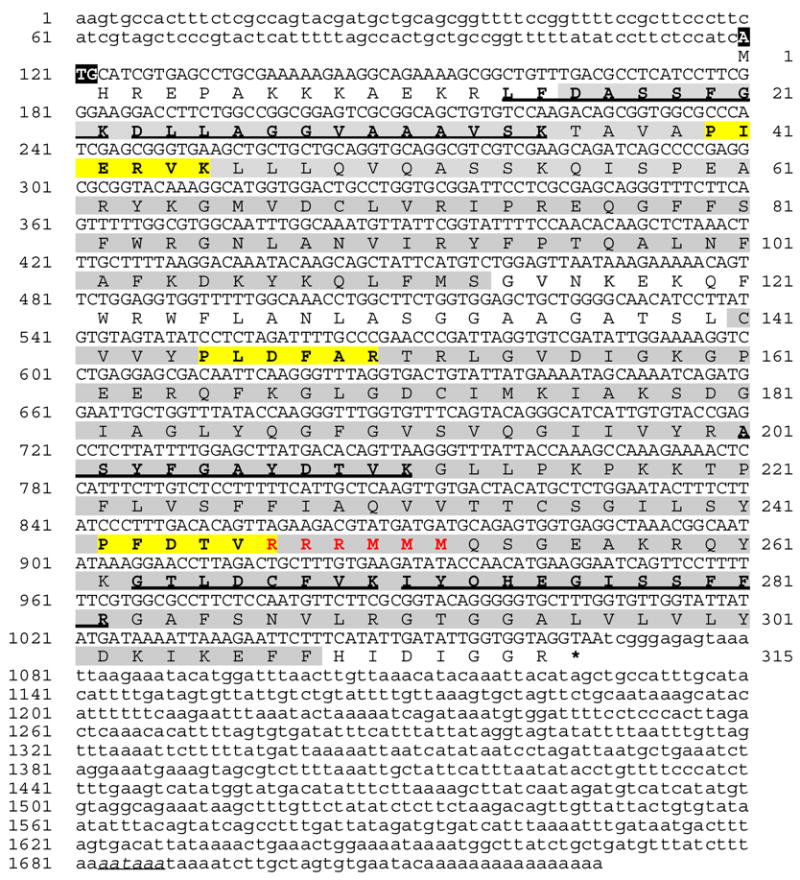
A. Complementary DNA and deduced amino acid sequences of human SFEC protein (GenBank™. accession number BC022032). Numbering of nucleotide base pairs is on left and numbering of amino acids is on right. The 1727-bp cDNA sequence is shown with the 945-bp ORF in uppercase letters and the 119-bp 5′ UTR and 660-bp 3′ UTR in lowercase letters. Peptide sequences originally obtained by mass spectrometry are bold underlined. Homology domains placing SFEC in the mitochondrial carrier protein family are shaded. These consist of three similar motifs (unit1: aa 16–118; unit2: aa141–217; unit3: aa218–308). The consensus motif of PX(D/E)XX(K/R) (bold in yellow block) lies in each repetitive motif at amino acids 40–45 (PIERVK), 145–150 (PLDFAR) and 242–247 (PFDTVR). This motif is characteristic for all mitochondrial carriers (Walker and Runswick, 1993; Nelson et al., 1998). SFEC contains a hexapeptide carrying the signature of RRRMMM at amino acid 247–252 (bold in red), a feature of all ADP/ATP carriers but absent from other mitochondrial carriers (Pebay-Peyroula et al., 2003).
Figure 4.
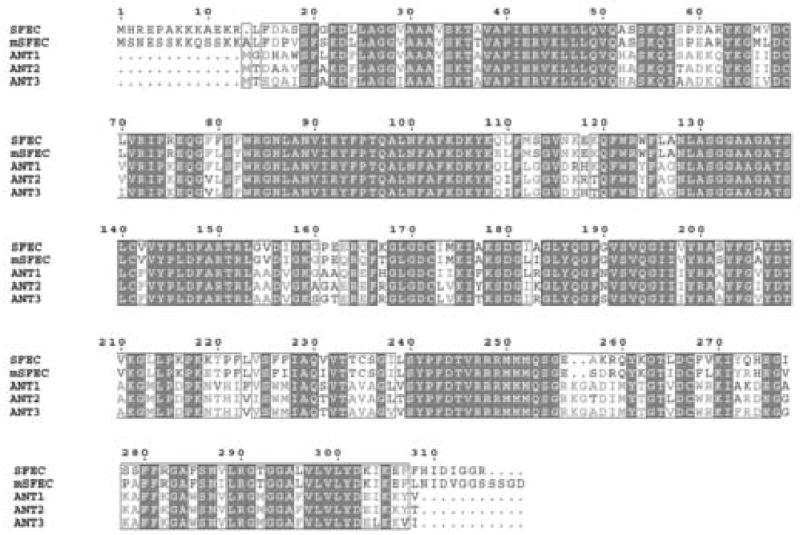
Alignment of human and mouse SFEC with other human adenine nucleotide carrier proteins (adenine nucleotide translocases, ANTs) using the conserved domain architecture retrieval tool (CDART, Geer et al., 2002). Both human and mouse SFEC differ from the human (as well as mouse) ANTs by their characteristic N and C terminal extensions.
The characteristic feature of SFEC in both mouse and human is the presence of unique N and C-termini domains (Figure 4). The conservation of these N and C extensions in these two species and their absence in AAC1-3 of both species indicates that SFEC is a novel member of the family of ADP/ATP carrier proteins. The thirteen amino acid N terminus in both mouse and human does not contain any known motifs but may be important in the fibrous sheath localization of this molecule in the sperm flagellum (see below).
SFEC (AAC4) is a testis abundant protein
Gene expression of SFEC on the Northern analysis and dot array using a 32P-labeled cDNA of the full length open reading frame (315 amino acids) demonstrated that the SFEC mRNA transcripts were expressed in the testis but not in spleen, thymus, prostate, small intestine, colon, leukocytes or ovary (Figure 5). Recombinant SFEC was expressed and purified (Figure 6A). Generation of an antibody against truncated SFEC (amino acid 4–120) was performed in rats. Western blot analysis demonstrated that the anti-SFEC antibody recognized the recombinant protein as well as a 32 kDa band in the isolated FS fraction and in human sperm protein extracts. This is the same molecular weight as the FS band originally microsequenced (Figure 6B). However, the anti-SFEC antibody did not recognize human heart mitochondrial proteins (Figure 6B) while anti-ANT1 antibody detected a 32 kDa ANT1 band (Figure 6C). No band was observed in the negative control experiment in which the anti-ANT1 antibody was replaced by normal goat serum (Figure 6C).
Figure 5.
