Abstract
Background
Late in the 20th Century, it was recognized that connective tissue structures in the orbit influence the paths of the extraocular muscles, and constitute their functional origins. Targeted investigations of these connective tissue “pulleys” led to the formulation of the active pulley hypothesis, which proposes that pulling directions of the rectus extraocular muscles are actively controlled via connective tissues.
Purpose
This review rebuts a series of criticisms of the active pulley hypothesis published by Jampel, and Jampel and Shi, in which these authors have disputed the existence and function of the pulleys.
Methods
The current paper reviews published evidence for the existence of orbital pulleys, the active pulley hypothesis, and physiologic tests of the active pulley hypothesis. Magnetic resonance imaging in a living subject, and histological examination of a human cadaver directly illustrate the relationship of pulleys to extraocular muscles.
Results
Strong scientific evidence is cited that supports the existence of orbital pulleys, and their role in ocular motility. The criticisms of have ignored mathematical truisms and strong scientific evidence.
Conclusions
Actively controlled orbital pulleys play a fundamental role in ocular motility. Pulleys profoundly influence the neural commands required to control eye movements and binocular alignment. Familiarity with the anatomy and physiology of the pulleys is requisite for a rational approach to diagnosing and treating strabismus using emerging methods. Conversely, approaches that deny or ignore the pulleys risk the sorts of errors that arise in geography and navigation from incorrect assumptions such as those of a flat (“platygean”) earth.
Keywords: extra-ocular muscle, Listing’s Law, pulley, strabismus
The paper in the current issue1 by Robert S. Jampel and Dian X. Shi is the latest polemic by the former author attacking the idea that connective tissue pulleys have an important role in ocular motility and strabismus. These criticisms offered by Jampel and Shi are repetitive, reiterating claims that Dr. Jampel has offered repeatedly in letters to the editor of Investigative Ophthalmology and Visual Science (IOVS)2,3. I have published replies to the editor of IOVS refuting Jampel's objections in the past 4,5, but in the interest of dispelling confusion in the ophthalmic community, I again take this opportunity to clarify this important issue.
Historical Background
Traditional views of the action of the extraocular muscles (EOMs) suppose them to travel via the shortest paths in the orbit from their origins in the orbital apex to their scleral insertions. It was further presumed, although not often explicitly stated, that the brain must calculate necessary tensions in all the EOMs to enable the eye to rotate to its desired positions. This neural calculation is an explicit part of the theory offered by Jampel and Shi1. The foregoing presumptions were embodied in the first effort to develop computational models of the forces in the EOMs that might be useful for planning strabismus surgery6. Unfortunately, this endeavor to quantitatively describe the mechanical behavior of the EOMs was unsuccessful. Each simulation calculated that the eye would inevitably turn to point the cornea directly at the orbital apex, and would then remain stuck in that position. Such a nonphysiologic outcome never occurs. Computational models required mechanical constraints on rectus EOM sideslip in secondary and tertiary gaze postitions7.
Confirming radiographic findings in monkeys8,9, innovative magnetic resonance imaging (MRI) studies performed in the late 1990s by Joel M. Miller in human beings demonstrated that the posterior paths of the rectus EOMs are remarkably stable in the orbit despite the largest possible ductions. This seminal finding has been replicated in hundreds of subjects subsequently. Nevertheless, this MRI demonstration of rectus EOM path stability did not distinguish the mechanisms responsible for it. Miller supposed that there might be two possible mechanical factors that might prevent rectus EOMs from side-slipping over the globe, and prevent the globe from flipping posteriorly within the orbit to point the cornea to the orbital apex10. Miller imagined that there might be connective tissue couplings between the EOM tendons and the globe that would prevent such disastrous outcomes. Alternatively, he supposed that there might be connective tissues suspended from the orbit functioning as pulleys that would control rectus EOM paths and stabilize them relative to the globe.
It was only our performance of MRI in strabismic patients before and after rectus EOM transposition that indicated that the connective tissues stabilizing the EOM paths could not be coupled to the globe, but had to be coupled to the orbit. This conclusion is certain because EOM paths remained stable relative the orbit secondary gazes even as little as one day following surgical lysis of all couplings between the EOMs and the globe itself10. This finding strongly suggested the existence of orbital pulleys for the rectus EOMs, although no anatomic pulleys were known at that time.
In 1995, our laboratory re-examined the anatomy of the entire orbit to determine which connective tissues might be responsible for stabilization of rectus EOM paths11. We designed rigorous anatomical techniques to avoid possible artifacts of handling and processing, and employed the then-new technique of digital microscopy. We studied orbital anatomy in humans and monkeys using serially sectioned, whole orbits that could not have been subjected to any artifacts arising from postmortem dissection since no such dissection was performed. Furthermore, we performed MRI of the specimens prior to any sectioning or imbedding, and thus could reliably conclude that no anatomic artifacts were responsible for the conclusions. We used MRI to digitally compensate for shrinkage artifacts of processing, a precaution never undertaken in prior studies. Our monkey and human orbital studies12–15 in more than two dozen whole orbits and accomplished over more than a dozen years of sustained effort, have been confirmed as well in rabbits, cows and dogs in our laboratory, and in rodents in other laboratories16. There exist in all species connective tissue sleeves encircling the rectus EOMs at the level of their perforation of posterior Tenon's fascia. Functional anatomical studies in humans indicate that these encirclements function as pulleys of the rectus EOMs, and influence their pulling directions. Subsequent studies have demonstrated that the inferior oblique muscle also has a soft pulley17.
