Abstract
Increased frequency of cavum septum pellucidum (CSP) has been inconsistently observed in schizophrenia, and little is known about its functional implications. We investigated whether patients with schizophrenia were more likely than healthy controls to have CSP, and among patients assessed the relationship between CSP, psychiatric symptoms, and selected neuropsychological functions. Seventy-seven patients with diagnoses of DSM-IV schizophrenia spectrum disorders and 55 healthy controls were studied and completed a 1.5 T MRI scan. Two raters, blind to group membership, determined the presence, length and grade of the CSP. A subset of participants also underwent neuropsychological testing. A CSP of at least 1 mm in length was present in 68.8% of patients and 76.4% of controls, and the groups did not differ significantly with respect to presence or absence, length, overall size, or percent with an abnormally large CSP (≥ 6 mm). Patients with an abnormally large CSP demonstrated poorer performance on measures of verbal learning and memory than patients with smaller CSP. Among patients, CSP length was significantly correlated with negative symptoms, verbal learning, and sentence comprehension. Among patients with abnormally large CSP, CSP length was correlated with reaction time on two conditions of a Continuous Performance Test. CSP, while prevalent, was not more frequent in our sample of patients with schizophrenia, and had few associations with symptom severity or neuropsychological deficits.
Keywords: Schizophrenia, MRI, Cavum Septum Pellucidum, Neuropsychology
1. Introduction
Cavum septum pellucidum (CSP), a space between the two leaflets of the septi pellucidi, is thought to be a neurodevelopmental anomaly, and its presence may be consistent with neurodevelopmental theories of schizophrenia. This structure is closely linked developmentally to the limbic system, which has been implicated in the etiology of the disorder. Increased prevalence of CSP in patients with schizophrenia spectrum disorder has, however, been inconsistently observed since it was originally described by DeGreef et al. ( 1992a, 1992b). Presence of CSP in patients with schizophrenia has been noted in female patients (Galarza et al., 2004), male patients (Uematsu and Kaiya, 1989), chronic patients (DeGreef et al., 1992a; DeLisi et al., 1993; Kwon et al., 1998; Scott et al., 1993), first episode patients (DeGreef et al., 1992b; Kasai et al., 2004; Kwon et al., 1998; Snyder et al., 1998), as well as childhood onset schizophrenia (Nopoulos et al., 1998), with prevalence rates ranging from 15% to 58% prevalence in patients and 2% to 30% in controls, in studies reporting a significant group difference. Studies that have not found a significant difference in frequency of CSP between patients with schizophrenia and healthy controls report variable prevalence rates of 18 – 88% in both groups (Fukuzako et al., 1996; Hagino et al., 2001; Jurjus et al., 1993; Kwon et al., 1998; Nopoulos et al., 1997).
One potential contributor to the variability seen across studies is the manner in which CSP has been measured. This has included defining CSP as being present if it is identified on at least one MRI slice (about 1 mm to 1.5 mm thick), using a grading system to determine overall size based on consideration of length and width, and considering a CSP abnormally large if it is greater than or equal to 6 mm in size. Using the latter definition, results have been mixed, with some groups finding an increased prevalence of large CSP in patients with schizophrenia (Kwon et al., 1998; Nopoulos et al., 1997), and another noting no differences between patients and healthy controls (Hagino et al., 2001). Of note, in the Nopoulos et al. (1997) study, all patients with large CSP were male. In another study, Nopoulos et al. (1996) found a greater right > left asymmetry and volume decrement in the left temporal lobe in patients with a large CSP, and decreased total brain, frontal and temporal lobe volumes in those without a large CSP.
Little is known about the functional implication of CSP. Studies have attempted to identify relationships between clinical and demographic variables (Jurjus et al., 1993; Mathew et al., 1985; Shioiri et al., 1996), symptoms (Mathew et al., 1985; Nopoulos et al., 2000), duration of illness (Fukuzako et al., 1996; Mathew et al., 1985), family history of illness (Uematsu and Kaiya, 1989), intellectual functioning in patients with large CSP (Nopoulos et al., 2000), and other brain morphometric changes (DeLisi et al., 1993; Galarza et al., 2004; Mathew et al., 1985; Rajarethinam et al., 2001). While findings have generally not supported relationships between these variables and presence of CSP (DeLisi et al., 1993; Galarza et al., 2004; Jurjus et al., 1993; Rajarethinam et al., 2001; Shioiri et al., 1996), Uematsu & Kaiya (Uematsu and Kaiya, 1989) reported that CSP was significantly related to family history of schizophrenia, Nopoulos et al. (2000) reported a significant inverse relationship between size of CSP and IQ scores, and Mathew et al. (1985) reported a significant correlation between age and septal area, and between duration of illness and septal area. Although the overall incidence of CSP between patients and controls was not found to differ, Fukuzako et al. (1996) noted that patients with a history of long-term institutionalization had a significantly higher incidence of CSP than those who had spent less than three years in hospital.