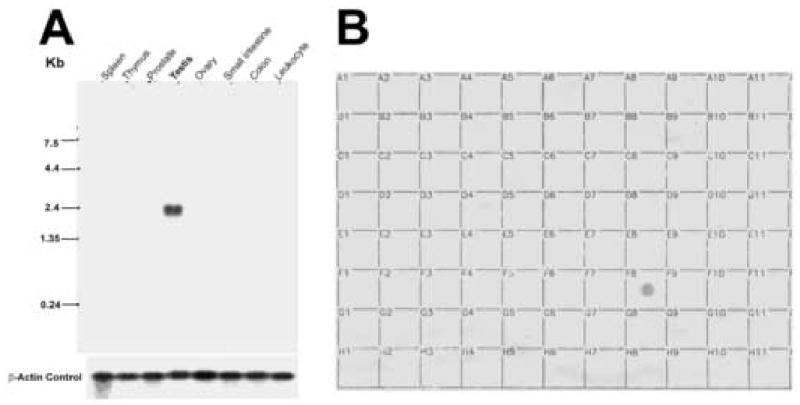
A, Northern blot (Clontech) containing poly(A)+ mRNA from human tissues ( 2 μg per lane) was hybridized with radiolabeled SFEC cDNA and exposed for 72 h. RNA markers are indicated on the left. A single SFEC transcript of 2.4 kb was apparent only in testis. The blot was subsequently stripped and rehybridized with human β-actin as a probe to assess the levels of RNA in each lane (lower panel). B, Dot blot containing poly(A)+ RNA from 76 human tissues (obtained from Clontech) was hybridized with a radiolabeled full length of SFEC cDNA, and the signal was visualized by autoradiography. A hybridization signal was found only in testis (box F8) after 72-h exposure. The distribution of mRNAs from 76 human tissues in the dot blot was as follows. A1, whole brain; B1, cerebral cortex; C1, frontal lobe; D1, parietal lobe; E1, occipital lobe; F1, temporal lobe; G1, paracentral gyrus of cerebral cortex; H1, pons; A2, left cerebellum; B2, right cerebellum; C2, corpus callosum; D2, amygdalla; E2, caudate nucleus; F2, hippocampus; G2, medulla oblongata; H2, putamen; A3, substantia nigra; B3, accumbens nucleus; C3, thalamus; D3, pituitary gland; E3, spinal cord; A4, heart; B4, aorta; C4, left atrium; D4, right atrium; E4, left ventricle; F4, right ventricle; G4, interventricular septum; H4, apex of the heart; A5, esophagus; B5, stomach; C5, duodenum; D5, jejunum; E5, ileum; F5, ilocecum; G5, appendix; H5, ascending colon; A6, transverse colon; B6, descending colon; C6, rectum; A7, kidney; B7, skeletal muscle; C7, spleen; D7, thymus; E7, peripheral blood lymphocytes; F7, lymph node; G7, bone marrow; H7, trachea; A8, lung; B8, placenta; C8, bladder; D8, uterus; E8, prostate; F8, testis; G8, ovary; A9, liver; B9, pancreas; C9, adrenal gland; D9, thyroid gland; E9, salivary gland; F9, mammary gland; A10, leukemia HL-60; B10, HeLa S3; C10, leukemia K-562; D10, leukemia MOLT-4; E10, Burkitt’s lymphoma Raji; F10, Burkitt’s lymphoma Daudi; G10, colorectal adenocarcinoma SW480; H10, lung carcinoma A549; A11, fetal brain; B11, fetal heart; C11, fetal kidney; D11, fetal liver; E11, fetal spleen; F11, fetal thymus; G11, fetal lung.
Figure 6.
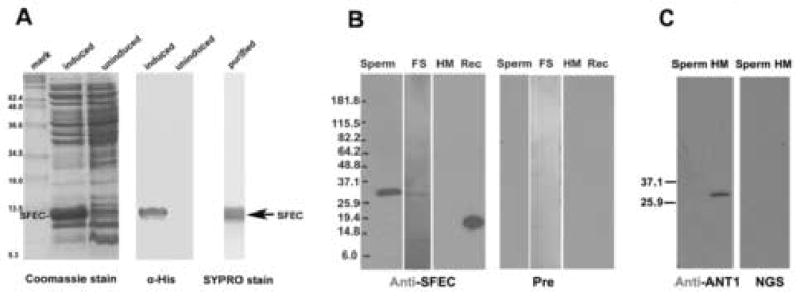
Expression, purification of SFEC and Western analysis with rat anti-recSFEC antibody demonstrating that SFEC is a component of FS in sperm protein extracts , undetected in heart mitochondrial.. A Coomassie blue stain of induced and uninduced truncated recombinant SFEC (117 amino acids; aa 4–120) expressed in BLR (DE3) host cell after cloning into the pET28b vector. Western analysis of recombinant proteins showing that anti-histidine antibody detects the expected molecular weight of ~13 kDa on the induced truncated SFEC protein. Affinity purified recombinant SFEC protein stained by SYPRO Ruby stain (Bio-Rad) confirming the purity of immunogen before injection into rats. B. Western blots of anti-recSFEC antibody on the human sperm (A), isolated FS (B), mitochondria from human heart (C) and recombinant SFEC (D) proteins. Immue serum recognized the recombinant SFEC and a band at 32kDa in human sperm proteins extracted by 1% Nonidet P40 and 0.5% sodium deoxycholate (A). Proteins from isolated FS contained the 32 kDa band (C) identical to that initially identified as SFEC by mass spectrometry (B). Human heart mitochondrial proteins, although having the highest amino acid sequence identity with SFEC (67%), were not recognized by rat anti-recSFEC antibody. Pre immune serum did not recognized any protein bands. C. Western blots of anti-human ANT1 antibody on human sperm and human heart mitochondrial proteins (HM). The human heart mitochondrial protein was recognized at 32kDa by anti-ANT1 antibody, serving as a positive control for the mitochondrial protein extract while sperm proteins were not recognized by goat anti-human ANT1 antibody. Normal goat serum controls did not recognize any proteins.
AAC4 (SFEC) localizes to the principal piece of the sperm flagellum
Indirect immunofluorescence localized SFEC to the principal piece of the sperm flagellum in 100% of sperm (Figure 7). Interestingly, in addition to the principal piece, the entire (21.5%, 83/386) or part of the mid piece (9.8%, 38/386) were also stained by anti-recombinant SFEC antibody. The restricted principal piece localization of SFEC in 69% of sperm differed from the known mitochondrial association of ANT family members. No staining was observed in the negative control experiment in which the anti-SFEC antibody was replaced by either pre-immune sera or immune sera pre-absorbed with recombinant SFEC protein (Figure 7).
Figure 7.