The active pulley hypothesis (APH) was a later elaboration upon the idea of pulleys, and was first announced in Stockholm in 199918. Additional anatomical investigations had revealed by that time that the orbital layer (OL) fibers of the rectus EOMs terminated well short of the scleral tendons, and instead inserted upon the connective tissue pulleys19. This finding caused me to suppose that the orbital layers of the EOMs might actively position the connective tissue pulleys. I further deduced that appropriate active positioning of the rectus EOM pulleys might be responsible for Listing's law (LL) of ocular torsion, since appropriate anteroposterior positioning of the pulleys would cause rectus EOM pulling directions to change systemically with eye position19. Admittedly, this was a revolutionary hypothesis4,20,21, but the APH has reconciled a wide variety of heretofore confusing observations and resolves numerous kinematic problems in the neural control of eye movements. The powerful feature of the APH is that it is highly quantitative, and could thus be disproven experimentally if false.
The APH makes very precise predictions about the locations of the EOMs, their changes with gaze, and their relationship to the highly quantitative LL. Furthermore, the APH makes strong predictions about what neural signals will and will not be found on the motor neurons controlling the cyclovertical EOMs. Since it was first proposed in 1999, the APH has been extensively and quantitatively tested, and has in each instance fulfilled its predictions regarding visually guided eye movements. It was for the APH that I received the 2003 Friedenwald Award of the Association for Research in Vision and Ophthalmology22. Several detailed reviews of the evidence supporting the APH have been recently published4,21,22, and the APH is now fundamental in modern textbooks on ocular motility15,23,24.
With the foregoing in mind, it should be difficult to take seriously the repetitive criticisms of Jampel and Shi. Their latest polemic categorically dismisses all the published evidence for the existence of the orbital pulleys, the validity of the APH, and makes numerous assertions about orbital anatomy and physiology that are grossly and obviously wrong. I will briefly detail these for the benefit of the reader.
Assertion of Rotational Commutativity is a Fundamental Error
In their polemic, Jampel and Shi make the amazing and totally incorrect assertion that "eye movements, as well as all human movements, are commutative." They imply that the order of rotations of biologic structures is not relevant to the final orientation of the structures1. Unfortunately, it is a fundamental mathematical truism that rotations of any sort or object are always non-commutative: the sequence of rotations has an important influence on the final orientation achieved, and there is simply no way around this unless the rotational axes themselves interact mutually25–27. Quaia and Optican have shown mathematically that eye movements can appear commutative to the brain, but only if they conform to LL, which says that the rotational axis of the eye changes by one-half of the current eye position25. Tweed showed mathematically that this assertion is the same as Listing's, but not Donder's, law28. Donder's law simply says that the torsional orientation of the eye is uniquely dependent upon horizontal and vertical eye position. Donder’s law says nothing about commutativity. Donder’s law is not the same as LL, which says that when the head is upright and stationary, the eye both complies with Donder's law and does so by rotation about a single axis, which lies in a unique plane called Listing’s plane. Unfortunately, if one is to correctly analyze three-dimensional (3-D) ocular rotations on a rigorous basis, non-commutative mathematics are totally unavoidable. If proper mathematics are omitted, one will reach erroneous conclusions in the same way as did Jampel and Shi.
Irrelevance of the Law of Reciprocal Innervation
Jampel and Shi complain that striated orbital layers (OLs) of the rectus EOMs cannot be opposed by elastic suspensions, but they believe instead that all striated muscles must have antagonist striated muscles. This statement is a curious and idiosyncratic invention of Jampel and Shi, and is without foundation. Striated muscles, such as those of the middle ear, need not have direct antagonists. The fact that there is reciprocal innervation of muscles that do act in agonist-antagonist pairs does not require that all muscles have antagonists. The logic of Jampel and Shi is a fallacy.
Rectus Pulleys are Properly Positioned
Jampel and Shi note that the APH requires that the distance between the pulley and the globe center be maintained equal to the distance between the globe center and the scleral insertion. This requirement is indeed essential to mechanical implementation of LL, but Jampel and Shi falsely assert confusion and lack of experimental evidence on the point. Detailed MRI studies in humans have demonstrated that the pulleys, in fact, are precisely positioned and precisely moved so as to implement the half-angle requirement of LL29. Any confusion on the point is attributable to Jampel and Shi.
Shi and Jampel assert that because the length and width of EOM tendons and the distance from the limbus vary, the innervation to the OL must vary precisely from EOM to EOM. Based on quantitative MRI in alert humans, we can be confident that on average the pulleys are, in fact, actively positioned as required to implement LL29. Of course, there are both individual variations in anatomy and in the degree to which eye movements conform to LL. If Jampel and Shi believe that the nervous system is smart enough to control explicitly all of ocular kinematics without the assistance of the orbital connective tissues, it would hardly be implausible to suppose that the brain might make the necessary smaller adjustments in innervation to EOMs to maintain proper pulley positions. A challenge for modern ocular motor neuroscience will be to correlate the innervation in the OL and global layers (GLs) of rectus EOMs with actual pulley positions achieved, but such an experiment is feasible.
Superior Rectus Tendon Does Not Need to Bunch Up
Jampel and Shi confusingly assert that GL fibers of the superior rectus would bunch up during lateral eye movement. This claim is absurd, and MRI studies in tertiary gaze positions show that the putative "bunching up" does not occur. This criticism is nonsense.
Misleading Statements Concerning Orbital Anatomy
Jampel and Shi went to some effort in their Figure 2 to mislead readers regarding orbital anatomy3. Repeating a similarly misleading presentation in a letter to the editor of IOVS by Jampel3, Jampel and Shi republish from Jampel’s earlier letter to the editor of IOVS in Fig. 2 a computed tomography (CT) x-ray of the orbits which fails to demonstrate pulleys. I have previously published a rebuttal5 to Jampel and Shi’s Fig. 2, and they are fully aware of that refutation. My rebuttal letter includes the present Fig. 1, a gadodiamide enhanced MRI of a living human medial rectus pulley5. Jampel and Shi seem to be willfully blind to the existence of evidence for pulleys, for they continue to assert that imaging does not demonstrate pulleys even after having seen my Fig. 1. Failure of Jampel and Shi’s CT to demonstrate soft tissues such as pulleys should surprise no one. The CT scan also fails to demonstrate any of the orbital nerves or blood vessels. These anatomic structures, as well as the pulleys, are invisible if not imaged properly. Proper technique involves demonstration of the pulleys using MRI. I also provide some previously unpublished contrast MRI images showing gaze-related anteroposterior shifts in the location of human horizontal rectus pulleys (Fig. 2). These images use gadodiamide contrast to demonstrate locations of the pulleys, and show that they move posteriorly with contraction of their respective EOMs. It is obvious that MRI has a decisive advantage over x-ray CT, which has very poor soft tissue resolution.