The present study examined whether CSP was more common in our relatively large sample of patients with schizophrenia as compared to healthy controls. Specifically, we evaluated CSP using several criteria employed in prior studies including presence on a single MRI scan slice, length of at least six millimeters (i.e., “abnormally large” CSP), as well as overall size of the CSP using a published grading system (Kwon et. Al., 1998). By analyzing these three features, we sought to determine whether variability in the literature was due at least in part to measurement issues. We also examined whether a relationship could be identified between the CSP length or grade and clinical symptoms or neuropsychological test performance.
2. Methods
2.1. Subjects
Subjects included 77 patients with schizophrenia (N=57), schizoaffective disorder (N=17), or psychosis NOS (N=3), diagnosed using the Structured Clinical Interview for DSM-IV (American Psychiatric Association, 1994) and 55 healthy controls. Controls with any Axis I diagnosis, and patients with a comorbid Axis I diagnosis were excluded from participation. Any participant with a history of neurological illness, head injury with loss of consciousness, systemic illness with potential cognitive sequelae, or current substance abuse or past substance dependence history was also excluded. Written informed consent was obtained from all participants prior to inclusion in the study.
2.2. Procedure
All participants completed an MRI scan for evaluation of the CSP. Subsets of these participants completed clinical symptom measures and a battery of neuropsychological tests.
2.2.1. Clinical Assessment
Patients were assessed with several clinical symptom measures. Positive and negative symptom severity was assessed using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984) and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1983). The Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1988) was used to assess the level of overall psychopathology severity.
2.2.2. Neuropsychological Assessment
The neuropsychological tests discussed below were administered as part of a comprehensive assessment in a single session of approximately three and a half hours duration. The tests were administered by an examiner who was unaware of the patient’s scores on clinical or CSP measures. Executive functions were assessed using the Wisconsin Card Sorting Test (WCST) categories completed and number of perseverative errors (Heaton et al., 1993). The California Verbal Learning Test II (CVLT-II) was administered to assess verbal learning and memory (Delis et al., 1987). Subjects were presented a list of 16 words and were required to recall as many words as possible. This was repeated for a total of five trials (CVLT-II, trials 1–5). Subjects were then required to recall as many words as possible from memory following the presentation of an interference list of 16 new words and again after a twenty minute delay (CVLT-II, 20’ delayed recall). The Thumb Finger Sequencing Test was administered to assess fine motor sequencing speed. Participants were asked to touch each finger to their thumb as many times as possible using their dominant, and then their nondominant hand. Two trials were completed for each hand, and the highest score for each hand was retained. Sentence Comprehension from the Boston Diagnostic Aphasia Examination and the Controlled Oral Word Fluency Test were used to assess expressive language ability. For Sentence Comprehension, participants are asked a series of yes/no questions; each question is rephrased and asked a second time, requiring both a correct yes and no response for the item to be scored correctly. Total number of correct items was used. For the Continuous Performance Test (CPT), there are three conditions. In the Simple Reaction Time condition, participants are required to press a large button on the CPT box every time they see a number 9 in the center of the screen. In the Vigilance and Distractibility conditions, they are asked to press the button every time they see the number nine directly after they see the number 1 in the center of the screen; in the Distractibility condition, they are also presented with numbers simultaneously to the left and right of the center. Accuracy (30 possible correct, adjusted for omissions and commissions) and reaction time are used.
2.2.3. Structural MRI
Whole brain MRI scans were obtained with a 1.5 Tesla General Electric (Milwaukee, Wis.) magnet using a three-dimensional spoiled gradient recall acquisition (SPGR; TE=13 msec, TR=38 msec, flip angle=45°, number of excitations=1), which yielded a series of 124 contiguous, 1.5-mm coronal slices with an in-plane field of view of 24 cm and a 256 x 256 matrix. Images are then realigned and resampled to 1 mm using BRAINS so that all brains are oriented similarly. CSP Ratings: Two raters, blind to group membership, determined the presence (on at least one 1 mm coronal slice), length (total number of contiguous slices on which it was present) (Nopoulos et al., 1997), and grade of the CSP (DeGreef et al., 1992b; Kim and Peterson, 2003; Kwon et al., 1998). The grade measure is based on consideration of length, width, and overall size, and ranges from 0 (absent) to 4 (very enlarged; see Figure 1). For 47 random cases, reviewed by two individual raters (H.B.C. and H.S.P.), the intraclass correlation for interrater reliability was calculated to be .98 for number of slices, and .93 for grade. Inter-rater disagreement was resolved through consensus.
Figure 1.
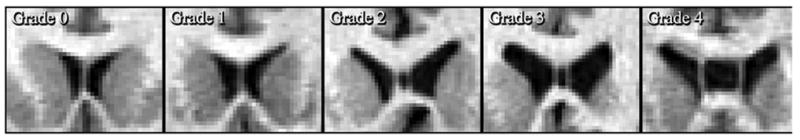
Illustrative examples of the different grades of the cavum septum pellucidum (CSP), ranging from Grade 0 (No CSP) to Grade 4 (Severe CSP). These figures are based on the grading system developed by DeGreef et al. (DeGreef et al., 1992b), using their definitions of severity.
