Abstract
INTRODUCTION
Objectives
Elderly patients are more likely to ingest prescription medications concurrently with botanical supplements, therefore, they may be vulnerable to herb-drug interactions. Phytochemical-mediated modulation of cytochrome P-450 (CYP) activity may underlie many herb-drug interactions. Some evidence suggests that CYP activity may decrease in the elderly. If so, herb-mediated changes in CYP activity may take on greater clinical relevance in this population. Single time-point, phenotypic metabolic ratios were used to determine whether long-term supplementation of St. John’s wort, garlic oil, Panax ginseng, and Ginkgo biloba affected CYP1A2, CYP2D6, CYP2E1, or CYP3A4 activity in elderly subjects.
METHODS
Twelve healthy volunteers between the ages of 60 and 76 (mean = 67 years) were randomly assigned to receive each supplement for 28 days followed by a 30-day washout period. Probe drug cocktails of midazolam, caffeine, chlorzoxazone, and debrisoquine were administered before and at the end of supplementation. Pre- and postsupplementation phenotypic ratios were determined for CYP3A4, CYP1A2, CYP2E1, and CYP2D6 using 1-hydroxymidazolam/midazolam serum ratios (1-hr), paraxanthine/caffeine serum ratios (6-hr), 6-hydroxychlorzoxazone/chlorzoxazone serum ratios (2-hr), and debrisoquine urinary recovery ratios (8-hr), respectively. The content of purported “active” phytochemicals was determined for each supplement.
RESULTS
Comparisons of pre- and post-St. John’s wort phenotypic ratios revealed significant induction of CYP3A4 (~140%) and CYP2E1 activity (~28%). Garlic oil inhibited CYP2E1 activity by approximately 22%. P. ginseng inhibition of CYP2D6 was statistically significant, but the magnitude of the effect (~7%) did not appear clinically relevant. None of the supplements tested in this study appeared to affect CYP1A2 activity.
CONCLUSIONS
Elderly subjects, like their younger counterparts, are susceptible to herb-mediated changes in CYP activity, especially those involving St. John’s wort. Pharmacokinetic herb-drug interactions stemming from alterations in CYP activity may adversely affect drug efficacy and/or toxicity. When compared to earlier studies that employed young subjects, the data suggest that some age-related changes in CYP responsivity to botanical supplementation may exist. Concomitant ingestion of botanical supplements with prescription medications, therefore, should be strongly discouraged in the elderly.
INTRODUCTION
The concomitant use of botanical supplements and conventional medications has engendered a growing medical concern over possible herb-drug interactions.[1–7] Recent surveys indicate that 24–36% of all consumers use botanical products on a regular basis.[1–3,8,9] Still other surveys indicate that herbal supplement usage is prevalent among patients taking prescription medications,[10–12] with many individuals greater than 65 years of age.[12–15] Given that the elderly constitute the largest demographic with regard to prescription medication use, they may also exhibit an increased risk for herb-drug interactions.
Phytochemical-mediated changes in human drug metabolizing enzyme activity may underlie many herb-drug interactions. Recently, a host of prospective clinical investigations have examined the ability of various botanical supplements to modulate human cytochrome P-450 (CYP) activity;[16–21] yet, none have examined these effects in elderly subjects. The practice of self-medicating with botanical supplements is common among elderly subjects,[1,13] and the products most frequently consumed include garlic, Ginkgo biloba, ginseng, and St. John’s wort.[9–12,14,15] Accordingly, these four supplements were selected for examination in the current study.
To date, St. John’s wort, a dietary supplement with antidepressant properties, appears to be the most problematic botanical with regard to CYP-mediated herb-drug interactions.[4–7] Hyperforin, a phytochemical component of St John’s wort, is a ligand for the steroid xenobiotic receptor (SXR) and thus acts as a potent inducer of CYP3A4 and MDR1 gene expression.[22,23] Due to the overwhelming number of prescription medications that are substrates for both CYP3A4 and the drug efflux pump, P-glycoprotein (the gene product of MDR1), their effectiveness can be greatly diminished when used concomitantly with St. John’s wort. Garlic, commonly used for treatment of hypercholesterolemia, also appears to modulate CYP activity in vivo. The evidence for garlic, however, is somewhat equivocal in that certain data suggests garlic may induce CYP3A4,[24] while other evidence indicates that it has little impact on CYP3A4,[17,19] but may inhibit CYP2E1.[17] Ginkgo biloba is frequently used as an alternative adjunctive treatment for mild senile dementia although its effectiveness is suspect.[25,26] Accordingly, its use is rising among elderly patients.[13–15,26] Studies in young volunteers indicate, however, that ginkgo’s effect on CYP activity is minimal, and similar findings have also been presented for Panax ginseng.[17,18,27] Nevertheless, the growing popularity of herbal supplement use together with evidence of herb-mediated changes in drug metabolism, suggest that elderly consumers may be especially vulnerable to herb-drug interactions. Moreover, age-related changes have been observed in the pharmacokinetics of several CYP substrates,[28–30] as well as certain CYP enzymes,[29,30] thus the question remains as to whether such effects may extend to botanical supplements and their effect on drug metabolism in the elderly.
Evaluating changes in the clearance of various probe drugs (medications that are substrates for specific CYP enzymes) has become a widely accepted methodology for assessing possible CYP-mediated drug-drug interactions.[31–33] Recently, a rapid and reliable in vivo screening method that utilizes single-time point phenotypic metabolic ratios was described for identifying botanical supplements capable of modulating CYP activity.[17] Although not intended to supplant traditional concentration-time profiles as a means of determining drug clearance, phenotypic metabolic ratios can provide reasonable estimates of probe drug clearance, thereby allowing in vivo evaluations of multiple CYP enzymes and multiple botanical supplements by means of a limited blood sampling scheme. This method was previously used to assess the effects of long-term supplementation with St. John’s wort, garlic oil, P. ginseng, and G. biloba on CYP1A2, CYP2D6, CYP2E1, and CYP3A4 activity in young, healthy volunteers. Botanical supplements containing extracts of Citrus aurantium, milk thistle, saw palmetto, and Echinacea purpurea have also been evaluated by this technique.[34] The purpose of the current study was to utilize single-time point phenotypic ratios as an instrument for determining the effects of St. John’s wort, garlic oil, P. ginseng, and G. biloba on CYP activity in elderly, healthy volunteers.
MATERIALS AND METHODS
Study subjects
The University of Arkansas for Medical Sciences Human Research Advisory Committee (Little Rock, AR) approved this study protocol and all participants provided written informed consent before commencing the study. Twelve elderly adults (6 males, 6 females) (age, mean ± SD = 67 ± 5.2 years; weight, 79.8 ± 14.4 kg) participated in the study and all subjects were in good health as indicated by medical history, routine physical examination, and clinical laboratory testing. All subjects were extensive metabolizers of CYP2D6 as confirmed by debrisoquine urinary recovery screenings.[31] All subjects were nonsmokers, ate a normal diet, and were not using botanical dietary supplements. With the exception of 2 female subjects taking conjugated estrogens/medroxyprogesterone acetate (Prempro™, 0.3 mg) no other participants were using prescription or nonprescription medications. Subjects were asked to abstain from alcohol, caffeine, fruit juices, cruciferous vegetables, and charbroiled meat throughout the study. Adherence to these restrictions was further emphasized five days before each probe drug administration. Subjects were also asked to refrain from taking prescription and nonprescription medications during supplementation periods, and any medication use during this time was brought to the investigators’ attention. Documentation of compliance to these restrictions was achieved through the use of a food/medication diary.