Fig. 2.
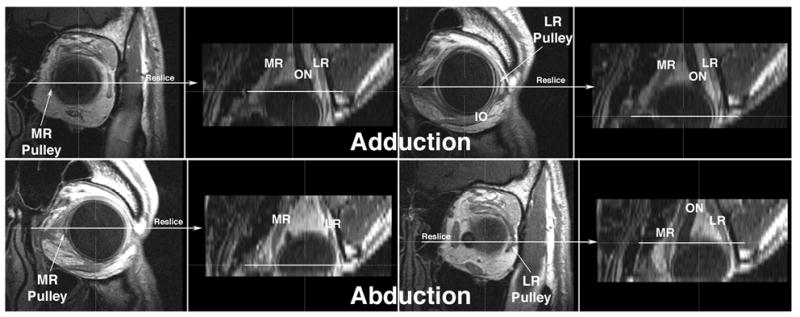
Gadodiamide enhanced, multiplanar T1 MRI of left orbit of normal human in adduction and abduction. Images were originally acquired in 18 contiguous 2 mm thick quasi-coronal planes perpendicular to the long axis of the orbit at 312 micron resolution. Using three-dimensional technique, the image stacks for each gaze direction were then interactively reconstructed in the planes indicated to produce the axial images shown. The horizontal rectus pulleys are recognized as dark rings In each case, the coronal images that include the medial rectus (MR) and lateral rectus (LR) pulleys are directly displayed for each gaze direction, and the corresponding location on the axial reconstruction is marked with a horizontal white line. Note that the MR pulley shifts posteriorly in adduction, and anteriorly in abduction. The LR pulley shifts anteriorly in adduction, and posteriorly in abduction.
Fig. 1.
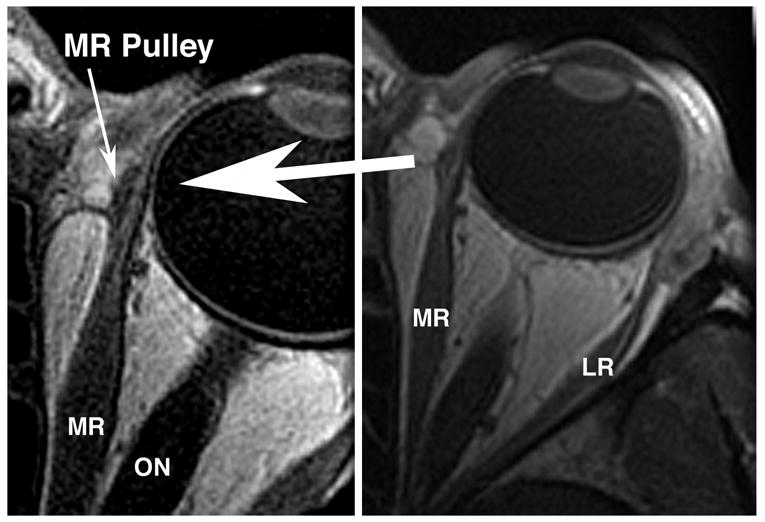
Gadodiamide-enhanced, T1 weighted axial magnetic resonance image (MRI) of a human left orbit in 2 mm thickness planes at 390 micron resolution at right, and 312 micron resolution at left, demonstrating the medial rectus (MR) pulley. Clearly seen at higher resolution at left, but not so evident at lower resolution, the MR pulley is evident as a less vascular sleeve around the enhancing, more vascular muscle. LR – lateral rectus muscle. ON – optic nerve. (With permission from IOVS5).
Overwhelming Histological Evidence for Pulleys
Jampel and Shi are critical of the past description of pulleys as being distributed structures. This is simply an anatomical fact, and the distributed nature of pulleys was probably one reason why they were discovered historically so late.
Jampel and Shi make the amazing assertion that there is no histological evidence for pulleys1. In fact, Jampel and Shi ignore a great deal of evidence for pulleys. We have published numerous micrographs demonstrating the existence of pulleys in humans11–15,17,19,24,30–32 (including Fig. 3 here) and several species of monkeys12,14,17,31,32; we have unpublished histological data showing pulleys in rabbit, dog, and cow, and are currently sectioning the whole orbit of a horse. Other laboratories have published evidence showing pulleys in rodents16,33. Obviously, different techniques must be used for different experimental purposes. Histological techniques, such as we have carefully employed over the past decade for the study of pulleys, provide tissue discrimination and optimal resolution30. Functional anatomy, the demonstration of the behavior of tissues in living organisms, can be accomplished with MRI, which we have done in numerous cases. Direct mechanical assessment of tissue properties requires the measurement of tissue stresses and strains, which no one has been able to do very satisfactorily in any orbital tissues to date. The 19th and early 20th century references cited by Jampel and Shi cannot refute our careful, computer-assisted anatomic reconstructions that conclusively and consistently show the existence of pulley tissues.
Fig. 3.