2.3. Data Analysis
Group differences in demographic characteristics were analyzed using analysis of variance (ANOVA) and nonparametric statistics, as appropriate. The relationships between CSP length (in millimeters) and grade, and clinical and cognitive variables were analyzed using Spearman correlations. Multiple analysis of variance (MANOVA) using Wilks’ Lambda was used to determine whether there was an overall group difference in neuropsychological functioning. MANOVA was followed by univariate ANOVA’s to evaluate group differences on the individual neuropsychological test scores. Significant ANOVA’s were followed by post-hoc analyses using Tukey’s least significant difference (LSD) test, permitting identification of specific group differences while controlling for potential Type I error. All tests used two-tailed comparisons with significance level set at p < .05. All statistical analyses were performed using either SAS (version 8) or SPSS (version 11).
3. Results
3.1. Demographic and Clinical Characteristics
Table 1 presents demographic and clinical characteristics of the patient and comparison groups. The groups were balanced for age, sex composition, and handedness, although as expected the controls demonstrated a higher estimated baseline level of intellectual functioning based on the Reading subtest from the Wide Range Achievement Test (Wilkinson, 1993). Nonetheless, estimated baseline intellect was within the average range for both groups. Descriptive statistics and results of statistical analyses for the neuropsychological variables are presented in Table 2. Healthy controls performed better than patients with schizophrenia on all neuropsychological domains assessed. All group differences remained significant when data were analyzed using WRAT-3 Reading score as a covariate.
Table 1.
Demographic/Clinical Characteristics for Patients with Schizophrenia Spectrum Disorder and Healthy Controls
| Variable | Patients (N=77) | Controls (N=55) | Analysis | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | t | P | |
| Age, years | 34.3 | 10.5 | 32.7 | 11.0 | 0.86 | 0.39 |
| WRAT Reading | 98.0 | 13.1 | 108.6 | 9.6 | 5.23 | 0.0001 |
| SAPS, total | 8.0 | 4.5 | ||||
| SANS, total | 9.7 | 4.5 | ||||
| BPRS, total | 46.5 | 11.1 | ||||
| N | % | N | % | χ2 | p | |
| Sex, male | 57 | 74.0 | 32 | 58.2 | 3.67 | 0.06 |
| Right-handed | 70 | 90.9 | 52 | 94.6 | 1.02 | 0.60 |
Abbreviations: SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; WRAT = Wide Range Achievement Test, 3rd edition.
Table 2.
Neuropsychological Performance for Patients with Schizophrenia Spectrum Disorder and Healthy Controls
| Variable | Patients | Controls | Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | S.D. | N | Mean | S.D. | t | P< | |
| Intellectual abilities | ||||||||
| WAIS-III Digit Span | 65 | 9.0 | 2.9 | 51 | 11.5 | 2.9 | 4.57 | 0.0001 |
| WAIS-III Digit Symbol | 70 | 7.0 | 2.6 | 53 | 11.5 | 3.1 | 8.82 | 0.0001 |
| Executive functions | ||||||||
| WCST, categories | 69 | 4.6 | 2.0 | 52 | 5.4 | 1.4 | 2.58 | 0.01 |
| WCST, perseverative errors | 69 | 16.0 | 12.0 | 52 | 11.2 | 9.6 | 2.39 | 0.02 |
| Verbal memory | ||||||||
| CVLT-II, trials 1–5 | 69 | 43.1 | 12.7 | 54 | 58.6 | 7.3 | 8.52 | 0.0001 |
| CVLT-II, short delayed recall | 69 | 8.6 | 3.5 | 54 | 12.6 | 2.3 | 7.55 | 0.0001 |
| CVLT-II, 20’ delayed recall | 69 | 9.1 | 3.7 | 54 | 13.0 | 2.1 | 7.42 | 0.0001 |
| Psychomotor speed: Thumb | ||||||||
| Finger Sequencing | ||||||||
| Right hand | 69 | 8.3 | 1.9 | 44 | 9.8 | 1.5 | 4.16 | 0.0001 |
| Left hand | 69 | 8.1 | 2.0 | 44 | 9.2 | 1.6 | 3.18 | 0.002 |
| Expressive language | ||||||||
| Sentence Comprehension | 69 | 10.3 | 1.9 | 44 | 11.4 | 0.8 | 4.35 | 0.0001 |
| Attention/Concentration: CPT | ||||||||
| Simple Reaction Time, Accuracy | 63 | 28.5 | 3.2 | 47 | 29.1 | 1.4 | 1.28 | 0.20 |
| Simple Reaction Time, RT | 63 | 348.8 | 94.7 | 47 | 300.6 | 76.0 | 2.87 | 0.005 |
| Vigilance, Accuracy | 63 | 26.8 | 5.5 | 47 | 29.0 | 2.3 | 2.78 | 0.007 |
| Vigilance, RT | 63 | 438.0 | 91.8 | 47 | 378.8 | 71.4 | 3.66 | 0.0004 |
| Distractibility, Accuracy | 59 | 20.3 | 11.6 | 47 | 27.4 | 2.7 | 4.56 | 0.0001 |
| Distractibility, RT | 59 | 474.0 | 90.8 | 47 | 403.9 | 67.1 | 4.56 | 0.0001 |
Abbreviations: WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; WCST, Wisconsin Card Sorting Test; CVLT-II, California Verbal Learning Test, Second edition; RT, Reaction Time (in milliseconds), CPT, Continuous Performance Test.