Supplementation and phenotyping procedure
The ability of garlic oil, P. ginseng, G. biloba, and St. John’s wort (Hypericum perforatum) to modulate human CYP activity was evaluated individually on four separate occasions in each subject. This was an open-label study randomized for supplementation sequence. Each supplementation period lasted twenty-eight days and was followed by a 30-day washout period. This randomly assigned sequence of supplementation followed by washout was repeated until each subject had received all four botanical supplements (Figure 1). To minimize intra-product variation in phytochemical content, single lots of each botanical supplement were purchased from the same vendor (Vitamer, Lake Forest, CA) Product labels were followed regarding the administration of garlic oil (500 mg, three times daily); P. ginseng (500 mg, three times daily, standardized to 5% ginsenosides); G. biloba (60 mg, four times daily, standardized to 24% flavone glycosides and 6% terpene lactones); and H. perforatum (300 mg, three times daily, standardized to 0.3% hypericin). Telephone and electronic mail reminders were used to facilitate compliance, while pill counts and supplementation usage records were used to verify compliance.
Figure 1.
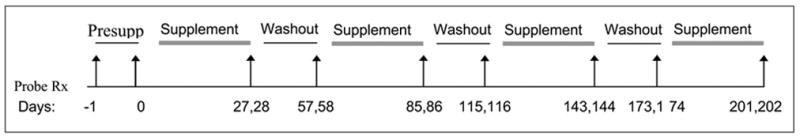
Supplementation and washout scheme. Arrows indicate days of probe drug administration.
On the day of each scheduled probe drug administration, all subjects reported to the General Clinical Research Center at the University of Arkansas for Medical Sciences. CYP1A2, CYP2D6, CYP2E1 and CYP3A4 phenotypes were assessed before (Days –1, 0) and at the end of each supplementation phase (Days 27, 28) (Figure 1). Forty-eight hours before supplementation (Day –1) each subject received an oral dose of caffeine (100 mg), and midazolam (8 mg). For the first two hours after midazolam administration, blood pressure, heart rate, respiration rate and pulse oximeter readings were obtained every 15 minutes. Blood samples (10 mL) were collected at 1 and 6 hours after probe drug administration and separated by centrifugation (1133 × g) to obtain serum for determining CYP3A4 and CYP1A2 activity. To avoid potential interference from midazolam and caffeine, CYP2E1 and CYP2D6 phenotypes were assessed twenty-four hours later.[35] The day before supplementation (Day 0), subjects emptied their bladder prior to receiving an oral dose of chlorzoxazone (500 mg) and debrisoquine (5 mg). Blood samples were then obtained at 2 hours and urine was collected for eight hours, at which time the volume was recorded and a 10-milliliter aliquot stored for analysis. All samples were stored frozen at −70°C until analyzed. Phenotypes were again assessed on supplementation Days 27 (CYP1A2, CYP3A4) and 28 (CYP2D6, CYP2E1). The CYP modulatory capability of each botanical supplement was evaluated by comparing individual differences in phenotype before and at the end of 28 days of supplementation.
Analytical methods
Serum concentrations of caffeine and paraxanthine were quantified by high performance liquid chromatography (HPLC) with ultraviolet absorbance detection per the method of Holland et al.[36] Chlorzoxazone and 6-hydroxychlorzoxazone serum concentrations were measured by HPLC using ultraviolet absorbance detection as previously described by Frye and Stiff.[37] The HPLC method described by Frye and Branch employing fluorescence detection was utilized for the quantitation of debrisoquine and 4-hydroxydebrisoquine in urine.[38] A previously described modification of the HPLC method of Sautou et al.[39] was used to determine serum concentrations of midazolam and 1-hydroxymidazolam.[17] To optimize the recovery of 6-hydroxychlorzoxazone and 1-hydroxymidazolam, serum samples (250 μL) for these probe drugs were incubated with β-glucuronidase (250 μL, 1800 units per mL) for 2.5 hours at 37°C.
Phytochemical analysis
The phytochemical content of each supplement was independently analyzed for specific “marker compounds” by high performance liquid chromatography (HPLC). Recent identification of hyperforin, a phytochemical component of Hypericum perforatum, as a nuclear receptor-mediated inducer of CYP3A4,[22,23] prompted us to determine the hyperforin content of St. John’s wort used in this study, as well as the concentration present in midazolam-containing serum samples. Quantitative determination of hyperforin, adhyperforin, and several other phytochemical components of St. John’s wort was achieved by HPLC using photodiode array detection with confirmation via mass spectrometry according to the method of Liu et al.[40] Hyperforin serum concentrations, obtained two hours after St. John’s wort administration on day 27, were determined by HPLC using solid-phase extraction and ultraviolet absorption detection per the method of Cui et al.[41]
Panax ginseng was analyzed for ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, and Rg at the National Center for Natural Products Research (University of Mississippi, University, MS.) by use of a proprietary gradient HPLC method. In brief, capsules were weighed and the contents transferred to a 15 mL polystyrene conical tube containing 3mL of water:acetonitrile (15:85). The samples were sonicated for 15 minutes, centrifuged at 1550 rpm, and the supernatant transferred to a 10 mL volumetric flask. After repeating this process twice, the volumetric flask was diluted to final volume with the extraction solution. Each sample was then filtered through a 0.45 μm Nylon membrane filter and a 50 μL aliquot was injected onto the HPLC column. The HPLC system consisted of a Waters 2695 Alliance Separations Module equipped with a 996 photodiode array detector (Waters Corp. Milford, MA). Ginsenosides were purchased from Chromadex (Santa Ana, CA) and separated on a Luna C18 column (150 × 3.0 mm; 5 μm particle size) (Phenomenex, Torrance, CA) using gradient elution at a flow rate of 0.7 mL/minute and a detection wavelength of 205 nm. The mobile phase consisted of (A) water:acetonitrile (85:15, v/v); (B) water:acetonitrile (25:75, v/v); (C) water:acetonitrile (50:50, v/v); and (D) methanol. The gradient elution as follows:100% A to 100% B in 30 minutes, and then to 100% C after an additional 30 minutes. Each run was followed by an 8 minute wash with 100% D and a re-equilibration period of 10 minutes. Peaks were assigned by a comparison of retention times and ultraviolet absorption spectra. Chromatographic data was collected and analyzed using the Millennium software system (Waters Corp., Milford, MA). Retention times for ginsenosides Re, Rg1, Rf, Rb1, Rb2, Rc, and Rd were 28, 29, 42.5, 46.3, 47.3, 48.3, and 50.5 minutes, respectively. Using standard curves covering the range of 22.5 to 160 μg/mL, relative standard deviations for inter- and intra-day analyses were less than 5%. The limit of quantitation for the various ginsenosides ranged from 22.5 μg/mL for Rb2 to 40 μg/mL for Rd. Percent recoveries for all ginsenosides exceeded 99%.