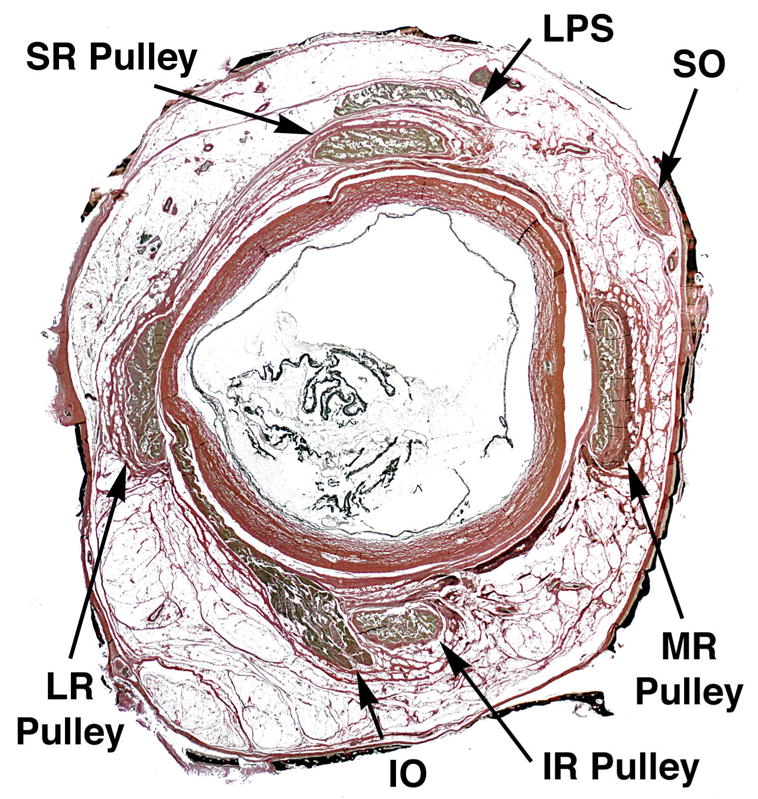
Quasi-coronal histological micrograph of a whole, serially sectioned right human orbit stained with van Gieson’s elastin stain, taken 11.40 mm posterior to the anterior corneal surface so as to intersect the rectus pulleys. Pulleys are seen as rings of connective tissue encircling the muscle bellies. At higher power, dense black deposits of elastin may be seen at the points where pulleys inflect the muscle paths in eccentric gaze positions. The method also stains the artificially thinned orbital bones black. IO – inferior oblique muscle. IR – inferior rectus muscle. LPS – levator palpebrae superioris muscle. LR – lateral rectus muscle. MR – medial rectus muscle. SR – superior rectus muscle. Note that the LPS lacks an encircling pulley.
Abundant Physiological Evidence for Pulleys
In their diatribe, Jampel and Shi ignore and deny the physiological evidence for the existence of orbital pulleys. The authors overlook the numerous published studies conclusively demonstrating that the rectus EOM paths are stabilized in the orbit in eccentric gazes8,29,34,35, that this stability is maintained even after disinsertion of the scleral tendons10,36, and even after enucleation of the entire globe37. This sort of functional anatomical data is decisive physiological evidence for pulleys. Jampel and Shi rely on the former's electrophysiologic studies from the 1960s and 1970s in rhesus monkeys. These studies were performed using techniques now regarded as outmoded, and which continue to support erroneous conclusions. Jampel’s studies were not quantitative, and did not employ well-defined kinematic coordinate systems as necessary for valid 3-D analysis of ocular kinematics. The theoretical concepts in instrumentation techniques necessary for 3-D kinematic analysis of eye movements did not become available until the late 1980s38. Consequently, the early physiologic studies to which Dr. Jampel refers could not have been guided by any of the concepts that are explained by the APH, and the studies were preceded by wide ablations of the superior and lateral orbital tissues likely to have obscured pulley function. Much better physiologic stimulation39 and recording40 experiments have very recently been performed in behaving primates, and these support the claimed role of pulleys in the regulation of ocular kinematics. There is now good reason to believe that the techniques employed by Dr. Jampel were inaccurate and insensitive, since in his hands they have uniquely failed to demonstrate the existence of ocular counter-rolling41, a robust vestibulo-ocular reflex readily investigated in numerous modern laboratories 42–50.
Jampel and Shi cite the study of Dimitrova et al. in which electrically evoked horizontal saccades in monkeys and cats were not affected much by removal of connective tissues around the lateral rectus muscle51. Of course, the APH did not predict any change under these conditions. A proper experimental test of the APH would have involved e tertiary gaze positions and analysis of the rotational axis of the ensuing eye movements. In that case, one would have predicted large changes from pulley ablation. Fortunately, appropriate electrophysiological tests of the APH experiments have been done in alert, behaving monkeys instrumented with 3-D magnetic search coils. Such experiments decisively demonstrate that horizontal saccades evoked by the electrical stimulation of the abducens nerve conform to LL just as predicted by the APH39, and contrary to what would be predicted by the obsolete view of Jampel and Shi. Furthermore, studies of the vertical rectus and oblique motor neurons in behaving monkeys demonstrate that none of these motor neurons carry the neural commands responsible for implementing LL40. It is now impossible to escape the logical conclusion that LL is mechanically implemented, not neurally specified. If the motor neurons do not carry the LL signal, then the brain does not implement LL. The recent, decisive physiological evidence completely and directly contradicts Jampel's overall thesis that the brain implements LL and all aspects of ocular kinematics. For those persuaded by scientific evidence, the controversy about the origin of Listing’s law is finished.
Jampel and Shih claim that there is no clinical evidence for the existence of pulleys. This statement can be made only by persons ignorant of modern orbital anatomy. The pulleys can be directly photographed and demonstrated at both surgery and at postmortem dissections11,15. Rectus pulleys degenerate with aging12,52. Heterotopic and unstable rectus pulleys are among the causes of incomitant strabismus24,53–58. Rectus pulley surgery is emerging as an effective clinical technique59,60. Jampel and Shi assert that following strabismus surgery, eye movements remain commutative and obey Donder's law. However, since eye movements were not commutative even before surgery, patients afterward will of course remain noncommutative. My laboratory routinely performs 3-D recordings of human eye movements using dual search coils and proper mathematical analysis 61,62. We are finding that strabismus surgery does alter ocular kinematics. As yet unpublished studies of 3-D ocular kinematics in my laboratory demonstrate large violations of LL following strabismus surgery.