3.2. Cavum Septum Pellucidum
As can be seen in Table 3, there was no significant difference between patients with schizophrenia and healthy controls in terms of CSP length, presence of CSP on at least one slice, or grade. Patients identified with abnormally large CSP did not differ from the other group in terms of percent diagnosed with schizophrenia (81.8% in abnormally large CSP group vs. 72.7%) or in terms of percent receiving typical antipsychotic medications (18.2 % in abnormally large CSP group vs. 19.6%).
Table 3.
Cavum septum pellucidum data for patients with schizophrenia and healthy controls
| Variable | Patients (N=77) | Controls (N=55) | Analysis | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | t | P | |
| # slices in which CSP appears | 3.3 | 4.8 | 2.8 | 2.1 | 0.78 | 0.44 |
| N | % | N | % | χ2 | P | |
| % with large CSP (≥ 6 one mm slices) | 11 | 14.3 | 5 | 9.1 | 0.81 | 0.37 |
| CSP present (1 one mm slice minimum) | 53 | 68.8 | 42 | 76.4 | 0.90 | 0.34 |
| Grade: | ||||||
| 0 (absent) | 24 | 31.2 | 13 | 23.6 | ||
| 1 (questionable/equivocal) | 19 | 24.7 | 21 | 38.2 | ||
| 2 (mild) | 19 | 24.7 | 12 | 21.8 | ||
| 3 (moderate) | 6 | 7.8 | 7 | 12.7 | ||
| 4 (large) | 9 | 11.7 | 2 | 3.6 | χ2 for grade: 5.98 | 0.20 |
3.3. Neuropsychological Functioning
Descriptive statistics and results of statistical analyses for the neuropsychological variables are presented in Table 4. We identified two groups of patients: those with an abnormally large CSP (≥ 6 mm; 9–10 subjects), and those with no or minimal CSP (≤ 2 mm; 28–31 subjects). Patients with an abnormally large CSP tended to have slightly higher clinical symptom ratings and score more poorly on neuropsychological tests than did the other group. However, the only significantly different performance between the groups was on indices of the CVLT-II; those with an abnormally large CSP scored significantly worse on CVLT acquisition (trials 1–5), short delayed free recall and long delayed free recall. Trends were noted (p = .06–.08) for the group with abnormally large CSP to have more negative symptoms, more perseverative errors on the WCST, and be less accurate on the vigilance condition of the Continuous Performance Test than the other patients with schizophrenia.
Table 4.
Clinical Descriptors and neuropsychological performance for patients with schizophrenia spectrum disorder with (≥ 6 mm) and without (≤ 2 mm) abnormally large CSP
| Variable | With Large CSP | Without Large CSP | Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | S.D. | N | Mean | S.D. | t | P< | |
| Clinical Variables | ||||||||
| SAPS, total | 9 | 9.7 | 3.0 | 29 | 8.2 | 4.5 | 0.91 | 0.37 |
| SANS, total | 9 | 11.8 | 2.9 | 29 | 8.6 | 4.7 | 1.92 | 0.06 |
| BPRS, total | 9 | 50.3 | 9.9 | 29 | 45.6 | 10.4 | 1.19 | 0.24 |
| Intellectual abilities | ||||||||
| WAIS-III Digit Span | 9 | 8.2 | 3.3 | 30 | 9.7 | 2.0 | 1.20 | 0.24 |
| WAIS-III Digit Symbol | 10 | 7.0 | 2.9 | 31 | 6.9 | 3.1 | 0.08 | 0.94 |
| Executive functions | ||||||||
| WCST, categories | 10 | 3.4 | 2.3 | 30 | 4.6 | 1.9 | 1.69 | 0.10 |
| WCST, perseverative errors | 10 | 23.0 | 10.8 | 30 | 15.5 | 11.7 | 1.78 | 0.08 |
| Verbal memory | ||||||||
| CVLT-II, trials 1–5 | 10 | 32.7 | 10.0 | 30 | 45.9 | 11.7 | 3.20 | 0.003 |
| CVLT-II, short delayed recall | 10 | 6.6 | 3.1 | 30 | 9.4 | 3.3 | 2.32 | 0.03 |
| CVLT-II, 20’ delayed recall | 10 | 6.6 | 3.3 | 30 | 9.8 | 3.1 | 2.52 | 0.02 |
| Psychomotor speed: Thumb | ||||||||
| Finger Sequencing | ||||||||
| Right hand | 10 | 7.9 | 2.6 | 30 | 8.5 | 1.4 | 0.70 | 0.50 |
| Left hand | 10 | 7.8 | 2.5 | 30 | 8.3 | 1.7 | 0.66 | 0.51 |
| Expressive language | ||||||||
| Sentence Comprehension | 10 | 10.1 | 1.2 | 30 | 10.7 | 1.8 | 1.01 | 0.32 |
| Attention/Concentration: CPT | ||||||||
| Simple Reaction Time, Accuracy | 9 | 29.6 | 0.7 | 28 | 28.4 | 3.5 | 1.63 | 0.11 |
| Simple Reaction Time, RT | 9 | 315.2 | 46.9 | 28 | 339.2 | 90.5 | 0.76 | 0.45 |
| Vigilance, Accuracy | 9 | 23.1 | 6.8 | 28 | 27.3 | 5.0 | 2.00 | 0.06 |
| Vigilance, RT | 9 | 423.0 | 77.8 | 28 | 453.8 | 100.1 | 0.95 | 0.35 |
| Distractibility, Accuracy | 9 | 11.7 | 13.7 | 26 | 16.2 | 12.1 | 1.04 | 0.30 |
| Distractibility, RT | 9 | 476.3 | 128.7 | 26 | 472.4 | 73.6 | 0.09 | 0.93 |
Abbreviations: WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; WCST, Wisconsin Card Sorting Test; CVLT-II, California Verbal Learning Test, Second edition; RT, Reaction Time (in milliseconds), CPT, Continuous Performance Test.