Sulfide content of the garlic oil preparation was determined via gas chromatography with flame ionization and mass spectrometry detection per the method of Lawson et al.[42] Simultaneous detection of terpene lactones and flavonoid aglycones in Ginkgo biloba was achieved by HPLC with evaporative light scattering detection as described by Li and Fitzloff.[43]
Phenotype Assessment
Serum ratios of 1-hydroxymidazolam/midazolam determined one hour after dosing were used to estimate CYP3A4 activity.[44] CYP1A2 phenotypes were determined from paraxanthine/caffeine serum ratios obtained at six hours.[31,32] CYP2E1 activity was estimated from 6-hyrdoxychlorzoxazone/chlorzoxazone serum ratios obtained 2 hours after dosing,[32] while CYP2D6 activity was assessed using eight-hour debrisoquine urinary recovery ratios: [4-hydroxydebrisoquine/(debrisoquine + 4-hydroxydebrisoquine)].[32,33]
Statistics
A repeated measures ANOVA model was fit for each phenotype response using SAS Proc Mixed software (SAS Institute, Inc. Cary, N.C.). Since pre-and post-supplementation phenotypic ratios were determined in each subject for all four supplements, a covariance structure existed for measurements within subjects. Sex, supplement, and supplement-by-sex terms were estimated for each phenotype using a Huynh-Feldt covariance structure fit. If supplement-by-sex interaction terms for a specific phenotypic measure were significant at the 5% level, the focus of the post-supplementation minus pre-supplementation response was assessed according to sex. If the supplement-by-sex interaction was not statistically significant, responses for both sexes were combined.
Additionally, a power analysis was performed to estimate the ability to detect significant post- minus pre-supplementation effects. All four phenotype models obtained at least 80% power at the 5% level of significance to detect a Cohen effect size of 1.0 to 1.32 standard deviation units.[45]
RESULTS
General Experimental Observations
All subjects completed the study and no serious adverse events occurred during the course of the investigation.
Effect of Supplementation on CYP Phenotype
The effects of chronic St. John’s wort, garlic oil, G. biloba, and P. ginseng supplementation on CYP phenotypic ratios are shown in Figures 2 through 5 and Table I. Twenty-eight days of St. John’s wort supplementation resulted in a 141% (range = 58%–725%) increase in the mean one-hour 1-hydroxymidazolam/midazolam serum ratio (p < 0.001) (Fig 2A, Table I), whereas chronic administration of garlic oil, G. biloba, or P. ginseng produced no significant changes in CYP3A4 phenotype (Fig 2B–D, Table I).
Figure 2.
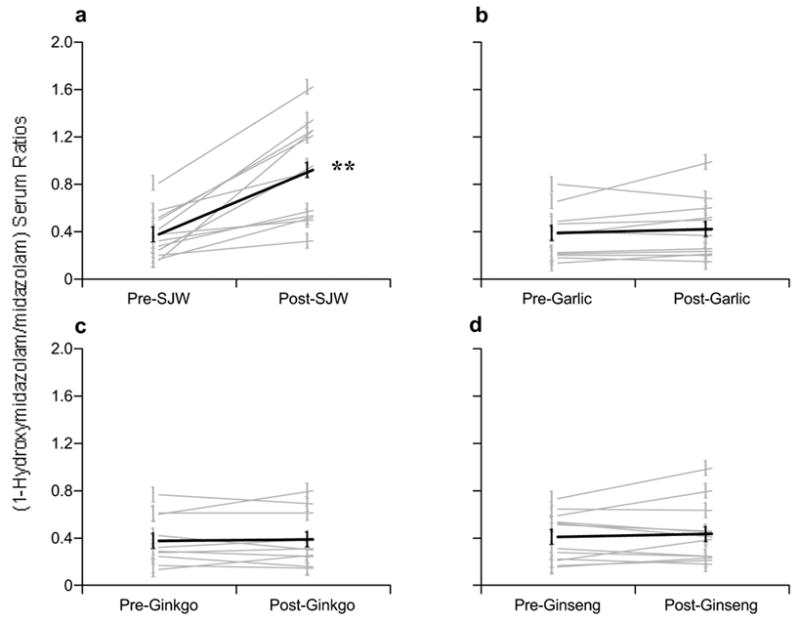
Comparison of pre- and post-supplementation phenotypic ratios (1-hydroxymidazolam/midazolam) for CYP3A4. (A) St. John’s wort, (B) Garlic oil, (C) Ginkgo biloba, (D) Panax ginseng. Gray circles = individual values, Black circles = group means. Astersisks = statistically significant difference from baseline.
Figure 5.
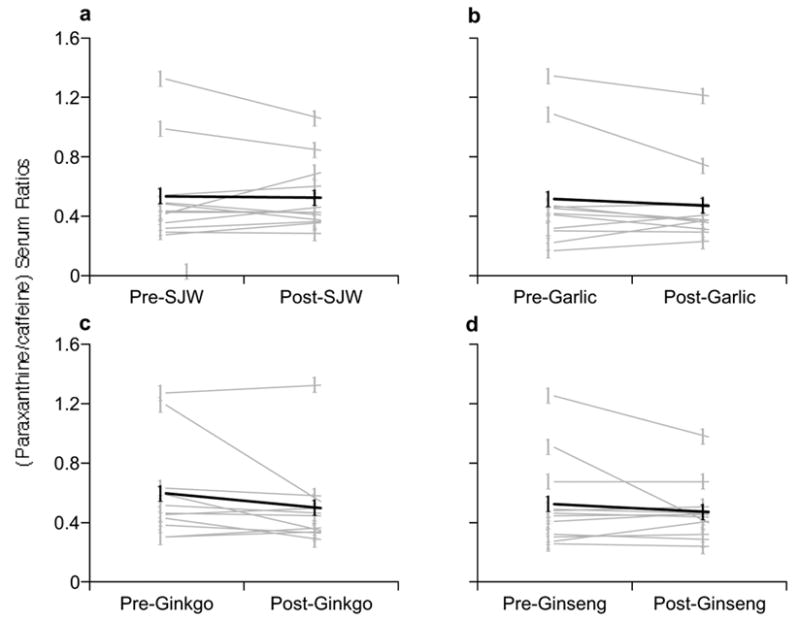
Comparison of pre- and post-supplementation phenotypic ratios (paraxanthine/caffeine) for CYP1A2. (A) St. John’s wort, (B) Garlic oil., (C) Ginkgo biloba, (D) Panax ginseng. Gray circles = individual values, Black circles = group means.
Table I.