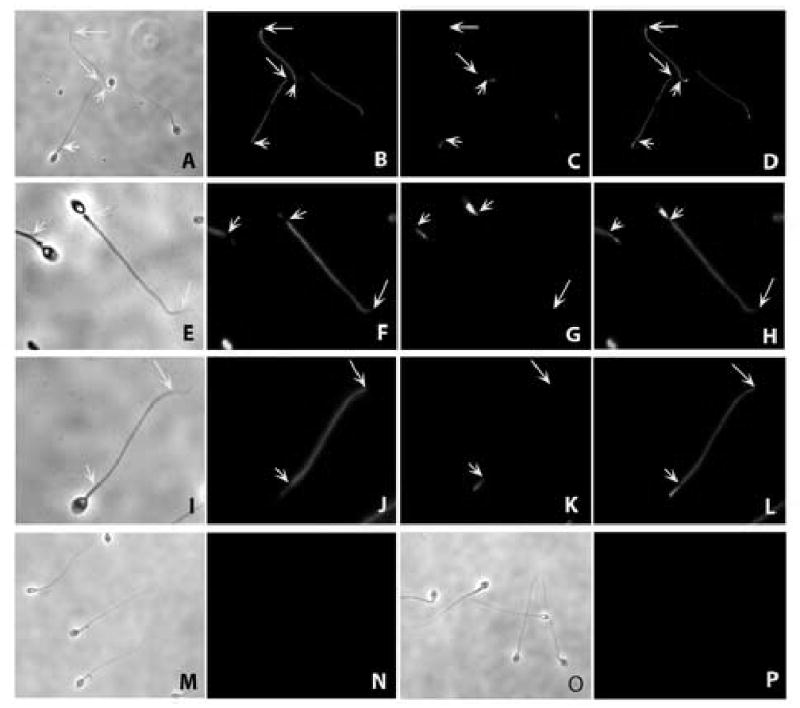
Indirect immunofluorescent localization of SFEC to the entire principal pieces of the human sperm flagellum using rat antiserum against recombinant human SFEC. A,E,I,M and O are phase contrast images of the corresponding FITC images B,F,J,N and P, respectively. The mid piece of the sperm flagellum was identified by labeling with Mito Tracker Red CMX Ros (C.G,K). D,H,L: Merged images of FITC with Mitotracker. The entire mid piece (J) or part of the middle piece (F) as well as the principal piece was recognized by anti-rat SFEC antibody. Pre-immune serum showed no immunofluorescence in human sperm (N). Post immune sera pre-absorbed with recombinant SFEC protein revealed no immunostaining (P). Short arrows indicate the posterior end of the mid piece whereas the long arrows indicate the posterior end of the principal piece. The principal piece is the region between long and short arrows where major immunostaining is seen (B, D, F, H, J and L).
Pyruvate kinase and aldolase localize to the human sperm principal piece
The glycolytic enzymes, aldolase (Figure 8) and pyruvate kinase (Figure 9), localized to the principal piece of the sperm flagella. No staining was found in the negative control experiment in which the anti-aldolase or pyruvate kinase antibodies were replaced by normal goat serum.
Figure 8.
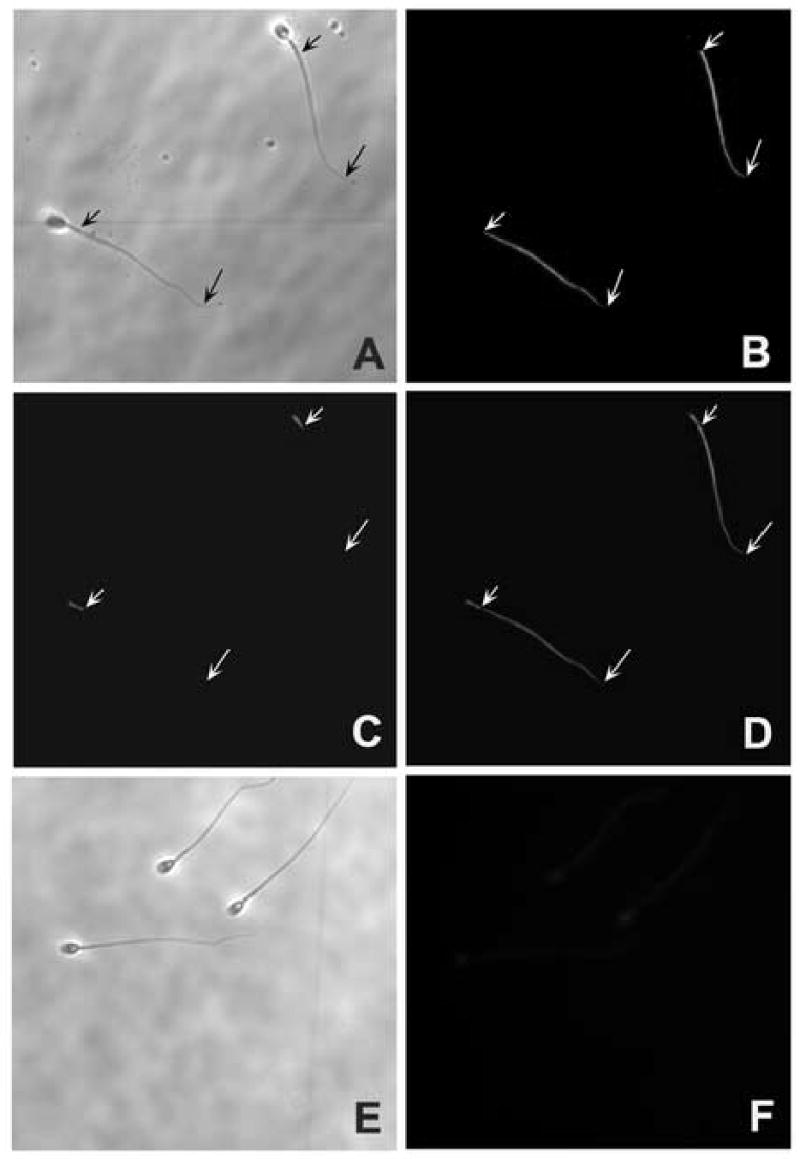
Indirect immunofluorescent localization of aldolase (rabbit muscle) to the entire principal pieces of the human sperm flagellum using goat anti-aldolase antibody (Novus Biologicals, Inc. Littleton, CO). A and E are phase contrast images of the corresponding FITC images B and F respectively. The mid piece of the sperm flagellum was identified by labeling with Mito Tracker Red CMX Ros (C). D: Merged images of FITC with Mitotracker. The entire mid piece principal piece (B and D) was recognized by anti-goat aldolase antibody. Normal goat serum showed no immunofluorescence in human sperm (F). Short arrows indicate the posterior end of the middle piece whereas the long arrows indicate the posterior end of the principal piece. The principal piece is the region between long and short arrows where major immunostaining is seen (B and D).
Figure 9.