Analyses were replicated using the same group with abnormally large CSP, and all other patients (i.e., CSP of 5 mm or less), and results only differed in that the SANS total difference, WCST perseverative error difference, and vigilance accuracy score, all trends in the initial analysis, became significant. Given the large discrepancy in group sizes (9 or 10 with abnormally large CSP vs. 54–60 without abnormally large CSP), we chose to present the more conservative results. When controls with and without an abnormally large CSP were compared on neuropsychological measures, no significant differences were seen.
3.4. Correlation of Cavum Septum Pellucidum with Other Variables
Table 5 presents correlations between CSP variables (i.e., CSP length and grade), clinical symptoms, and neuropsychological performance in subgroups of the patients with schizophrenia. For 65 patients with schizophrenia on whom data was available, there were no significant correlations between BPRS total score or total SAPS score and either measure of CSP. Total SANS score was positively correlated with the length of the CSP. Further, for the patients with schizophrenia on whom neuropsychological data was available (see table for specific Ns, range: 63–70 participants), there were no significant correlations between test performance and CSP grade. Number of slices showing CSP was negatively correlated with CVLT-II acquisition and performance on a test of sentence comprehension. When correlations were examined in the very small subset of patients demonstrating an abnormally large CSP, no significant correlations were seen between symptoms or test performance and CSP grade. There was a significant positive correlation between reaction time for the simple (r = 0.71, p = .03) and vigilance (r = 0.78, p = .01) conditions of the CPT and length of the CSP. Finally, when correlations were examined in healthy controls for whom neuropsychological data was available (N: 44–54 participants), there was a significant negative correlation between reaction time for the simple condition of the CPT (r = −0.29, p = .05) and length, while no significant correlations were found for CSP grade.
Table 5.
Spearman correlations between CSP variables and clinical symptoms and neuropsychological test scores in patients with schizophrenia spectrum disorder
| Variable | N | CSP Slices | P | CSP Grade | P |
|---|---|---|---|---|---|
| Clinical Symptoms | |||||
| BPRS Total Score | 65 | 0.22 | 0.08 | 0.15 | 0.25 |
| SANS Total Score | 65 | 0.26 | 0.03 | 0.10 | 0.42 |
| SAPS Total Score | 65 | 0.10 | 0.42 | 0.12 | 0.32 |
| Neuropsychological Performance | |||||
| WAIS-III Digit Span | 65 | −0.13 | 0.28 | −0.10 | 0.41 |
| WAIS-III Digit Symbol | 70 | −0.05 | 0.66 | −0.08 | 0.52 |
| WCST, categories | 69 | −0.16 | 0.20 | −0.07 | 0.57 |
| WCST, perseverative errors | 69 | 0.21 | 0.08 | 0.20 | 0.09 |
| CVLT-II, trials 1–5 | 69 | −0.30 | 0.01 | −0.21 | 0.08 |
| CVLT-II, 20’ delayed recall | 69 | −0.22 | 0.07 | −0.15 | 0.21 |
| Right hand thumb finger sequencing | 69 | −0.03 | 0.83 | 0.01 | 0.93 |
| Left hand thumb finger sequencing | 69 | −0.07 | 0.55 | 0.03 | 0.82 |
| Sentence Comprehension | 69 | −0.27 | 0.03 | −0.18 | 0.14 |
| Continuous Performance Test
Simple Reaction Time, Accuracy Simple Reaction Time, RT Vigilance, Accuracy |
63 63 63 |
0.04 0.10 −0.09 |
0.77 0.45 0.47 |
0.11 0.10 −0.11 |
0.38 0.44 0.38 |
| Vigilance, RT | 63 | −0.04 | 0.78 | −0.01 | 0.91 |
| Distractibility, Accuracy | 59 | −0.15 | 0.25 | −0.13 | 0.32 |
| Distractibility, RT | 59 | 0.09 | 0.51 | 0.05 | 0.71 |
Abbreviations: WAIS-III, Wechsler Adult Intelligence Scale, 3rd edition; WCST, Wisconsin Card Sorting Test; CVLT-II, California Verbal Learning Test, 2nd edition; RT, Reaction Time (in milliseconds).