Jampel and Shi claim that recovery of ischemic mononeuropathies associated with diabetes are not associated with changes in orbital connective tissues1. The APH never supposed any such changes to be necessary, although MRI does demonstrate a small shift in the medial rectus pulley position in superior oblique palsy63. There is no doubt that neurological lesions can cause strabismus, as we have repeatedly confirmed as in congenital fibrosis of the extraocular muscles64. Connective tissue lesions, such as pulley abnormalities, can be another cause of strabismus.
The Central Nervous System Does Not Explain Everything
Jampel and Shi conclude their polemic with the claim that the structural organization of the orbit is uniform from individual to individual, and that only neural commands vary so as to cause strabismus. This claim of anatomical uniformity is patently absurd, and be falsified with only one of innumerable counter-examples 54,57. It is easy to find clinical examples of dystopic orbits with abnormal pulley positions associated both with craniofacial disorders and with other abnormalities. Strabismus is prevalent in craniosynostosis, particularly large V and A patterns65,66, yet responds poorly to oblique EOM surgery67. Rectus EOM paths may be markedly abnormal in craniosynostosis53,68, imparting abnormal pulling directions. We propose that because the EOM pulley array is anchored to the bony orbit at discrete points12, bony abnormality alters EOM pulling directions by malpositioning pulleys. Typically, the heterotopic array of rectus pulleys is ex- or intorted, not necessarily symmetrically. Computer simulations suggest rectus pulley malpositioning as in craniosynostosis can produce incomitant strabismus54,57. Extorsion of the pulley array is associated with V patterns, and intorsion associated with A patterns58.
Jampel and Shi describe the existence of autonomic nerves within the orbit. We are better aware than most investigators of orbital autonomic innervation, having in our laboratory traced such innervation from ganglia to the smooth muscle in the orbital connective tissue system of monkeys and humans32. The APH does not require a contribution from smooth muscle to implement LL. The existence of autonomic control of orbital smooth muscle does not deny the APH, although smooth muscle may provide a mechanism for some minor modulations of pulley position and connective tissue stiffnesses.
We can agree with Jampel and Shi that neural factors may influence Listing’s plane, but we propose that this occurs via reconfiguration of the EOM pulleys. For example, there are good experimental observations of ocular extorsion and temporal tilting of Listing’s plane during convergence 69,70 associated with torsional repositioning of the rectus EOM pulley array 71 and alteration of trochlear motor neuron discharge in monkeys 72.
The vestibulo-ocular reflex (VOR) does not conform to LL61. Our recent study evaluated human EOMs during ocular counter-rolling, an otolith-mediated, static torsional VOR that can be evoked in an MRI scanner by decubitus positioning 73. The coronal plane positions of the rectus EOMs shifted torsionally in the same direction as ocular torsion evoked by ocular counter-rolling. Torsion of the rectus pulley array was roughly half of the ocular torsion reported by others in the same decubitus position. Oblique EOMs exhibited cross section changes consistent with their putative roles in torsional repositioning of rectus pulleys 73. This finding, considered in context of saccade kinematics during the VOR 61, suggests that the array of rectus pulleys resembles a kind of “inner gimbal” conforming to Listing’s half angle kinematics for visually guided movements such as fixations and saccades, but which is rotated by the oblique EOMs to implement eye movements such as the VOR. Recordings of trochlear motor neuron discharge suggest that ocular extorsion during convergence is neurally commanded 72.
We have proposed that the central neural signals correlated with all types of eye movements would be expected to reflect effects of torsional reconfiguration of rectus pulleys during the VOR. Recordings from burst neurons in monkeys appear compatible with torsional shift of rectus pulleys transverse to the EOM axes induced by static head tilt 74. Preferred directions of saccadic superior colliculus neurons shift in the opposite direction, and by slightly more than half the amount, of static head tilt 48. In this sense, the brain does control matters.
Regardless of the ocular motor subsystem involved, torsional rectus pulley shifts during the VOR would preserve the advantage of apparent commutativity of the peripheral ocular motor apparatus for concurrent saccades and pursuit. This commutativity would be valuable even though higher-level sensorimotor transformations must account for 3-D geometrical effects of eye and head orientation 48,75–77. In context of realistic EOM pulleys, sensorimotor integration of saccades does not require explicit neural computation of ocular torsion. This simplification solves some complexity, but moves other kinematic issues to a higher level. When head movements are involved, neural consideration of torsion is geometrically unavoidable for target localization in space 76,78.
The weight of the evidence indicates that several aspects of ocular kinematics are primarily implemented by a complex mechanical articulation, rather than by complex neural commands to a simpler mechanical arrangement. This insight alters the interpretation of common situations, and offers hope of surgical solutions to clinical disorders that might earlier have been believed to have neural origins. Oblique EOM function is not critical for LL79, although oblique EOM tone might set initial orientation of Listing’s plane. This is supported by the finding in chronic superior oblique paralysis that LL is observed, albeit with temporal tilting of Listing’s plane 80,81, and that this temporally tilted Listing’s plane is not changed by vergence 82.
Philosophy of Science
The foregoing contemporary issues in the neuroscience of eye movements critically involve an understanding of the pulleys and other mechanical determinants of eye movements. The field of ocular motility cannot be integrated with contemporary neuroscience if strabismologists ignore the most fundamental anatomical basis of ocular motility, such as the pulleys. Jampel and Shi would have us retreat from modern anatomy in this manner. It therefore may be perplexing to readers that Jampel and others persist in denying this powerful and eminently useful new concept, even as the alternative traditional hypothesis has apparently become untenable. To understand this situation, one must appreciate that the APH is a “revolution” in the scientific sense20,83.