Pre- and Postsupplementation Phenotypic Ratios (means, 95% confidence intervals)
| Phenotypic Ratio (CYP) | Supplement | Presupplementation (mean and 95% CI) | Postsupplementation. (mean and 95% CI) | Difference (mean and 95% CI) |
|---|---|---|---|---|
| OH-MDZ/MDZ (CYP3A4) | St. John’s wort | 0.379 (0.250 to 0.507) | 0.914 (0.633 to 1.195) | 0.535 (0.309 to 0.761)* |
| Ginkgo biloba | 0.372 (0.235 to 0.508) | 0.389 (0.230 to 0.547) | 0.017 (−0.040 to 0.073) | |
| Garlic oil | 0.375 (0.247 to 0.502) | 0.422 (0.260 to 0.584) | 0.047 (−0.030 to 0.125) | |
| Panax ginseng | 0.404 (0.276 to 0.532) | 0.433 (0.272 to 0.594) | 0.029 (−0.054 to 0.112) | |
| DMX/CFE (CYP1A2) | St. John’s wort | 0.526 (0.315 to 0.736) | 0.520 (0.364 to 0.676) | −0.006 (−0.099 to 0.088) |
| Ginkgo biloba | 0.587 (0.347 to 0.826) | 0.501 (0.294 to 0.709) | −0.086 (−0.227 to 0.056) | |
| Garlic oil | 0.511 (0.279 to 0.743) | 0.465 (0.286 to 0.644) | −0.046 (−0.124 to 0.032) | |
| Panax ginseng | 0.518 (0.320 to 0.716) | 0.466 (0.334 to 0.597) | −0.052 (−0.168 to 0.063) | |
| OH-CZX/CZX (CYP2E1) | St. John’s wort | 0.393 (0.295 to 0.491) | 0.496 (0.378 to 0.613) | 0.103 (0.036 to 0.169)* |
| Ginkgo biloba | 0.427 (0.304 to 0.550) | 0.459 (0.345 to 0.572) | 0.032 (−0.038 to 0.102) | |
| Garlic oil | 0.423 (0.305 to 0.542) | 0.331 (0.220 to 0.443) | −0.092 (−0.150 to −0.034)* | |
| Panax ginseng | 0.441 (0.326 to 0.556) | 0.429 (0.294 to 0.564) | −0.012 (−0.087 to 0.064) | |
| HDEB/(HDEB+DEB) (CYP2D6) | St. John’s wort | 0.623 (0.499 to 0.746) | 0.634 (0.520 to 0.748) | 0.012 (−0.018 to 0.041) |
| Ginkgo biloba | 0.613 (0.494 to 0.732) | 0.602 (0.454 to 0.750) | −0.011 (−0.059 to 0.037) | |
| Garlic oil | 0.595 (0.452 to 0.739) | 0.581 (0.427 to 0.735) | −0.014 (−0.062 to 0.033) | |
| Panax ginseng | 0.611 (0.465 to 0.756) | 0.566 (0.418 to 0.714) | −0.045 (−0.070 to −0.019)* |
CI = confidence interval, OH-MDZ = 1-hydroxymidazolam, MDZ = midazolam, DMX = paraxanthine, CFE = caffeine, OH-CZX = 4-hydroxychlorzoxazone, CZX = chlorzoxazone, HDEB = 6-hydroxydebrisoquine, DEB = debrisoquine
= p < 0.05
Similar to its effect on CYP3A4, St. John’s wort produced significant increases in CYP2E1 activity (p = 0.006) after 28 days of use as evidenced by a 26% rise in the mean 6-hydroxychlorzoxazone/chlorzoxazone serum ratio between pre- and post-supplementation periods (Fig 4A). Conversely, garlic oil produced a significant decrease (p = 0.005) of 22% in 6-hydroxychlorzoxazone/chlorzoxazone serum ratios intimating that CYP2E1 activity was inhibited (Figure 4B). Chronic administration of G. biloba and P. ginseng had no modulatory effects on CYP2E1 (Fig 4C, D).
Figure 4.
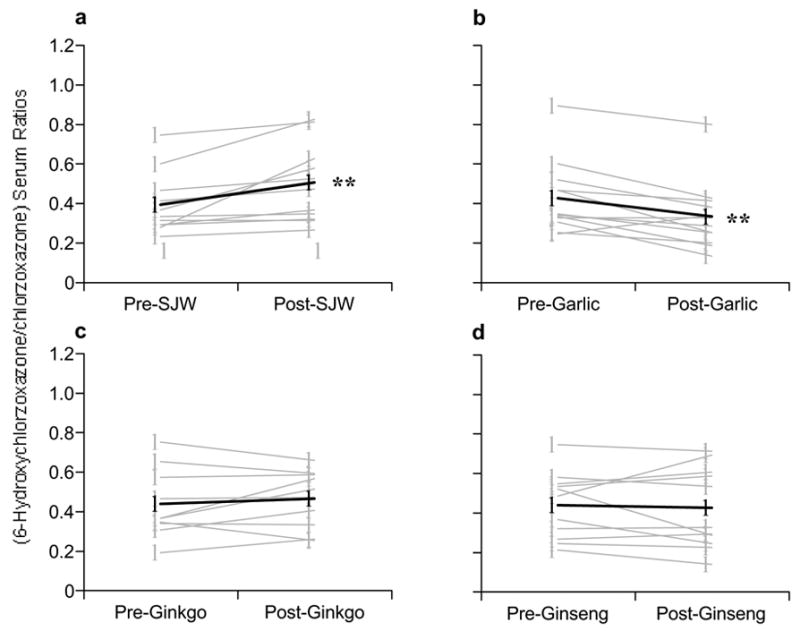
Comparison of pre- and post-supplementation phenotypic ratios (6-hydroxychlorzoxazone/chlorzoxazone) for CYP2E1. (A) St. John’s wort, (B) Garlic oil., (C) Ginkgo biloba, (D) Panax ginseng. Gray circles = individual values, Black circles = group means. Asterisks = statistically significant difference from baseline.
Twenty-eight days of P. ginseng administration produced a statistically significant decrease (p = 0.003) in debrisoquine urinary recovery ratios of 7% (Fig 3D, Table I), however, this effect was not considered clinically relevant. No significant changes in CYP2D6 phenotype were observed for St. John’s wort, garlic oil, or G. biloba.
Figure 3.