4. Discussion
The literature to date has been inconsistent in establishing whether presence or enlargement of the CSP has an increased association with schizophrenia, and in identifying its functional significance. Examination of this structure has been of particular interest in schizophrenia, given the potential role of the CSP as a marker of early developmental neuropathologic changes, its close relationship to the limbic system, and its association with a broad range of developmental problems, including mental retardation, developmental delay, seizures, and macro/microcephaly (Bodensteiner and Schaefer, 1990; Bodensteiner et al., 1998; Schaefer et al., 1994). The current study failed to find a significant increase in presence of CSP or enlargement of CSP between patients with schizophrenia spectrum disorder and healthy controls. No relationships between CSP grade and any variable examined were found. Examination of associations between CSP variables (i.e., length and grade) in patients and controls indicated a significant positive correlation between reaction time for the simple and vigilance conditions of the CPT and length of the CSP for patients, and a significant negative correlation between reaction time for the simple condition of the CPT and CSP length in healthy controls. When patients with schizophrenia spectrum disorder with and without an abnormally large CSP were compared, those with an abnormally large CSP tended to have slightly higher clinical symptom ratings and score more poorly on neuropsychological tests. The only statistically significantly difference between groups was on indices of the CVLT-II.
CSP has been reported in 58–85% of healthy subjects, using 1.5 mm contiguous slices (Kwon et al., 1998; Nopoulos et al., 1997). That 76% of our healthy subjects had a CSP of at least 1 mm in length is consistent with this finding. In the two studies above, CSP were reported in 77% and 59% of patients with schizophrenia, respectively, while our study found 69% of the patients with schizophrenia to have a CSP of at least 1 mm in length. A number of different ways of characterizing abnormal CSP have been examined, including simply presence of CSP, number of contiguous slices on which the CSP is found, grade (i.e., overall size) of CSP, and classification of CSP as abnormally “large” (i.e., greater or equal to 6 mm). We used all of the above-described methods to examine CSP in this paper, and none of them resulted in a significant difference between healthy controls and patients with schizophrenia. Using the distinction of abnormally large CSP, as defined by Kwon et al (Kwon et al., 1998), 14.3% of patients with schizophrenia and 9.1% of healthy controls met criteria; looking at the highest grade (i.e., 4) of CSP revealed an incidence of 11.7% in patients with schizophrenia vs. 3.6% of healthy controls. In general, these prevalence rates are lower than those reported in other studies of schizophrenia (30–32% patients vs. 7–10% controls) (DeGreef et al., 1992a, 1992b; DeLisi et al., 1993; Kwon et al., 1998; Nopoulos et al., 1997).
The reason for the discrepancies between studies with respect to CSP prevalence and correlates in schizophrenia remains unclear, although the present study suggests that the way in which CSP is measured may be contributory. In our patient sample, no relationship was found between any demographic, clinical or cognitive variable and grade of CSP, despite correlations with CSP length, as well as symptom and cognitive differences between patients with abnormal relative to normal CSP based on length. Changes in MRI protocols over time, with smaller slice thicknesses and improvements in resolution, also may have contributed to some of the discrepancies that have been reported between various studies. Further, it is possible that certain characteristics of patient samples may be more commonly associated with CSP, and that differential distribution of such characteristics between studies may account for some of the variability in findings. For example, a small number of studies have reported associations between CSP and a variety of demographic and historic variables, such as family history of schizophrenia and duration of institutionalization (Fukuzako et al., 1996; Mathew et al., 1985; Nopoulos et al., 2000; Uematsu and Kaiya, 1989), though evidence for a relationship with duration of illness has been largely negative (Hagino et al., 2001; Nopoulos et al., 2000; Shioiri et al., 1996).