The work of Thomas Kuhn on the philosophy of science provides good insight into the current situation84. Kuhn has examined in detail the circumstances of “scientific revolutions,” fundamental shifts in the paradigms used to conduct science. Examples of such paradigm shifts include the revolution in astronomy when the notion of a heliocentric solar system supplanted the ancient idea that all celestial bodies rotate about a flat earth. Other such revolutions are the atomic theory, and the neuron theory. In each case, practitioners of the older paradigm found it impossible to accept new paradigms and the observations underlying them. Entrenched practitioners of older paradigms found it difficult even to communicate scientifically with followers of the new paradigm because, among other reasons, even the nosology of the older paradigms carried mechanistic implications. I have elsewhere emphasized how traditional terminology in ocular motility can strongly biases our thinking in ocular motility85. For these and other reasons, adherents to older paradigms have typically been unwilling to consider new observations as valid scientific evidence at all. I contend that the same phenomenon underlies Jampel and Shi’s denial that any evidence supports the APH. They simply cannot accept that our abundant data constitutes “evidence.”
The historical studies of Thomas Kuhn demonstrate that competing scientific paradigms cannot co-exist in harmony, and that new scientific paradigms supplant old ones for various reasons84. Kuhn considers this process of change a “scientific revolution.” Historically, the most common reason for the ascendance of a new scientific paradigm is that it better predicts new phenomena than the old paradigm does. I predict this as the reason for ultimate widespread acceptance of the APH, since it quantitatively explains numerous fundamental and clinical phenomena in ocular motility inexplicable under the traditional paradigm. For example, the APH correctly predicted phenomena such as recently confirmed by Klier et al.86 and Ghasia et al.40 that are impossible with the traditional paradigm. Paraphrasing Kuhn, “There is nothing more practical than a good theory84.” The notions of pulleys and their physiologic behavior allow previously mysterious causes of strabismus to be diagnosed53,55–57, and effective surgeries to be performed59. This practical value includes the emerging range of surgical treatments of disorders of pulleys that could never have been contemplated under the traditional paradigm that fails to recognize the existence of these structures.
The Platygean View
The analogy of the flat (“platygean”) earth paradigm is instructive. The distinction between flat and spherical earth paradigms is of no practical importance for navigating the streets within a city, so members of the Flat Earth Society (http://www.alaska.net/~clund/e_djublonskopf/Flatearthsociety.htm) do not become lost as a result of their peculiar paradigm. Flat earth navigational theory results in appreciable but not necessarily disastrous errors for aircraft flying from state to state. However, flat earth theory is of no navigational value at all for space travel. Consequently, while the Flat Earth Society lists a wide range of evidence for their odd minority view, most travelers have abandoned the flat earth paradigm. Like navigating within a city using a flat earth paradigm, traditional views of EOM action give practically acceptable results for some clinical situations. Jampel and Shi’s paradigm simply cannot account for many contemporary scientific findings.
In spite of overwhelming contrary evidence, Jampel and Shi deny the existence of the orbital connective tissue pulleys that are now so well documented and which alone can account for so many crucial phenomena in ocular motility. I would not presume that scientific facts or logic would be sufficient to convince Jampel and Shi to abandon their antiquated position. I would hope, however, that more open-minded readers would not uncritically accept the baseless and silly denials of the facts by Jampel and Shi. We must embrace a modern paradigm, or risk becoming hopelessly lost platygeans on the spherical globe.
Footnotes
Support: Supported by U.S. Public Health Service, National Eye Institute: grants EY13583, EY08313, and EY00331. J. Demer received an unrestricted award from Research to Prevent Blindness and is Leonard Apt Professor of Ophthalmology.
References
- 1.Jampel RS, Shi DX. Evidence against mobile pulleys on the rectus muscles and inferior oblique muscle: Central nervous system controls ocular kinematics. J Peadiatr Ophthalmol Strabismus. 2006;43 doi: 10.3928/01913913-20060901-04. in press. [DOI] [PubMed] [Google Scholar]
- 2.Jampel RS. Pulley and globe stability. Invest Ophthalmol Vis Sci. 2006 e-letter. [Google Scholar]
- 3.Jampel RS. The superior rectus is not coupled to the superior oblique pulley. Invest Ophthalmol Vis Sci e-letter. 2006 [Google Scholar]
- 4.Demer JL. Ocular motility in a time of revolutionary paradigm shift. Invest Ophthalmol Vis Sci. 2006 doi: 10.1111/j.1442-9071.2006.01390.x. e-letter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demer JL. Author response: The superior rectus is not coupled to the superior oblique pulley. Invest Ophthalmol Vis Sci. 2006 e-letter. [Google Scholar]
- 6.Robinson DA. A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol. 1975;14:801–825. [PubMed] [Google Scholar]
- 7.Miller JM, Semmlow JL, Welkowitz W. Computer model of binocular alignment; Sixth Annual Conference, IEEE Engineering in Medicine and Biology Society; New York, NY. 1984. [Google Scholar]
- 8.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–40. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller JM, Robins D. Extraocular muscle sideslip and orbital geometry in monkeys. Vision Res. 1987;27:381–392. doi: 10.1016/0042-6989(87)90087-3. [DOI] [PubMed] [Google Scholar]
- 10.Miller JM, Demer JL, Rosenbaum AL. Effect of transposition surgery on rectus muscle paths by magnetic resonance imaging. Ophthalmology. 1993;100:475–487. doi: 10.1016/s0161-6420(93)31618-0. [DOI] [PubMed] [Google Scholar]