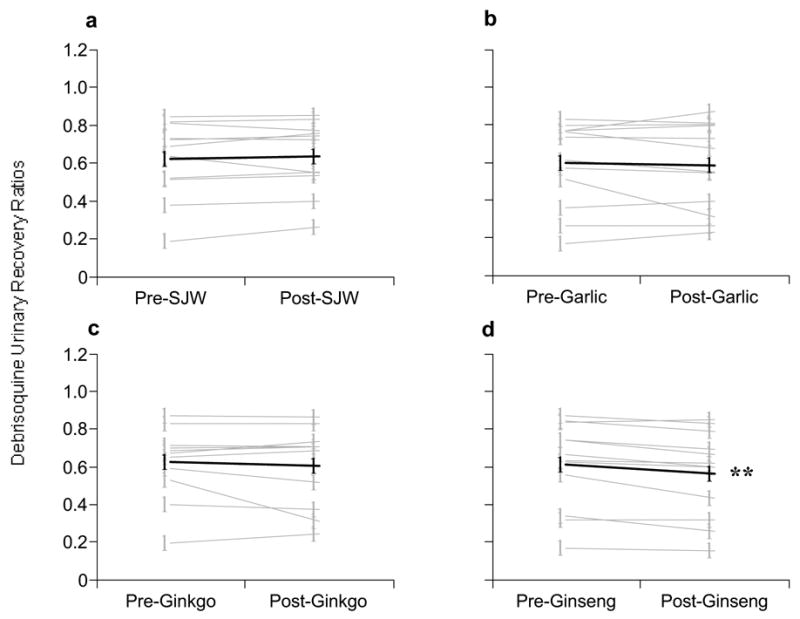
Comparison of pre- and post-supplementation phenotypic ratios (8-hour debrisoquine urinary recovery ratios) for CYP2D6. (A) St. John’s wort, (B) Garlic oil., (C) Ginkgo biloba, (D) Panax ginseng. Gray circles = individual values, Black circles = group means.
While moderate differences in pre- and post-supplementation paraxanthine/caffeine serum ratios were observed for specific individuals, no statistically significant differences in mean values were noted, suggesting that 28 days of supplementation had little effect on CYP1A2 activity (Fig 5, A–D).
Throughout the study period no statistically significant differences in mean baseline phenotypic ratios were observed for CYP1A2, CYP2D6, CYP2E1, or CYP3A4. In, addition, no sex-related changes in CYP phenotypes were noted for any of the supplements.
Phytochemical content
Table II depicts the content of various phytochemicals present in the supplements used for this study. Based on a hyperforin content of 5.34 mg/g, each subject ingested approximately 4.8 mg daily. Mean serum hyperforin concentrations determined on day 28, one hour after oral midazolam administration, were 51.3 ± 10.7 ng/mL. No significant difference in hyperforin serum concentration was noted between males (51.2 ± 10.4 ng/mL) and females (51.3 ± 12.1 ng/mL). Serum concentrations of phytochemicals present in the other supplements were not determined.
Table II.
Content of phytochemical marker compounds for botanical supplements.
| Supplement | Compound | Content (mg/capsule) | Daily Dose (mg) |
|---|---|---|---|
| St. John’s wort | Hyperforin | 1.60 | 4.80 |
| Adhyperforin | 0.25 | 0.75 | |
| Hypericin | 0.09 | 0.27 | |
| Pseudohypericin | 0.23 | 0.69 | |
| Quercetin | 2.15 | 6.45 | |
| Rutin | 2.09 | 6.27 | |
| Hyperoside | 1.45 | 4.35 | |
| Isoquercitrin | 1.38 | 4.14 | |
| Quercitrin | 0.98 | 2.94 | |
| Biapigenin | 0.27 | 0.81 | |
| Panax ginseng | Ginsenosides | ||
| Rb1 | 7.39 | 22.2 | |
| Rb2 | 6.8 | 20.4 | |
| Rg1 | 2.34 | 7.02 | |
| Rd | 2.04 | 6.12 | |
| Re | 1.50 | 4.50 | |
| Rc | 1.35 | 4.05 | |
| Rf | 0.94 | 2.82 | |
| Total | 22.4 | 67.1 | |
| Ginkgo biloba | Terpene Lactones | ||
| Bilobalide | 1.58 | 6.32 | |
| Ginkgolide A | 2.99 | 11.96 | |
| Ginkgolide B | 1.82 | 7.28 | |
| Ginkgolide C | 0.80 | 3.20 | |
| Ginkgolide J | 0.23 | 0.92 | |
| Total | 7.42 | 29.68 | |
| Flavonol glycosides | |||
| Quercetin | 14.90 | 59.60 | |
| Kaempferol | 8.67 | 34.68 | |
| Isorhamnetin | 2.36 | 9.44 | |
| Total | 25.93 | 103.72 | |
| Garlic oil | Sulfides | ||
| Allyl Methyl Sulfide | 0.03 | 0.09 | |
| Allyl Sulfide | 0.12 | 0.36 | |
| Methyl Propenyl Sulfide | 0.02 | 0.06 | |
| Diallyl Disulfide | 0.15 | 0.45 | |
| Allyl Methyl Trisulfide | 0.03 | 0.09 | |
| Allyl Trisulfide | 0.22 | 0.66 | |
| Isopropenyl Disulfde | 0.01 | 0.03 | |
| Diallyl Tetrasulfide | 0.04 | 0.12 | |
| Total | 0.62 | 1.86 |
DISCUSSION
The study provides further support that single time-point phenotypic ratios can provide a practical method for identifying herb-drug interactions that involve CYP modulation in vivo. Previously, this approach had been used to document CYP3A4 induction as evidenced by significant elevations in 1-hydroxymidazolam/midazolam ratios following St. John’s wort supplementation.[17] The single-time point phenotypic ratio approach confirmed what other investigators observed with St. John’s wort when more conventional “area-under-the-curve” methods were utilized to determine midazolam clearance.[16] The method also demonstrated that an absence of change in mean phenotypic ratios following botanical supplementation could be interpreted as a lack of effect on CYP activity. Such was the case with milk thistle, Citrus aurantium, Ginkgo biloba, Panax ginseng, and saw palmetto extracts[17,34]—the latter three examples being confirmed by other investigators using area-under-the-curve assessments.[18–21] More recently, this methodology has demonstrated that goldenseal supplementation inhibits CYP2D6 and CYP3A4 activity in vivo.[46] Thus, a range of herb-mediated effects on CYP activity (e.g. induction, inhibition, or no effect) can be differentiated with single time-point phenotypic ratios. It must be emphasized, however, that single-time point phenotypic ratios simply provide estimates of probe drug clearance. Yet, even with this limitation, the method’s distinct advantage lies in an ability to evaluate multiple CYP enzymes and multiple botanical supplements in vivo while using a limited blood-sampling scheme.
These findings are in close agreement with those reported previously for a cohort of young volunteers who underwent an identical study protocol.[17] The present results suggest that, like their younger counterparts, elderly subjects are equally susceptible to botanical-mediated changes in CYP activity, especially those involving St. John’s wort. A 141% increase in the mean phenotypic ratio following a 28-day course of St. John’s wort (Figure 2A) is a testament to the magnitude of CYP3A4 induction among elderly users of this botanical. A comparison of St. John’s wort’s effect on CYP3A4 phenotypic ratios for both young and elderly subjects is depicted in Figure 6. It is interesting to note the similarity in magnitude of the effect (98% in young versus 141% in elderly) despite a 2.5 fold difference in daily-administered hyperforin dose (12.2 mg in young versus 4.8 mg in elderly). Moreover, this discrepancy in hyperforin dosing yielded closely matching serum concentrations (42.6 ng/mL in young versus 51.2 ng/mL in elderly).