To our knowledge, there is only one previous study (Nopoulos et al., 2000) which examined the relationship between CSP and cognition, using the Wechsler Adult Intelligence Scale – Revised Full Scale, Verbal, and Performance IQ. Within the group of patients with abnormally large CSP, they found a significant correlation between CSP size and all three IQ indices. These correlations were not observed in either the patients without an abnormally large CSP, or in the healthy control group. Our study found that patients with an abnormally large CSP performed more poorly on measures of verbal learning and memory than did those patients without an abnormally large CSP. We also observed significant negative correlations between length of the CSP in the total sample of patients and performance on tests of receptive language and verbal learning and memory. The latter finding is particularly interesting in light of the fact that the septum pellucidum is part of the limbic system, with an important role in linkage between many structures, including the hypothalamus, hippocampus, amygdala, habenula, and brainstem reticular formation (Bodensteiner et al., 1998; Bruyn, 1977). In normal fetal development, the septum pellucidum is first solid, then cleaves to form a fluid-filled cavity along its length (the CSP); near the time of birth, these two leaflets fuse back together. Fusion of the two leaflets is believed to be dependent on normal development of a variety of other surrounding structures, including the hippocampus and the corpus callosum, as well as the cerebral hemispheres. Disturbance of any or all of these structures might result in an enlarged CSP. The fact that the patients with an abnormally large CSP also demonstrated poorer performance in verbal learning and memory on the CVLT-II, a task requiring intact medial temporal lobe integrity, lends support to the notion that the hippocampi in patients with enlarged CSP may not have developed normally either. This interpretation is consistent with the findings of Kasai et al (2004), indicating an association with larger CSP and smaller left parahippocampal gyrus gray matter volumes in patients in their first episode of schizophrenia. Future studies examining the relationship between large CSP and medial temporal lobe structures, the corpus callosum, and other brain regions thought to be linked to the septum pellucidum warrant further consideration.
Consistent with the Nopoulos et al. (2000) study, we found a tendency for the patients with an abnormally large CSP to have somewhat more severe negative symptoms on the SANS. In addition, correlation analysis indicated a significant positive relationship between CSP length and SANS total score in the total sample of patients. Overall, these findings support an association between large CSP and more severe negative symptoms in patients with schizophrenia.
There are some limitations of this paper to be considered. There were only a small number of patients with an abnormally large CSP, which may limit the power to detect differences in cognitive and clinical status. Further investigations comparing larger samples of patients with clearly defined normal and abnormally large CSP will be necessary to determine whether the latter is particularly associated with cognitive deficits and more severe psychopathology. We also were lacking information on institutionalization and family history of psychiatric illness for a large number of patients in our sample. We therefore cannot rule out the possibility that there are some family and historic variables that may be associated with abnormally large CSP in our patients with schizophrenia. Further research into the impact of historical information on CSP morphology and prevalence rates will be important.
In summary, our patients with schizophrenia and healthy controls did not differ with respect to the prevalence of CSP, regardless of how the CSP was measured, be it presence (i.e., 1 mm at minimum), total length, or grade of the CSP (i.e., overall size). These findings suggest that the presence of CSP is a normal variant in most healthy controls and most patients with schizophrenia. Nonetheless, an abnormally large CSP in patients with schizophrenia appears to be associated with greater symptom severity and cognitive deficits in areas such as intellectual functioning and verbal learning and memory. Future studies of the CSP in schizophrenia will likely benefit from inclusion of more substantial samples of patients with abnormally large CSP.
Acknowledgments
Supported by grants from the National Alliance for Research on Schizophrenia and Depression, the Hitchcock Foundation, the National Alliance for Medical Image Computing (NIBIB U54 grant EB005149), the Ira DeCamp Foundation, and the New Hampshire Hospital. We thank Nancy Koven and Jo Cara Pendergrass for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders - IV. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) Iowa University; Iowa City: 1984. [PubMed] [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Bodensteiner J, Schaefer G. Wide cavum septum pellucidum: A marker of disturbed brain development. Pediatric Neurology. 1990;6:391–394. doi: 10.1016/0887-8994(90)90007-n. [DOI] [PubMed] [Google Scholar]
- Bodensteiner J, Schaefer G, Craft J. Cavum septi pellucidi and cavum vergae in normal and developmentally delayed populations. Journal of Child Neurology. 1998;13:120–121. doi: 10.1177/088307389801300305. [DOI] [PubMed] [Google Scholar]
- Bruyn G. Agenesis septi pellucidi, cavum septi pellucidi, cavum vergae and cavum veli interpositi. In: Vinken PF, Bruyn GW, editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 1977. pp. 299–366. [Google Scholar]