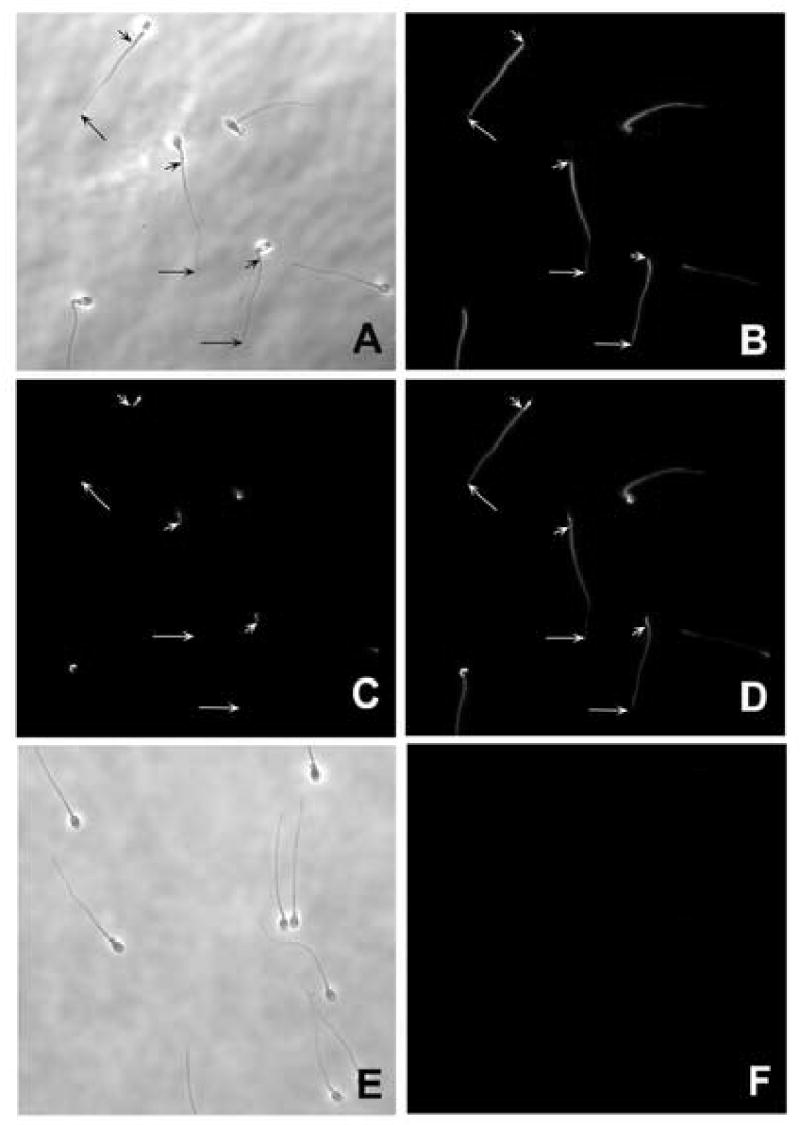
Indirect immunofluorescent localization of pyruvate kinase (rabbit muscle) to the entire principal pieces of the human sperm flagellum using goat anti-pyruvate kinase antibody (Novus Biologicals, Inc. Littleton, CO). A and E are phase contrast images of the corresponding FITC images B and F respectively. The mid piece of the sperm flagellum was identified by labeling with Mito Tracker Red CMX Ros (C). D: Merged images of FITC with Mitotracker. The entire middle piece principal piece (B and D) was recognized by anti-goat pyruvate kinase antibody. Normal goat serum showed no immunofluorescence in human sperm (F). Short arrows indicate the posterior end of the mid piece whereas the long arrows indicate the posterior end of the principal piece. The principal piece is the region between long and short arrows where major immunostaining is seen (B and D).
Prediction of SFEC structure
Protein Data Base crystal structure entry 1okc (Pebay-Peyroula et al., 2003) is a bovine mitochondrial ADP/ATP carrier in complex with carboxyatractyloside, an ANT inhibitor. The sequence identity of 1okc to SFEC and to other ANTs ranges from 56–90%, making 1okc suitable for homology modeling. The high conservation of sequences in the ATP/ADP binding pocket among SFEC, 1okc and other human ANTs predicts an identical ATP/ADP pocket in SFEC and other human ANTs (Figure 10). A substitution of tryptophane for tyrosine-124 in the entrance or “ladder” to the ATP cleft is a marked difference between human SFEC and the other three human ANTs (Figure 10).
Figure 10.
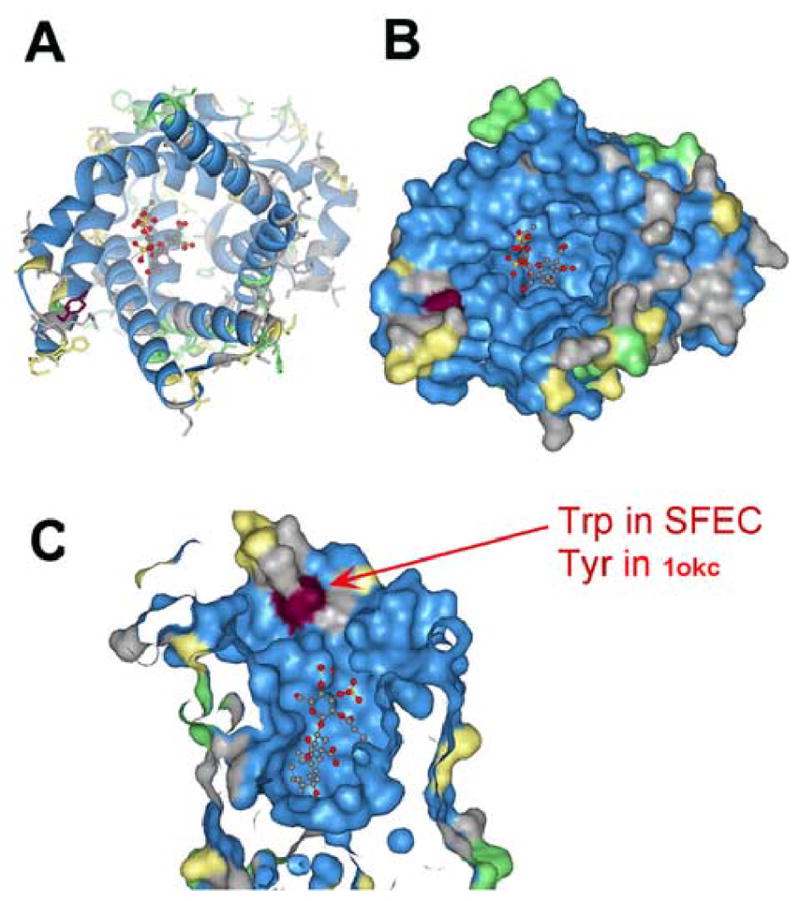
Modeling of the structure of SFEC by comparison with a known ANT1 (1okc) demonstrated that one amino acid, tryptophan in SFEC replaces tyrosine in ANT1 in the entrance to the binding niche. This architecture may afford an opportunity for selective inhibition. A. Ribbon presentation, B. Surface presentation, C. Surface presentation zoomed into binding niche. Blue: identical amino acid; Grey-yellow: Strong or weak homologous amino acid; Green: no homology.