- 11.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–36. [PubMed] [Google Scholar]
- 12.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 13.Oh SY, Poukens V, Cohen MS, Demer JL. Structure-function correlation of laminar vascularity in human rectus extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42:17–22. [PubMed] [Google Scholar]
- 14.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16. [PubMed] [Google Scholar]
- 15.Demer JL. Extraocular muscles. In: Jaeger EA, Tasman PR, editors. Duane's Clinical Ophthalmology. Philadelphia: Lippincott; 2000. pp. 1–23. Ch. 1. [Google Scholar]
- 16.Khanna S, Porter JD. Evidence for rectus extraocular muscle pulleys in rodents. Invest Ophthalmol Vis Sci. 2001;42:1986–1992. [PubMed] [Google Scholar]
- 17.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 18.Demer JL. Orbital connective tissues in binocular alignment and strabismus. In: Lennerstrand G, Ygge Y, editors. Advances in Strabismus Research: Basic and Clinical Aspects. London: Portland Press; 2000. pp. 17–32. [Google Scholar]
- 19.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 20.Demer JL. The orbital pulley system: A revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 21.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 23.Liegh RJ, S ZD. The Neurology of Eye Movements. New York: 2006. [Google Scholar]
- 24.Demer JL. Anatomy of Strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. 3. London: Elsevier; 2005. pp. 849–861. [Google Scholar]
- 25.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]
- 26.Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35:1727–39. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- 27.Tweed DB, Haslwanter TP, Happe V, Fetter M. Non-commutativity in the brain. Nature. 1999;399:261–263. doi: 10.1038/20441. [DOI] [PubMed] [Google Scholar]
- 28.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–27. doi: 10.1016/0042-6989(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 29.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–88. [PubMed] [Google Scholar]
- 30.Miller JM, Demer JL, Poukens V, Pavlowski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J Vis. 2003;3:240–251. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- 31.Kono R, Poukens V, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790–2799. doi: 10.1167/iovs.04-1147. [DOI] [PubMed] [Google Scholar]
- 32.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 33.Felder E, Bogdanovich S, Rubinstein NA, Khana TS. Structural details of rat extraocular muscles andd three-dimensional reconstruction of the rat inferior rectus muscle and muscle-pulley interface. Vis Res. 2005;45:1945–1955. doi: 10.1016/j.visres.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys inferred from muscle paths. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 35.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 36.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 37.Detorakis ET, Engstrom RE, Straatsma BR, Demer JL. Functional anatomy of the anophthalmic socket: Insights from magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2003;44:4307–4313. doi: 10.1167/iovs.03-0171. [DOI] [PubMed] [Google Scholar]
- 38.Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J Neurophysiol. 1987;58:832–49. doi: 10.1152/jn.1987.58.4.832. [DOI] [PubMed] [Google Scholar]
- 39.Klier EM, Meng H, Angelaki DE. Abducens nerve/nucleus stimulation produces kinematically correct three-dimensional eye movements. Soc Neurosci Abstr. 2005:abstract #475.4. [Google Scholar]
- 40.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Jampel RW, Shi DX. The absence of so-called compensatory ocular countertorsion. The response of the eyes to head tilt. Arch Ophthalmol. 2002;120:1331–1340. doi: 10.1001/archopht.120.10.1331. [DOI] [PubMed] [Google Scholar]
- 42.Bockisch CJ, Haslwanter T. Three-dimensional eye position during static roll and pitch in humans. Vision Res. 2001;41:2127–2137. doi: 10.1016/s0042-6989(01)00094-3. [DOI] [PubMed] [Google Scholar]
- 43.Markham CH, Diamond SG. Ocular counterrolling in response to static and dynamic tilting: Implications for human otolith function. J Vestib Res. 2002–2003;12:127–134. [PubMed] [Google Scholar]
- 44.Suzuki Y, Kase M, Kato H, Fukushima K. Stability of ocular counterrolling and Listing's plane during static roll-tilts. Invest Ophthalmol Vis Sci. 1997;38:2103–2111. [PubMed] [Google Scholar]
- 45.Collewijn H, Van dSJ, Ferman L, Jansen TC. Human ocular counterroll: assessment of static and dynamic properties from electromagnetic scleral coil recordings. Exp Brain Res. 1985;59:185–96. doi: 10.1007/BF00237678. [DOI] [PubMed] [Google Scholar]
- 46.Schworm HD, Ygge J, Pansell T, Lennerstrand G. Assessment of ocular counterroll during head tilt using binocular video oculography. Invest Ophthalmol Vis Sci. 2002;43:662–667. [PubMed] [Google Scholar]
- 47.Averbuch-Heller L, Rottach KG, Zivotofsky AZ, Suarez JI, Pettee AD, Remler BF, Leigh RJ. Torsional eye movements in patients with skew deviation and spasmodic torticollis: responses to static and dynamic head roll. Neurology. 1997;48:506–514. doi: 10.1212/wnl.48.2.506. [DOI] [PubMed] [Google Scholar]
- 48.Frens MA, Suzuki Y, Scherberger H, Hepp K, Henn V. The collicular code of saccade direction depends on the roll orientation of the head relative to gravity. Exp Brain Res. 1998;120:283–290. doi: 10.1007/s002210050402. [DOI] [PubMed] [Google Scholar]
- 49.Crawford JD, Tweed DB, Vilis T. Static ocular counterroll is implemented through the 3-D neural integrator. J Neurophysiol. 2003;90:2777–2784. doi: 10.1152/jn.00231.2003. [DOI] [PubMed] [Google Scholar]
- 50.Pansell T, Tribukait A, Bolzani R, Schworm HD, Ygge J. Drift of ocular torsion during sustained head tilt. Strabismus. 2005;13:115–121. doi: 10.1080/09273970500216408. [DOI] [PubMed] [Google Scholar]