Figure 6.
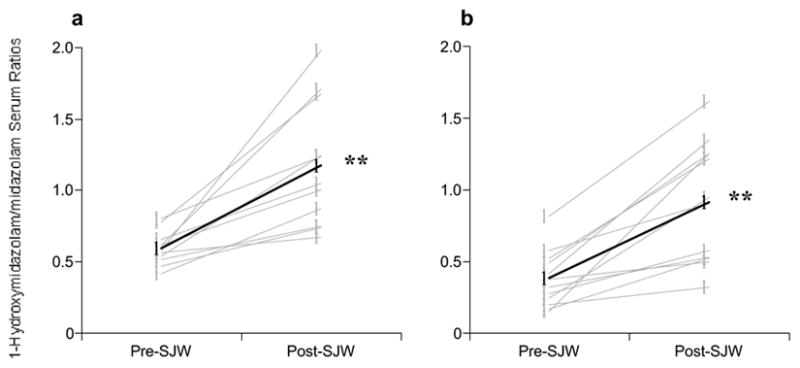
Effect of St. John’s wort on CYP3A4 phenotype in young (A) and elderly (B) subjects. Mean age for young subjects was 25 years, mean age for elderly subjects was 67 years. (See Gurley et al.17 for description of original study involving young volunteers.) HMDZ = 1-hydroxymidazolam, MDZ = midazolam. Gray circles = individual values, Black circles = group means. Asterisks = statistically significant difference from baseline. Reprinted with permission from Elsevier Press.
It is unknown whether hyperforin exhibits age-related differences in pharmacokinetics; however, similar serum concentrations between the two age groups, despite disparate doses, suggest that hyperforin clearance may be reduced in elderly subjects. Age-related reductions in the hepatic clearance of several medications have been noted previously.[28–30] Such changes are believed to result from reduced liver blood flow and/or diminished liver weight in the elderly, and not necessarily from alterations in CYP activity.[28–30] If such is the case with hyperforin, elderly consumers may be more vulnerable to the CYP3A4 inductive effects of St. John’s wort. In other words, relatively small doses of hyperforin may produce significant induction of CYP3A4 activity in elderly consumers. This is important since most St. John’s wort products do not provide a label claim for hyperforin content, but instead are standardized to another phytochemical, hypericin. Hypericin, although easily quantifiable, has no antidepressive activity or ability to induce CYP3A4. Moreover, St. John’s wort supplements standardized for hypericin exhibit considerable variability with regard to hyperforin content.[40,47–49] The practical implications of these findings would seem to render all St. John’s wort products as potent inducers of CYP3A4 in elderly patrons. Such a judgment is not unreasonable considering that hyperforin is a high-affinity ligand for the orphan nuclear receptor, SXR, and that activation of CYP3A4 can possibly occur at plasma concentrations as low as 15 ng/mL.[23]
In addition to its effect on CYP3A4, our data indicate that St. John’s wort appears to induce CYP2E1 in elderly subjects. This finding also corroborates the results of an earlier study in young individuals.[17] However, unlike the profound increase in CYP2E1 metabolic ratios reported for younger subjects, the magnitude of the effect was tempered in the elderly (110% increase in young versus 26% increase in elderly). A tempering of CYP2E1 induction could reflect individual variability in enzyme response or it may point toward an age-related reduction in CYP2E1 activity. Liver biopsies from surgical patients indicate that normal aging does not affect the activity of human CYP2E1,[50] however, other evidence suggests that CYP2E1 activity may decrease with age,[29,30] and that induction of the enzyme could also be affected by advanced age.[51,52] Unlike CYP3A4, CYP2E1 induction is not SXR-mediated; therefore, if hyperforin is the inducing agent then it must act through other mechanisms, perhaps as a ligand for other transcription factors or via posttranslational stabilization of the enzyme.[53] Other phytochemicals besides hyperforin may also be involved (see Table 2). Regardless of the induction mechanism, no interactions involving St. John’s wort and other CYP2E1 substrates have been reported. This lack of interaction may stem from inadequate reporting of suspected cases, or the paucity of orally administered drugs that are CYP2E1 substrates. Nevertheless, several inhalation anesthetics (e.g. enflurane, sevoflurane, methoxyflurane) are metabolized by CYP2E1 and their halogenated byproducts can produce liver injury.[54] Thus, a potential interaction may exist between these agents and St. John’s wort.
An almost 22% decrease in mean 6-hydroxychlorazoxazone/chlorzoxazone ratios suggests that CYP2E1 activity was inhibited by garlic oil. This was similar to the 40% reduction in CYP2E1 phenotype observed previously in young volunteers supplemented with garlic oil for 28 days.[17] Inhibition of human and murine CYP2E1 has been demonstrated for a variety of organosulfur compounds present in garlic preparations, particularly the allyl sulfides,[55–58] which are the chief constituents of steam distilled garlic oil products.[42] The difference in extent of inhibition between the two age groups hints at either an age-related diminution in CYP2E1 responsivity or a dissimilarity in allyl sulfide content between the garlic supplements. None of the other CYP enzymes (i.e., CYP1A2, CYP2D6, or CYP3A4) appeared to be affected by the garlic oil supplement, which corroborates our earlier findings in younger subjects. Taken together, our data suggests that garlic oil supplements pose a minimal risk for CYP-mediated herb-drug interactions.
A number of case reports have documented possible interactions between G. biloba and warfarin.[11,12] Such an interaction is particularly relevant to elderly patients on anticoagulant therapy. This interaction appears attributable to the inhibition of platelet activating factor by various ginkgolides.[59] A CYP-mediated explanation for the Ginkgo/warfarin interaction seems less plausible based on the present data. These current findings mirror those reported by our group in an earlier study of G. biloba supplementation in young adults.[17] In addition, our results corroborate those of Duche et al, who observed that 13 days of G. biloba extract administration to human volunteers had no effect on the pharmacokinetics of antipyrine, a non-specific probe of hepatic microsomal drug oxidation.[60] Furthermore, Markowitz et al noted that 14 days of G. biloba supplementation in normal volunteers did not affect the pharmacokinetics of the CYP2D6 substrate, dextromethorphan, or the CY3A4 substrate, alprazolam.[18] Taken together these findings indicate that herb-drug interactions attributable to G. biloba are not the result of phytochemical-mediated effects on CYP isoforms.