Discussion
SFEC (AAC4) localized to the flagellar principal piece
Indirect immunofluorescence revealed that SFEC, a unique ADP/ATP carrier protein characterized by extensions of N- and C- termini, localized solely to the flagellar principal piece in 69% of human sperm. These immuno-localization studies were performed more than 10 times using Mito-tracker labeling to determine the precise distal boundary of the mid-piece mitochondrial sheath. SFEC’s localization solely to the principal piece in the majority of the sperm flagella was confirmed in all cases. Together with the recovery of SFEC microsequences in the isolated preparation of human fibrous sheath ribs and columns, this evidence establishes that this member of the ADP/ATP carrier protein family, whose other members are found associated with the inner mitochondrial membrane, is located in the non-mitochondrial region of the human sperm flagellum.
This unique localization of SFEC in the human sperm flagellar cytoskeleton was not anticipated in the previous report of Dolce et al., (2005) who showed that ACC4 (SFEC) functions in the C14ADP/ATP liposome assay as an active ADP/ATP carrier to catalyze an electrophoretic exchange between ADP3− and ATP-4−. By real time PCR AAC4 was identified only in human liver, brain and testis (Dolce et al., 2005). Dolce and associates also found that GFP-fused AAC4 co-localized to mitochondria in CHO cells leading to the conclusion that AAC4 has properties of a classical mitochondrial adenine nucleotide translocase. It is possible that AAC4 functions in brain and liver as a mitochondrial protein, since it does possess a functionally active ADP/ATP binding domain that is nearly identical to ACC1-3 as well as six transmembrane domains. The non-mitochondrial, cytoskeletal location of SFEC in the sperm fibrous sheath may be due to a specific interaction mediated by the unique N and C terminal regions. The previous non-mitochondrial localization of a yeast AAC protein family member, peroxisomal adenine nucleotide transporter (Ant1p), in the peroxisomal membrane (Palmieri et al., 2001; Lasorsa et al., 2004) suggested a possible role in transporting cytosolic ATP into the peroxisomal lumen in exchange for AMP generated in the oxidation of fatty acids (Palmieri et al 2001).
SFEC Co-localizes with Glycolytic Enzymes
Studies since the 1960s have demonstrated that glycolytic enzymes cofractionate with an insoluble sperm component (Mohri et al, 1965; Storey and Kayne, 1975, 1978; Gillis and Tamblyn, 1984). The identification of glycolytic enzymes, including the A isoform of aldolase 1 (ALDOA) and LDHA, in isolated mouse fibrous sheath (Krisfalusi et al, 2006) coupled with the identification of glycolytic enzymes not previously reported in purified human fibrous sheaths (Table 1 and 2, this study) support the concept that this unique cytoskeletal element in the principal piece of the mammalian sperm flagellum has evolved as a site for glycolysis. These observations extend the earlier localizations of hexokinase (Travis et al., 1998, Mori et al., 1998; Travis et al., 2001) and GAPHD-2 (Westhoff and Kamp, 1997; Welch et al., 2000) in the fibrous sheath of the sperm principal piece as well as demonstrations that glycolysis occurs in the principal piece compartment, separate from oxidative phosphorylation (Miki et al, 2004; Mukai and Okuno, 2004). Recently, a proteomic analysis of mouse sperm flagella identified glycolytic enzymes including aldolase 1, triosephosphate isomerase, phosphoglycerate kinase 2 and glycerol 3-phosphate dehydrogenase (Cao et al., 2006). However, in that study there was no determination of subcellular localization and the protein fraction examined by mass spectrometry contained all sperm tail components such as mitochondria, outer dense fibers, axoneme and fibrous sheath due to technical difficulties in the isolation of each component from mouse sperm (Cao et al., 2006). The present findings confirm in the human that the fibrous sheath is a compartment for glycolysis perhaps serving as a “scaffold” to which glycolytic enzymes bind and detach or as a stable multi-protein complex. This concept that glycolysis is organized in the mammalian sperm flagellum parallels recent studies that identified enzymatic activities for phosphoglycerate mutase (PGM), enolase and pyruvate kinase (PK) in isolated Chlamydomonas flagellum (Mitchell et al., 2005), while another proteomic study of Chlamydomona flagella identified these glycolytic enzymes as well as aldolase, GAPDH, and phosphoglycerate kinase (Pazour et al., 2005). These findings have led to the conclusion that ATP synthesis throughout the Chlamydomonas flagellar compartment is essential for motility.
Functionally related enzymes in the glycolytic pathway may be spatially juxtaposed in the fibrous sheath to increase the efficiency of flux of metabolites through the pathway, a process traditionally referred to a channeling. In Drosophila flight muscles glycolytic enzymes that catalyze consecutive reactions along the glycolytic pathway have been co-localized along sarcomeres at M-lines and Z discs and this co-localization is required for normal flight (Wojtas et al., 1997; Sullivan et al., 2003). In flight muscles aldolase, glycerol-3-phosphate dehydrogenase (GPDH), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), triose phosphate isomerase, phosphoglycerate kinase and phosphoglycerol mutase (PGLYM) have an identical pattern of co-localization. In mutants null for GPDH, the other enzymes do not co-localize, indicating interdependency of localization (Sullivan et al., 2003). Glycolytic enzymes identified in the fibrous sheath of the sperm flagellum and their association with the sperm’s cytoskeleton provides a readily accessible model in which to further study enzyme-enzyme interactions in these pathways.
Evidence of Polyol and Lactate pathways in the fibrous sheath suggest several anaerobic pathways converge in the distal flagellum
The present finding of sorbitol dehydrogenase (SDH) in the isolated fibrous sheath suggests that the principal piece of the sperm flagellum may utilize sorbitol originating in the seminal plasma (O’Shea and Wales, 1965; Murdoch and White, 1968; Frenkel et al., 1975) and in female reproductive tract secretions (Casslen and Nilsson, 1984) as an energy source for sperm motility. SDH lies within the polyol metabolic pathway in which glucose is reduced first to sorbitol by aldose reductase, and the resulting sorbitol is subsequently oxidixed by SDH to fructose (Hers, 1956; King and Mann, 1958). The polyol pathway has been reported in the testis (Kobayashi et al., 2002) and glucose, fructose and sorbitol are known energy sources for sperm metabolism (Frenkel et al., 1975; Leese et al., 1981). The glucose transporter isoform GLUT5 has been shown to function also as a fructose transporter and to be highly expressed in human testis and localized to the plasma membrane of mature spermatozoa including the entire sperm flagellum (Burant et al., 1992).