- DeGreef G, Bogerts B, Falkai P, Greve B, Lantos G, Ashtari M, Lieberman J. Increased prevalence of the cavum septum pellucidum in magnetic resonance scans and post-mortem brains of schizophrenic patients. Psychiatry Research. 1992a;45:1–13. doi: 10.1016/0925-4927(92)90009-s. [DOI] [PubMed] [Google Scholar]
- DeGreef G, Lantos G, Bogerts B, Ashtari M, Lieberman J. Abnormalities of the septum pellucidum on MR scans in first-episode schizophrenic patients.[see comment] AJNR: American Journal of Neuroradiology. 1992b;13:835–840. [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- DeLisi LE, Hoff AL, Kushner M, Degreef G. Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatry Research. 1993;50:193–199. doi: 10.1016/0925-4927(93)90030-l. [DOI] [PubMed] [Google Scholar]
- Fukuzako T, Fukuzako H, Kodama S, Hashiguchi T, Takigawa M. Cavum septum pellucidum in schizophrenia: a magnetic resonance imaging study. Psychiatry & Clinical Neurosciences. 1996;50:125–128. doi: 10.1111/j.1440-1819.1996.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Galarza M, Merlo AB, Ingratta A, Albanese EF, Albanese AM. Cavum septum pellucidum and its increased prevalence in schizophrenia: A neuroembryological classification. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:41–46. doi: 10.1176/jnp.16.1.41. [DOI] [PubMed] [Google Scholar]
- Hagino H, Suzuki M, Kurokawa K, Mori K, Nohara S, Takahashi T, Yamashita I, Yotsutsuji T, Kurachi M, Seto H. Magnetic resonance imaging study of the cavum septi pellucidi in patients with schizophrenia. American Journal of Psychiatry. 2001;158:1717–1719. doi: 10.1176/appi.ajp.158.10.1717. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test Manual Revised and Expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Jurjus GJ, Nasrallah HA, Olson SC, Schwarzkopf SB. Cavum septum pellucidum in schizophrenia, affective disorder and healthy controls: A magnetic resonance imaging study. Psychological Medicine. 1993;23:319–322. doi: 10.1017/s0033291700028403. [DOI] [PubMed] [Google Scholar]
- Kasai K, McCarley RW, Salisbury DF, Onitsuka T, Demeo S, Yurgelun-Todd D, Kikinis R, Jolesz JA, Shenton ME. Cavum septi pellucidi in first-episode schizophrenia and first-episode affective psychosis: an MRI study. Schizophrenia Research. 2004;71:65–76. doi: 10.1016/j.schres.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Peterson BS. Cavum septi pellucidi in Tourette syndrome. Biological Psychiatry. 2003;54:76–85. doi: 10.1016/s0006-3223(02)01830-9. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, Yurgelun-Todd D, Tohen M, Kikinis R, Jolesz FA, McCarley RW. MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. American Journal of Psychiatry. 1998;155:509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Partain CL, Prakash R, Kulkarni MV, Logan TP, Wilson WH. A study of the septum pellucidum and corpus callosum in schizophrenia with MR imaging. Acta Psychiatrica Scandinavica. 1985;72:414–421. doi: 10.1111/j.1600-0447.1985.tb02634.x. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Krie A, Andreasen NC. Enlarged cavum septi pellucidi in patients with schizophrenia: Clinical and cognitive correlates. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:344–349. doi: 10.1176/jnp.12.3.344. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Swayze V, Andreasen NC. Pattern of brain morphology in patients with schizophrenia and large cavum septi pellucidi. Journal of Neuropsychiatry & Clinical Neurosciences. 1996;8:147–152. doi: 10.1176/jnp.8.2.147. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WT, Andreasen NC. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biological Psychiatry. 1997;41:1102–1108. doi: 10.1016/S0006-3223(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Giedd JN, Andreasen NC, Rapoport JL. Frequency and severity of enlarged cavum septi pellucidi in childhood-onset schizophrenia. American Journal of Psychiatry. 1998;155:1074–1079. doi: 10.1176/ajp.155.8.1074. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham D. The Brief Psychiatric Rating Scale (BPRS): Recent developments in ascertainment and scaling. Psychopharmacology Bulletin. 1988;24:97–99. [PubMed] [Google Scholar]
- Rajarethinam R, Miedler J, DeQuardo J, Smet CI, Brunberg J, Kirbat R, Tandon R. Prevalence of cavum septum pellucidum in schizophrenia studied with MRI. Schizophrenia Research. 2001;48:201–205. doi: 10.1016/s0920-9964(00)00110-9. [DOI] [PubMed] [Google Scholar]
- Schaefer G, Bodensteiner J, Thompson J. Subtle anomalies of the septum pellucidum and neurodevelopmental deficits. Developmental Medicine & Child Neurology. 1994;36:554–559. doi: 10.1111/j.1469-8749.1994.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Scott TF, Price TR, George MS, Brillman J, Rothfus W. Midline cerebral malformations and schizophrenia. Journal of Neuropsychiatry & Clinical Neurosciences. 1993;5:287–293. doi: 10.1176/jnp.5.3.287. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Oshitani Y, Kato T, Murashita J, Hamakawa H, Inubushi T, Nagata T, Takahashi S. Prevalence of cavum septum pellucidum detected by MRI in patients with bipolar disorder, major depression and schizophrenia. Psychological Medicine. 1996;26:431–434. doi: 10.1017/s0033291700034838. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bogerts B, Wu H, Bilder RM, Deoras KS, Lieberman JA. Absence of the adhesio interthalamica as a marker of early developmental neuropathology in schizophrenia: An MRI and postmortem histologic study. Journal of Neuroimaging. 1998;8:159–163. doi: 10.1111/jon199883159. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Kaiya H. Midsagittal cortical pathomorphology of schizophrenia: A magnetic resonance imaging study. Psychiatry Research. 1989;30:11–20. doi: 10.1016/0165-1781(89)90167-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The wide range achievement test-third edition (WRAT-III) administration manual. Wide Range, Inc; Wilmington: 1993. [Google Scholar]