Like G. biloba, Panax ginseng appears to have little effect on the activities of the specific CYP isoforms investigated in this study. Although we observed a statistically significant decrease in debrisoquine urinary recovery ratios (~7%) after P. ginseng supplementation, this seemingly minor inhibitory effect on CYP2D6 is not likely to be clinically relevant. These findings too are analogous to those reported in young subjects supplemented with P. ginseng for either 14 or 28 days.[17,20] Therefore, P. ginseng supplementation seems unlikely to pose clinically significant interactions with drugs that are substrates for CYP1A2, CYP2D6, CYP2E1, or CYP3A4. Its effect on other prominent CYP isoforms, however, remains to be evaluated in vivo. Clinical evidence from warfarin patients hints at a possible interaction between ginseng species and CYP2C9 substrates. Janetzky reported a case involving a patient on warfarin therapy in which the International Normalized Ratio (INR) was reduced during P. ginseng supplementation.[61] More recently, a randomized trial involving 20 subjects receiving warfarin, demonstrated that 14 days of P. quinquefolius also reduced both INR values and warfarin plasma concentrations.[62] CYP2C9 is the principal isoform involved in the biotransformation of warfarin (more particularly the active enantiomer, S-warfarin) and the aforementioned reports hint strongly at a ginseng-mediated induction of this enzyme. The use of ginseng supplements, therefore, should be discouraged in patients on warfarin therapy.
CONCLUSION
Prescription drug use is greatest among elderly adults and recent surveys indicate that usage of botanical supplements is rapidly increasing among this population. Our data demonstrate that botanical dietary supplements can modulate drug metabolism and, in turn, adversely affect the pharmacokinetics of a variety of medications used in the elderly. Therefore, botanical supplements, when taken concomitantly with conventional medications, can give rise to serious herb-drug interactions. This may be especially true when multiple drugs and multiple supplements are ingested concurrently. St. John’s wort is particularly problematic on account of its ability to induce CYP3A4, an enzyme involved in the biotransformation of more than 50% of all prescription medications. Other supplements (e.g. ginseng species) may also pose a risk for pharmacokinetic herb-drug interactions in the elderly. Accordingly, health care providers should question their patients about dietary supplement use and discourage concomitant administration of botanical supplements with prescription medications.
Acknowledgments
Supported through grants from the NIH/NIA (RO3AG17733-01) and NIH/NCRR to the General Clinical Research Center of the University of Arkansas for Medical Sciences (M01RR14288).
The authors would like to acknowledge Dr. Ikhlas Khan (University of Mississippi, National Center for Natural Products Research), and Dr. Mark Roman and Brian Schaneberg (Chromadex, Inc., Clearwater, FL) for analysis of supplements containing Panax ginseng, Ginkgo biloba, and garlic oil.
The authors have no financial or other interests to disclose.
References
- 1.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 2.Klepser TB, Doucette WR, Horton MR, et al. Assessment of patient’s perceptions and beliefs regarding herbal therapies. Pharmacotherap. 2000;20:83–87. doi: 10.1592/phco.20.1.83.34658. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: A systematic review. Drugs. 2001;61:2163–2175. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ang-Lee MK, Moss J, Yuan C. Herbal medicines and perioperative care. JAMA. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 6.Brazier NC, Levine MAH. Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther. 2003;10:163–169. doi: 10.1097/00045391-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gurley BJ, Hagan DW. Herbal and dietary supplement interactions with drugs. In: McCabe BJ, Frankel EH, Wolfe JJ, editors. Handbook of Food-Drug Interactions. Boca Raton: CRC Press; 2003. pp. 259–293. [Google Scholar]
- 8.Nelson L, Perrone J. Herbal and alternative medicine. Emerg Med Clin North Am. 2000;18:709–722. doi: 10.1016/s0733-8627(05)70154-1. [DOI] [PubMed] [Google Scholar]
- 9.Gunther S, Patterson RE, Kristal AR, et al. Demographic and health-related correlates of herbal and specialty supplement use. J Am Diet Assoc. 2004;104:27–34. doi: 10.1016/j.jada.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Skinner CM, Rangasami J. Preoperative use of herbal medicines: a patient survey. Br J Anaesth. 2002;89:792–795. [PubMed] [Google Scholar]
- 11.Rhee SM, Garg VK, Hershey CO. Use of complementay and alternative medicines by ambulatory patients. Arch Intern Med. 2004;164:1004–1009. doi: 10.1001/archinte.164.9.1004. [DOI] [PubMed] [Google Scholar]
- 12.Adusumilli PS, Ben-Porat L, Pereira M, et al. The prevalence and predictors of herbal medicine use in surgical patients. J Am Coll Surg. 2004;198:583–590. doi: 10.1016/j.jamcollsurg.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Foster DF, Phillips RS, Hamel B. Alternative medicine use in older Americans. J Am Geriatr Soc. 2000;48:1560–1565. doi: 10.1111/j.1532-5415.2000.tb03864.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen RJ, Ek K, Pan CX. Complementary and alternative medicine (CAM) use by older adults: a comparison of self-report and physician chart documentation. J Gerontol. 2002;57A:M223–M227. doi: 10.1093/gerona/57.4.m223. [DOI] [PubMed] [Google Scholar]
- 15.Dergal JM, Gold JL, Laxer DA, et al. Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging. 2002;19:879–886. doi: 10.2165/00002512-200219110-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Gorski JC, Hamman MA, et al. The effects of St. John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317–326. [PubMed] [Google Scholar]
- 17.Gurley BJ, Gardner SF, Hubbard MA, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–282. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz JS, Donovan JL, DeVane CL, et al. Multiple-dose administration of Ginkgo biloba did not affect cytochrome P-450 2D6 or 3A4 activity in normal volunteers. J Clin Psychopharmacol. 2003;23:576–581. doi: 10.1097/01.jcp.0000095340.32154.c6. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz JS, DeVane CL, Chavin KD, et al. Effects of garlic (Allium sativum L.) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin Pharmacol Ther. 2003;74:170–177. doi: 10.1016/S0009-9236(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GD, Rosito G, Mohustsy MA, et al. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 21.Gorski JC, Huang S-M, Pinto A, et al. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Wentworth JM, Agostini M, Love J, et al. St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 23.Moore LB, Goodwin B, Jones SA, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Nat Acad Sci. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piscitelli SC, Burstein AH, Welden N, et al. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 25.Gold PE, Cahill L, Wenk GL. Sci Am. 2003. Apr, The lowdown on Ginkgo biloba; pp. 86–91. [DOI] [PubMed] [Google Scholar]