- 51.Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–3815. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- 52.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 53.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 54.Clark RA, Demer JL, Miller JM, Rosenbaum AL. Heterotopic rectus extraocular muscle pulleys simulate oblique muscle dysfunction. Abstracts of the American Association for Pediatric Ophthalmology and Strabismus. 1997:39. [Google Scholar]
- 55.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–78. [PubMed] [Google Scholar]
- 56.Demer JL, Kono R, Wright W, Oh SY, Clark RA. Gaze-related orbital pulley shift: A novel cause of incomitant strabismus. In: de Faber JT, editor. Progress in Strabismology. Lisse: Swets and Zeitlinger; 2002. pp. 207–210. [Google Scholar]
- 57.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren (Netherlands): Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 58.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–47. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 59.Clark RA, Ariyasu R, Demer JL. Medial rectus pulley posterior fixation is as effective as scleral posterior fixation for acquired esotropia with a high AC/A ratio. Am J Ophthalmol. 2004;137:1026–1033. doi: 10.1016/j.ajo.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Clark RA, Ariyasu R, Demer JL. Medial rectus pulley posterior fixation: A novel technique to augment recession. J AAPOS. 2004;8:451–456. doi: 10.1016/j.jaapos.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809. doi: 10.1167/iovs.05-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crane BT, Tian J, Demer JL. Human angular vestibulo-ocular reflex initiation: Relationship to Listing's Law. Ann NY Acad Sci. 2005;1039:1–10. doi: 10.1196/annals.1325.004. [DOI] [PubMed] [Google Scholar]
- 63.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–12. [PubMed] [Google Scholar]
- 64.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 65.Limon de Brown E, Monasterio FO, Feldman MS. Strabismus in plagiocephaly. J Pediatr Ophthalmol Strabismus. 1998;25:180–190. doi: 10.3928/0191-3913-19880701-08. [DOI] [PubMed] [Google Scholar]
- 66.Khan SH, Nischal KKFD, Hayward RD, Walker J. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003;87:999–1003. doi: 10.1136/bjo.87.8.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coats DK, Paysse EA, Stager DR. Surgical management of V-pattern strabismus and oblique dysfunction in craniofacial dysostosis. JAAPOS. 2000;4:338–342. doi: 10.1067/mpa.2000.110337. [DOI] [PubMed] [Google Scholar]
- 68.Velez FG, Thacker NTBM, Rosenbaum AL. Cause of V pattern strabismus in craniosynostosis: a case report. Br J Ophthalmol. 2004;88:1598–1599. doi: 10.1136/bjo.2004.048413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapoula Z, Bernotas M, Haslwanter T. Listing's plane rotation with convergence: role of disparity, accommodation, and depth perception. Exp Brain Res. 1999;126:175–186. doi: 10.1007/s002210050727. [DOI] [PubMed] [Google Scholar]
- 70.Mok D, Ro A, Cadera W, Crawford JD, Vilis T. Rotation of Listing's plane during vergence. Vision Res. 1992;32:2055–2064. doi: 10.1016/0042-6989(92)90067-s. [DOI] [PubMed] [Google Scholar]
- 71.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 72.Mays LE, Zhang Y, Thorstad MH, Gamlin PD. Trochlear unit activity during ocular convergence. J Neurophysiol. 1991;65:1484–91. doi: 10.1152/jn.1991.65.6.1484. [DOI] [PubMed] [Google Scholar]
- 73.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 74.Scherberger H, Cabungcal J-H, Hepp K, Suzuki Y, Straumann D, Henn V. Ocular counterroll modulates the preferred direction of saccade-related pontine burst neurons in the monkey. J Neurophysiol. 2001;86:935–949. doi: 10.1152/jn.2001.86.2.935. [DOI] [PubMed] [Google Scholar]
- 75.Klier EM, Crawford JD. Human oculomotor system acounts for 3-D eye orientation in the visual-motor transformation for saccades. J Neurophysiol. 1998;80:2274–2294. doi: 10.1152/jn.1998.80.5.2274. [DOI] [PubMed] [Google Scholar]
- 76.Crawford JD, Martinez-Trujillo JC, Kleier EM. Neural control of three-dimensional eye and head movements. Cur Opin Neurosci. 2003;13:655–662. doi: 10.1016/j.conb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Van Opstal AJ, Hepp K, Hess BJ, Straumann D, Henn V. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science. 1991;252:1313–5. doi: 10.1126/science.1925545. [DOI] [PubMed] [Google Scholar]
- 78.Crawford JD, Guitton D. Visual-motor transformations required for accurate and kinematically correct saccades. J Neurophysiol. 1997;78:1447–1467. doi: 10.1152/jn.1997.78.3.1447. [DOI] [PubMed] [Google Scholar]
- 79.Porrill J, Warren PA, Dean P. A simple control laws generates Listing's positions in a detailed model of the extraocular muscle system. Vision Res. 2000;40:3743–3758. doi: 10.1016/s0042-6989(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 80.Wong AMF, Sharpe JA, Tweed D. Adaptive neural mechanism for Listing's law revealed in patients with fourth nerve palsy. InvestOphthalmol Vis Sci. 2002;43:1796–1803. [PubMed] [Google Scholar]
- 81.Straumann D, Steffen H, Landau K, Bergamin O, Mudgil AV, Walker MF, Guyton DG, Zee DS. Primary position and Listing's law in acquired and congenital trochlear nerve palsy. Invest Ophthalmol Vis Sci. 2003;44:4282–4292. doi: 10.1167/iovs.02-1181. [DOI] [PubMed] [Google Scholar]
- 82.Migliaccio AA, Cremer PD, Sw ST, Halmagyi GM. Vergence-mediated changes in Listing's plane do not occur in an eye with superior oblique palsy. Invest Ophthalmol Vis Sci. 2004;45:3043–3047. doi: 10.1167/iovs.04-0014. [DOI] [PubMed] [Google Scholar]
- 83.Haslwanter T. Mechanics of eye movements: implications of the "orbital revolution". Ann N Y Acad Sci. 2002;956:33–41. doi: 10.1111/j.1749-6632.2002.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 84.Kuhn TS. The Structure of Scientific Revolutions. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 85.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–59. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 86.Klier EM, Meng H, Angelaki DE. Three-dimensional kinematics at the level of the oculomotor plant. J Neurosci. 2006;26:2732–2737. doi: 10.1523/JNEUROSCI.3610-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