Under aerobic conditions, glycolysis is a prelude to the citric acid cycle of mitochondrial respiration. However, if the oxygen concentration is not sufficient, as in rapidly contracting muscle, pyruvate is converted into lactate, or ethanol, as in yeast under anaerobic conditions (alcoholic fermentation), with only a small net energy yield from glucose as compared with the yield from oxidative phosphorylation. The finding of both LDHA and LDHC in the isolated fibrous sheath indicates that pyruvate is further catabolized by LDHA or/and LDHC to form lactate generating nicotinamide adenine dinuleotide (NAD+) when the amount of oxygen is limited in the distal sperm flagellum. Identification of both LDHA (somatic form) and LDHC (testis specific form) as well as GAPDH-2 (testis specific form) and GAPDH (somatic form) in the fibrous sheath support the concept that both somatic and testis specific glycolytic isoforms are compartmentalized in the principal piece of the flagellum.
The mitochondria, where ATP generation occurs from oxidative phosphorylation, are localized solely in the sperm mid-piece. Yet the flagellum extends another 45 μm or so beyond the annulus at the distal end of the mid piece in human sperm. The localization within the fibrous sheath of the enzymes of glycolysis, the anaerobic LDH pathway wherein pyruvate is converted to lactate and the polyol pathway leads to the conclusion that the principal piece provides for convergent pathways of anaerobic ATP production along the flagellum beyond the mid-piece.
SFEC’s role in the principal piece of the sperm flagellum
SFEC (ACC4) has been shown to be an active adenine nucleotide carrier (Dolce et al., 2005). This is in accord with the computer model for SFEC (Fig. 10) that predicts identical conformations of the ATP/ADP binding pocket among SFEC and the other three human AACs. It is not yet apparent if SFEC functions in the distal flagellum as an ATP reservoir, an ATP carrier, and/or contains sites of protein-protein interaction with specific enzymes in the glycolytic pathway. One prediction from our model that enzymes in the fibrous sheath glycolytic pathway will be spatially linked posits that SFEC may directly interact with those specific enzymes, eg. phosphoglycerate kinase and pyruvate kinase, that catalyse ATP yielding reactions. The homology model for SFEC (Fig 10) predicts unique residues at the entrance to the nucleotide binding pocket of SFEC that are absent in other human ADP/ATP carriers, suggesting contraceptive opportunities for selective drug targeting.
The presence of glycolytic pathway enzymes together with a unique adenine nucleotide translocase SFEC in the fibrous sheath suggest new possibilities for energy production and translocation mechanisms in the principal piece of the sperm flagellum related to motility and capacitation. Protein phosphorylation is essential for capacitation (Visconti and Kopf, 1998) and the presence of local ATP generating mechanisms in proximity to ATP utilization in the capacitation dependent phosphorylation pathways suggest possible pathways and protein-protein interactions for SFEC. The reliance of the sperm on glycolysis as the principal source of ATP for motility (Miki et al, 2004; Mukai and Okuno, 2004) suggests that the sperm has evolved mechanisms for adapting to low oxygen tensions, a feature likely to occur during transport through varying regions of the tortuous female reproductive tract. This thought is in concert with the presence of a unique soluble adenylate cyclase in the sperm, sAC, which is bicarbonate sensitive (Chen et al., 2000). sAC shows higher homology to cyclases of cyanobacteria than to somatic cyclases raising the possibility that it represents an “ancestral cyclase” retained in the sperm. By analogy, SFEC, with its unique N and C terminus extensions and its expression in the sperm, is hypothesized to be an ancestral isoform of the ACC family leading to the prediction that ACCDs which associate with the cytoskeleton and with glycolytic enzyme complexes remain to be found in other organisms and model systems.
Acknowledgments
We thank Drs Robert Bloodgood and others at the Center for Research in Contraceptive and Reproductive Health for discussion and critique of the project. We also thank J. Kalmund for EM technical assistance at the Bonn University in Germany and P. Anderson for helping Prep Cell protein purification. This work was supported in part by the Bonfor to YHK (BONFOR 105/11) from Bonn University in Germany, the NIH Fogarty International Center Grant D43 TW/HD 00654, NIH U54 HD29099, P30 28934, the Andrew W. Mellon Foundation and Schering AG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boer PH, Adra CN, Lau YF, McBurney MW. The testis-specific phosphoglycerate kinase gene pgk-2 is a recruited retroposon. Mol Cell Biol. 1987;7:3107–12. doi: 10.1128/mcb.7.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, McLaren A. An electrophoretically detectable modification of glucosephosphate isomerase in mouse spermatozoa. J Reprod Fertil. 1981;63:169–73. doi: 10.1530/jrf.0.0630169. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–6. [PubMed] [Google Scholar]
- Bergman K, Goodenough UW, Goodenough DA, Jawitz J, Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol. 1975;67:606–22. doi: 10.1083/jcb.67.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5:801–810. doi: 10.1074/mcp.M500322-MCP200. [DOI] [PubMed] [Google Scholar]
- Casslen B, Nilsson B. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea. Am J Obstet Gynecol. 1984;150:877–81. doi: 10.1016/0002-9378(84)90466-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–8. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Chung FZ, Tsujibo H, Bhattacharyya U, Sharief FS, LI SS. Genomic organization of human lactate dehydrogenase-A gene. Biochem J. 1985;231:537–41. doi: 10.1042/bj2310537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens AL, Runswick MJ, Walker JE. DNA sequences of two expressed nuclear genes for human mitochondrial ADP/ATP translocase. J Mol Biol. 1989;206:261–80. doi: 10.1016/0022-2836(89)90477-4. [DOI] [PubMed] [Google Scholar]
- Dehal P, Predki P, Olsen AS, Kobayashi A, Folta P, Lucas S, Land M, Terry A, Ecale Zhou CL, Rash S, Zhang Q, Gordon L, Kim J, Elkin C, Pollard MJ, Richardson P, Rokhsar D, Uberbacher E, Hawkins T, Branscomb E, Stubbs L. Human chromosome 19 and related regions in mouse: conservative and lineage-specific evolution. Science. 2001;293:104–11. doi: 10.1126/science.1060310. [DOI] [PubMed] [Google Scholar]
- Dentler WL. Linkages between microtubules and membranes in cilia and flagella. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. Plenum Press; New York: 1990. pp. 31–64. [Google Scholar]
- Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–7. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Sluse-Goffart CM, Fux JP, Sluse FE, Liebecq C. Kinetic mechanism of the exchanges catalysed by the adenine-nucleotide carrier. Eur J Biochem. 1980;106:1–6. doi: 10.1111/j.1432-1033.1980.tb05990.x. [DOI] [PubMed] [Google Scholar]
- Eddy EM, O’Brien DA, Fenderson BA, Welch JE. Intermediate filament--like proteins in the fibrous sheath of the mouse sperm flagellum. Ann N Y Acad Sci. 1991;637:224–39. doi: 10.1111/j.1749-6632.1991.tb27312.x. [DOI] [PubMed] [Google Scholar]
- Edwards YH, Grootegoed JA. A sperm-specific enolase. J Reprod Fertil. 1983;68:305–10. doi: 10.1530/jrf.0.0680305. [DOI] [PubMed] [Google Scholar]
- Edwards Y, West L, Van Heyningen V, Cowell J, Goldberg E. Regional localization of the sperm-specific lactate dehydrogenase, LDHC, gene on human chromosome 11. Ann Hum Genet. 1989;53:215–9. doi: 10.1111/j.1469-1809.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Ellison JW, Li X, Francke U, Shapiro LJ. Rapid evolution of human pseudoautosomal genes and their mouse homologs. Mamm Genome. 1996;7:25–30. doi: 10.1007/s003359900007. [DOI] [PubMed] [Google Scholar]
- Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263:15335–41. [PubMed] [Google Scholar]
- Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436. doi: 10.1016/0012-1606(75)90411-x. [DOI] [PubMed] [Google Scholar]
- Force A, Viallard JL, Grizard G, Boucher D. Enolase isoforms activities in spermatozoa from men with normospermia and abnormospermia. J Androl. 2002;23:202–10. [PubMed] [Google Scholar]
- Frenkel G, Peterson RN, Freund M. Oxidative and glycolytic metabolism of semen components by washed guinea pig spermatozoa. Fertil Steril. 1975;26:144–7. doi: 10.1016/s0015-0282(16)40934-9. [DOI] [PubMed] [Google Scholar]
- Fujita A, Nakamura K, Kato T, Watanabe N, Ishizaki T, Kimura K, Mizoguchi A, Narumiya S. Ropporin, a sperm-specific binding protein of rhophilin, that is localized in the fibrous sheath of sperm flagella. J Cell Sci. 2000;113:103–12. doi: 10.1242/jcs.113.1.103. [DOI] [PubMed] [Google Scholar]
- Fulcher KD, Mori C, Welch JE, O’Brien DA, Klapper DG, Eddy EM. Characterization of Fsc1 cDNA for a mouse sperm fibrous sheath component. Biol Reprod. 1995;52:41–9. doi: 10.1095/biolreprod52.1.41. [DOI] [PubMed] [Google Scholar]
- Fundele R, Winking H, Illmensee K, Jagerbauer EM. Developmental activation of phosphoglycerate mutase-2 in the testis of the mouse. Dev Biol. 1987;124:562–6. doi: 10.1016/0012-1606(87)90510-0. [DOI] [PubMed] [Google Scholar]
- Geer LY, Domrachev M, Lipman DJ, Bryant SH. CDART: protein homology by domain architecture. Genome Res. 2002;12:1619–23. doi: 10.1101/gr.278202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis BA, Tamblyn TM. Association of bovine sperm aldolase with sperm subcellular components. Biol of Reprod. 1984;31(1):25–35. doi: 10.1095/biolreprod31.1.25. [DOI] [PubMed] [Google Scholar]
- Gitlits VM, Toh BH, Loveland KL, Sentry JW. The glycolytic enzyme enolase is present in sperm tail and displays nucleotide-dependent association with microtubules. Eur J Cell Biol. 2000;79:104–11. doi: 10.1078/S0171-9335(04)70012-6. [DOI] [PubMed] [Google Scholar]
- Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- Haraguchi Y, Chung AB, Torroni A, Stepien G, Shoffner JM, Wasmuth JJ, Costigan DA, Polak M, Altherr MR, Winokur ST, Wallace DC. Genetic mapping of human heart-skeletal muscle adenine nucleotide translocator and its relationship to the facioscapulohumeral muscular dystrophy locus. Genomics. 1993;16:479–85. doi: 10.1006/geno.1993.1214. [DOI] [PubMed] [Google Scholar]
- Hers HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202–3. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- Iwata T, Popescu NC, Zimonjic DB, Karlsson C, Hoog JO, Vaca G, Rodriguez IR, Carper D. Structural organization of the human sorbitol dehydrogenase gene (SORD) Genomics. 1995;26:55–62. doi: 10.1016/0888-7543(95)80082-w. [DOI] [PubMed] [Google Scholar]
- Izzo P, Costanzo P, Lupo A, Rippa E, Paolella G, Salvatore F. Human aldolase A gene. Structural organization and tissue-specific expression by multiple promoters and alternate mRNA processing. Eur J Biochem. 1988;174:569–78. doi: 10.1111/j.1432-1033.1988.tb14136.x. [DOI] [PubMed] [Google Scholar]
- Kamp G, Busselmann G, Lauterwein J. Spermatozoa: models for studying regulatory aspects of energy metabolism. Experientia. 1996;52:487–94. doi: 10.1007/BF01919321. [DOI] [PubMed] [Google Scholar]
- Kim YH, de Kretser DM, Temple-Smith PD, Hearn MT, McFarlane JR. Isolation and characterization of human and rabbit sperm tail fibrous sheath. Mol Hum Reprod. 1997;3:307–13. doi: 10.1093/molehr/3.4.307. [DOI] [PubMed] [Google Scholar]
- Kim YH, Jha KN, Mandal A, Vanage G, Farris E, Snow PL, Klotz K, Naaby-Hansen S, Flickinger CJ, Herr JC. Translation and assembly of CABYR coding region B in fibrous sheath and restriction of calcium binding to coding region A. Dev Biol. 2005;286:46–56. doi: 10.1016/j.ydbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- King TE, Mann T. Sorbitol dehydrogenase in spermatozoa. Nature. 1958;182:868–9. doi: 10.1038/182868a0. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981;290:449–54. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kaneko T, Iuchi Y, Matsuki S, Takahashi M, Sasagawa I, Nakada T, Fujii J. Localization and physiological implication of aldose reductase and sorbitol dehydrogenase in reproductive tracts and spermatozoa of male rats. J Androl. 2002;23:674–83. [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O’brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–8. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- Ku DH, Kagan J, Chen ST, Chang CD, Baserga R, Wurzel J. The human fibroblast adenine nucleotide translocator gene. Molecular cloning and sequence. J Biol Chem. 1990;265:16060–3. [PubMed] [Google Scholar]
- Lasorsa FM, Scarcia P, Erdmann R, Palmieri F, Rottensteiner H, Palmieri L. The yeast peroxisomal adenine nucleotide transporter: characterization of two transport modes and involvement in DeltapH formation across peroxisomal membranes. Biochem J. 2004;381:581–5. doi: 10.1042/BJ20040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ, Astley NR, Lambert D. Glucose and fructose utilization by rat spermatozoa within the uterine lumen. J Reprod Fertil. 1981;61:435–7. doi: 10.1530/jrf.0.0610435. [DOI] [PubMed] [Google Scholar]
- Levy SE, Chen YS, Graham BH, Wallace DC. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene. 2000;254:57–66. doi: 10.1016/s0378-1119(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Orlando A, Kanous KS. The flagellar beat of rat sperm is organized by the interaction of two functionally distinct populations of dynein bridges with a stable central axonemal partition. J Cell Sci. 1992;102:249–60. doi: 10.1242/jcs.102.2.249. [DOI] [PubMed] [Google Scholar]
- Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, Retief JD, Coonrod SA, Kinter M, Sherman N, Cesar F, Flickinger CJ, Herr JC. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–97. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–5. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Maquat LE, Chilcote R, Ryan PM. Human triosephosphate isomerase cDNA and protein structure. Studies of triosephosphate isomerase deficiency in man. J Biol Chem. 1985;260:3748–53. [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–6. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Pedersen LB, Feely M, Rosenbaum JL, Mitchell DR. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol Biol Cell. 2005;16:4509–18. doi: 10.1091/mbc.E05-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri H, Mohri T, Ernster L. Isolation and enzymatic properties of the midpiece of bull spermatozoa. Exp Cell Res. 1965;38:217–246. doi: 10.1016/0014-4827(65)90400-3. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Welch JE, Gotoh H, Goulding EH, Fujioka M, Eddy EM. Mouse spermatogenic cell-specific type 1 hexokinase (mHk1-s) transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol Reprod Dev. 1998;49:374–85. doi: 10.1002/(SICI)1098-2795(199804)49:4<374::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Murdoch RN, White IG. Studies of the metabolism of human spermatozoa. J Reprod Fertil. 1968;16:351–61. doi: 10.1530/jrf.0.0160351. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen S, Mandal A, Wolkowicz MJ, Sen B, Westbrook VA, Shetty J, Coonrod SA, Klotz KL, Kim YH, Bush LA, Flickinger CJ, Herr JC. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236–54. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Fujita A, Murata T, Watanabe G, Mori C, Fujita J, Watanabe N, Ishizaki T, Yoshida O, Narumiya S. Rhophilin, a small GTPase Rho-binding protein, is abundantly expressed in the mouse testis and localized in the principal piece of the sperm tail. FEBS Lett. 1999;445:9–13. doi: 10.1016/s0014-5793(99)00087-3. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Felix CM, Swanson JM. Highly conserved charge-pair networks in the mitochondrial carrier family. J Mol Biol. 1998;277:285–308. doi: 10.1006/jmbi.1997.1594. [DOI] [PubMed] [Google Scholar]
- O’Shea T, Wales RG. Metabolism of sorbitol and fructose by ram spermatozoa. J Reprod Fertil. 1965;10:353–68. doi: 10.1530/jrf.0.0100353. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Alberio S, Pisano I, Lodi T, Meznaric-Petrusa M, Zidar J, Santoro A, Scarcia P, Fontanesi F, Lamantea E, Ferrero I, Zeviani M. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet. 2005;14:3079–3088. doi: 10.1093/hmg/ddi341. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Rottensteiner H, Girzalsky W, Scarcia P, Palmieri F, Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–59. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- Regen DM, Pilkis SJ. Sensitivity of pathway rate to activities of substrate-cycle enzymes: application to gluconeogenesis and glycolysis. J Theor Biol. 1984;111:635–58. doi: 10.1016/s0022-5193(84)80259-3. [DOI] [PubMed] [Google Scholar]
- Schiebel K, Weiss B, Wohrle D, Rappold G. A human pseudoautosomal gene, ADP/ATP translocase, escapes X-inactivation whereas a homologue on Xq is subject to X-inactivation. Nat Genet. 1993;3:82–7. doi: 10.1038/ng0193-82. [DOI] [PubMed] [Google Scholar]
- Slim R, Levilliers J, Ludecke HJ, Claussen U, Nguyen VC, Gough NM, Horsthemke B, Petit C. A human pseudoautosomal gene encodes the ANT3 ADP/ATP translocase and escapes X-inactivation. Genomics. 1993;16:26–33. doi: 10.1006/geno.1993.1135. [DOI] [PubMed] [Google Scholar]
- Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- Storey BT, Kayne FJ. Energy metabolism of spermatozoa. V. The Embden-Myerhof pathway of glycolysis: activities of pathway enzymes in hypotonically treated rabbit epididymal spermatozoa. Fertility & Sterility. 1975;26(12):1257–1265. [PubMed] [Google Scholar]
- Storey BT, Kayne FJ. Energy metabolism of spermatozoa. VII. Interactions between lactate, pyruvate and malate as oxidative substrates for rabbit sperm mitochondria. Biology of Rreproduction. 1978;18(4):527–536. doi: 10.1095/biolreprod18.4.527. [DOI] [PubMed] [Google Scholar]
- Sullivan DT, MacIntyre R, Fuda N, Fiori J, Barrilla J, Ramizel L. Analysis of glycolytic enzyme co-localization in Drosophila flight muscle. J Exp Biol. 2003;206:2031–8. doi: 10.1242/jeb.00367. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, Kopf GS, Moss SB. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9:263–76. doi: 10.1091/mbc.9.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276:7630–6. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H, Tani K, Fujii H, Miwa S. Expression of L- and M-type pyruvate kinase in human tissues. Genomics. 1988;2:86–9. doi: 10.1016/0888-7543(88)90112-7. [DOI] [PubMed] [Google Scholar]
- Turner RM, Johnson LR, Haig-Ladewig L, Gerton GL, Moss SB. An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82. Genomic organization, protein kinase A-RII binding, and distribution of the precursor in the sperm tail. J Biol Chem. 1998;273:32135–41. doi: 10.1074/jbc.273.48.32135. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Liberty GA, Mohan J, Winfrey VP, Olson GE, Carr DW. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol Endocrinol. 1999;13:705–17. doi: 10.1210/mend.13.5.0278. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- Walker JE, Runswick MJ. The mitochondrial transport protein superfamily. J Bioenerg Biomembr. 1993;25:435–46. doi: 10.1007/BF01108401. [DOI] [PubMed] [Google Scholar]
- Welch JE, Brown PL, O’Brien DA, Magyar PL, Bunch DO, Mori C, Eddy EM. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J Androl. 2000;21:328–38. [PubMed] [Google Scholar]
- Welch JE, Schatte EC, O’Brien DA, Eddy EM. Expression of a glyceraldehyde 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells. Biol Reprod. 1992;46:869–78. doi: 10.1095/biolreprod46.5.869. [DOI] [PubMed] [Google Scholar]
- Westhoff D, Kamp G. Glyceraldehyde 3-phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa. J Cell Sci. 1997;110:1821–9. doi: 10.1242/jcs.110.15.1821. [DOI] [PubMed] [Google Scholar]
- Wojtas K, Slepecky N, von Kalm L, Sullivan D. Flight muscle function in Drosophila requires colocalization of glycolytic enzymes. Mol Biol Cell. 1997;8:1665–75. doi: 10.1091/mbc.8.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. ADP-dependent microtubule translocation by flagellar inner-arm dyneins. Cell Struct Funct. 2000;25:263–7. doi: 10.1247/csf.25.263. [DOI] [PubMed] [Google Scholar]