- 26.Kales HC, Blow FC, Welsh DE, et al. Herbal products and other supplements: use by elderly veterans with depression and dementia and their caregivers. J Geriatr Psychiatry Neurol. 2004;17:25–31. doi: 10.1177/0891988703261998. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GD, Rosito G, Mohustsy MA, et al. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 28.Kinirons MT, Crome P. Clinic pharmacokinetic considerations in the elderly. Clin Pharmacokinet. 1997;33:302–312. doi: 10.2165/00003088-199733040-00005. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka E. In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther. 1998;23:247–255. doi: 10.1046/j.1365-2710.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–184. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 31.Fuhr U, Rost KL. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics. 1994;4:109–116. doi: 10.1097/00008571-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Frye RF, Matzke GR, Adedoyin A, et al. Validation of the five-drug “Pittsburg cocktail” approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther. 1997;62:365–376. doi: 10.1016/S0009-9236(97)90114-4. [DOI] [PubMed] [Google Scholar]
- 33.Streetman DS, Bertino JS, Nafziger AN. Phenotyping of drug metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Gurley BJ, Gardner SF, Hubbard MA, et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Palmer JL, Scott RJ, Gibson A, et al. An interaction between cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A) Br J Clin Pharmacol. 2001;52:555–561. doi: 10.1046/j.0306-5251.2001.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland DT, Godfredsen KA, Page T, et al. Simple high performance liquid chromatography method for the simultaneous determination of serum caffeine and paraxanthine following rapid sample preparation. J Chromatogr (B) 1998;707:105–110. doi: 10.1016/s0378-4347(97)00590-2. [DOI] [PubMed] [Google Scholar]
- 37.Frye RF, Stiff DD. Determination of chlorzoxazone and 6-hydroxychlorzoxazone in human plasma and urine by high-performance liquid chromatography. J Chromatogr (B) 1996;686:291–296. doi: 10.1016/s0378-4347(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 38.Frye RF, Branch RA. Improved high-performance liquid chromatographic determination of debrisoquine and 4-hydroxydebrisoquin in human urine following direct injection. J Chromatogr (B) 1996;677:178–182. doi: 10.1016/0378-4347(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 39.Sautou V, Chopineau J, Terrisse MP, et al. Solid-phase extraction of midazolam and two of its metabolites from plasma for high performance liqui chromatographic analysis. J Chromatogr (B) 1991;571:298–304. doi: 10.1016/0378-4347(91)80459-p. [DOI] [PubMed] [Google Scholar]
- 40.Liu FF, Ang CYW, Heinze TM, et al. Evaluation of major active components in St. John’s wort dietary supplements by high-performance liquid chromatography with photodiode array detection and electrospray mass spectrometric confirmation. J Chromatogr (A) 2000;888:85–92. doi: 10.1016/s0021-9673(00)00555-0. [DOI] [PubMed] [Google Scholar]
- 41.Cui Y, Gurley B, Ang CYW, et al. Determination of hyperforin in human plasma using solid-phase extraction and high-performance liquid chromatography with ultraviolet detection. J Chromatogr (B) 2002;780:129–135. doi: 10.1016/s1570-0232(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 42.Lawson L, Wang Z, Hughes BG. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 1991;57:363–370. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Fitzloff JF. Simultaneous determination of terpene lactones and flavonoid aglycones in Ginkgo biloba by high-performance liquid chromatography with evaporative light scattering detection. J Pharm Biomed Anal. 2002;30:67–75. doi: 10.1016/s0731-7085(02)00201-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhu B, Ou-Yang DS, Cheng Z, et al. Single plasma sampling to predict oral clearance of CYP3A probe midazolam. Acta Pharmacol Sinica. 2001;22:634–638. [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates Publishers; 1988. pp. 19–42. [Google Scholar]
- 46.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4 phenotypes. Clin Pharmacol Ther. 2005 doi: 10.1016/j.clpt.2005.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Los Reyes GC, Koda RT. Determining hyperforin and hypericin content in eight brands of St. John’s wort. Am J Health-Syst Pharm. 2002;59:545–547. doi: 10.1093/ajhp/59.6.545. [DOI] [PubMed] [Google Scholar]
- 48.Ganzera M, Zhao J, Khan IA. Hypericum perforatum—chemical profiling and quantitative results of St. John’s wort products by an improved high-erformance liquid chromatography method. J Pharm Sci. 2002;91:623–630. doi: 10.1002/jps.10057. [DOI] [PubMed] [Google Scholar]
- 49.Wurglics M, Westerhoff K, Kaunzinger A, et al. Batch-to-batch reproducibility of St. John’s wort preparations. Pharmacopsychiatry. 2001;34:S152–S156. doi: 10.1055/s-2001-15453. [DOI] [PubMed] [Google Scholar]
- 50.Hunt CM, Strater S, Stave GM. Effect of normal aging on the activity of human hepatic cytochrome P450IIE1. Biochem Pharmacol. 1990;40:1666–1669. doi: 10.1016/0006-2952(90)90470-6. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima T, Wang RS. Induction of cytochrome P450 by toluene. Int J Biochem. 1994;26:1333–1340. doi: 10.1016/0020-711x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 52.Koop DR, Tierney DJ. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. Bioessays. 1990;12:429–435. doi: 10.1002/bies.950120906. [DOI] [PubMed] [Google Scholar]
- 53.Chien JY, Thummel KE, Slattery JT. Pharmacokinetic consequences of induction of CYP2E1 by ligand stabilization. Drug Metab Dispos. 1997;25:1165–1175. [PubMed] [Google Scholar]
- 54.Kenna JG, Jones RM. The organ toxicity of inhaled anesthetics. Anesth Analg. 1995;81:S51–S66. doi: 10.1097/00000539-199512001-00008. [DOI] [PubMed] [Google Scholar]
- 55.Loizou GD, Crocker J. The effects of alcohol and diallyl sulphide on CYP2E1 activity in humans: a phenotyping study using chlorzoxazone. Hum Exp Toxicol. 2001;20:321–327. doi: 10.1191/096032701680350587. [DOI] [PubMed] [Google Scholar]
- 56.Haber D, Siess M, Canivenc-Lavier M, et al. Differential effects of dietary diallyl sulfide and diallyl disulfide on rat intestinal and hepatic drug-metabolizing enzymes. J Toxicol Environ Health. 1995;44:423–434. doi: 10.1080/15287399509531971. [DOI] [PubMed] [Google Scholar]
- 57.Siess M, Le Bon A, Canivenc-Lavier M, et al. Modification of hepatic drug-metabolizing enzymes in rats treated with alkyl sulfides. Cancer Lett. 1997;120:195–201. doi: 10.1016/s0304-3835(97)00309-1. [DOI] [PubMed] [Google Scholar]
- 58.Yang CS, Chhabra SK, Hong J, et al. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr. 2001;131:1041S–1045S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- 59.Chung KF, Dent G, McCuster M, Guinot P, Page CP, Barnes PJ. Effect of ginkgolide mixture (BN 52063) in antagonizing skin and platelet responses to platelet activating factor in man. Lancet. 1987;1:248–251. doi: 10.1016/s0140-6736(87)90066-3. [DOI] [PubMed] [Google Scholar]
- 60.Duche JC, Barre J, Guinot P, Duchier J, Cournot A, Tillement JP. Effect of Ginkgo biloba extract on microsomal enzyme induction. Int J Clin Pharm Res. 1989;9:165–168. [PubMed] [Google Scholar]
- 61.Janetzky K, Morreale AP. Probable interaction between warfarin and ginseng. Am J Health-Syst Pharm. 1997;54:692–693. doi: 10.1093/ajhp/54.6.692. [DOI] [PubMed] [Google Scholar]
- 62.Yuan C-S, Wei G, Dey L, Karrison T, et al. American ginseng reduces warfarin’s effect in healthy patients: a randomized controlled trial. Ann Intern Med. 2004;141:23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]


