Abstract
Habituation enables the organism to attend selectively to novel stimuli by diminishing no-longer necessary responses to repeated stimuli. Because the prefrontal cortex (PFC) has a core role in monitoring attention and behavioral control especially under novelty, neural habituation responses may be modified in drug addiction, a psychopathology that entails PFC abnormalities in both structure and function. Sixteen cocaine abusers and 12 gender-, race-, education-, and intelligence-matched healthy control subjects performed an incentive sustained attention task twice, under novelty and after practice, during functional magnetic resonance imaging. For cocaine abusers practice effects were noted in the PFC (including anterior cingulate cortex/ventromedial rostral PFC, dorsolateral PFC, and medial frontal gyrus) and cerebellum (signal attenuations/decreases: return to baseline); activations in these regions were associated with craving, frequency of use, and length abstinence. In the control subjects practice effects were instead restricted to posterior brain regions (precuneus and cuneus) (signal amplifications/increases: deactivation away from baseline). Also, only in the cocaine abusers, increased speed of behavioral performance between novelty to practice was associated with a respective attenuation of activation in the thalamus. Overall, we report for the first time a differential pattern of neural responses to repeated presentation of an incentive sustained attention task in cocaine addiction. Our results suggest a disruption in drug addiction of neural habituation to practice that possibly encompasses opponent anterior vs. posterior brain adaptation to the novelty of the experience: overly expeditious for the former but overly protracted for the latter. Overall, cocaine addicted individuals may be predisposed to an increased challenge when required to maintain alertness as a task progresses, not able to optimally utilize a prematurely habituating PFC to compensate with an increased attribution of salience to a desired reward.
Keywords: fMRI BOLD, adaptation, practice effects, prefrontal cortex, alertness, drug abuse, drug addiction, salience, reward
Introduction
Habituation, the ability to cease responding to irrelevant (or no longer novel) events in an environment with multiple sensory stimuli, is dependent on the integrity of the medial prefrontal cortex (PFC) in rodents (Broersen and Uylings, 1999). In human studies, results have similarly implicated the PFC in determining the allocation of attentional resources to novel events (Daffner et al., 2003) and in a learning-related transition from novelty to practice (reviewed in (Kelly and Garavan, 2005)). Because structural and functional abnormalities in the PFC have been implicated in the core clinical characteristics of drug addiction (reviewed in (Goldstein et al., 2006; Goldstein and Volkow, 2002)), in the current study we hypothesized that cocaine addiction would modulate neural habituation to practice. Indeed, lack of normal satiety (i.e., habituation) to cocaine itself has been previously suggested to be at the core of the drug addiction process (Volkow and Fowler, 2000), and this impaired habituation may generalize to other non drug-related situations.
This suggestion that addicted individuals’ drug-related satiety/habituation neural processes are compromised is consistent with several conceptual accounts of drug addiction. These models emphasize the role of reward processing and opponent responses (e.g., diminishing effect of pleasant vs. increasing effect of unpleasant responses as addiction progresses) (Solomon and Corbit, 1973) (Koob and Bloom, 1988; Koob et al., 1997) and of incentive sensitization of drug wanting (but not liking) (Robinson and Berridge, 2003) as we recently reviewed (Goldstein et al., 2006). This suggestion derives also from recent animal and human studies. In rodents, cocaine treatment appears to interfere with the retention of the habituation process (see between-session cocaine induced anti-habituation effect on locomotor activity, (Carey et al., 2003), although see (Ahmed et al., 1996) for negative results with another psychostimulant, d-amphetamine). In humans, drug addiction has frequently been associated with specific cognitive-behavioral impairments that may be associated with compromised habituation, encompassing learning and memory deficits (reviewed in (Volkow et al., 2002)), and impulsivity and perseverative behavior (Bechara et al., 2002);(Clark et al., 2006);(Petry, 2003);(von Geusau et al., 2004). More direct evidence offers an evoked response psychophysiological study, where 10 abstinent chronic cocaine abusers, but not alcohol abusers or control subjects, had diminished repetition-related auditory P50 amplitude and suppression (Fein et al., 1996). Although these studies provide strong evidence for the potential disruption of the neural correlates underlying habituation/practice effects in human cocaine addiction, no studies to date have used functional magnetic resonance imaging (fMRI) to explicitly probe the effect of repetition on brain responsiveness in drug addicted individuals.
We have previously modeled drug addiction as characterized by Impaired Response Inhibition and Salience Attribution (I-RISA) where salience is hypothesized to be grossly biased toward the drug and at the expense of other potentially but no-longer rewarding stimuli, with a concomitant decrease in the ability to inhibit the maladaptive drug use (Goldstein and Volkow, 2002). In a separate fMRI study we recently provided evidence for the I-RISA model, showing a disrupted neural processing of the salience of a secondary reward in cocaine addicted individuals (Goldstein et al., 2007). In this recent study, sustained monetary reward was associated with a robust and complex neuronal activation pattern in healthy control subjects, encompassing the PFC. The cocaine abusers instead had reduced regional activations in the between group analyses or less sensitivity to differences between the monetary conditions in the within group analyses (Goldstein et al., 2007). In the current study our goal was to clarify whether these previous results could be affected by differences between the groups in neural responses to practice effects and habituation, processes that are inherent in the performance of any neuroimaging task (Garavan et al., 2000) but that to date have not been investigated in drug addiction. Thus, to examine the potential effect of habituation on fMRI responses to a sustained attention task in cocaine addicted individuals as compared to matched healthy control subjects we undertook secondary analyses of the separately published data (Goldstein et al., 2007). We hypothesized a change in the neural habituation responses to practice (repetition) on this incentive sustained attention task as a function of cocaine addiction; we specifically hypothesized that this change would encompass the PFC.
Materials and Methods
Participants
Twenty-eight medically healthy subjects participated in the study, 16 cocaine abusers and 12 matched control subjects. There were no group differences in gender (4 females in both groups), race (11 and 10 African-Americans in the cocaine and control groups, respectively), and education (mean±SD, cocaine: 12.8±2.7 years vs. control: 14±1.3 years, t(21)=−1.66, p>0.1). Subjects were also matched on right hand dominance (Oldfield, 1971), verbal and non-verbal measures of general intellectual functioning [as measured by the Wide Range Achievement Test III - Reading Scale (Wilkinson, 1993), and the Wechsler Abbreviated Scale of Intelligence - Matrix Reasoning Scale (Wechsler, 1999), respectively], and self-reported depression (Beck et al., 1996). Significant differences between the groups were observed in age (42.8±4.6 years in cocaine abusers vs. 37.6±7.1 years in control subjects, t(26)=2.3, p < 0.05) and cigarette smoking (11 current smokers/1 past smoker/4 never smokers in cocaine abusers vs. no current/2 past/10 never smokers in control subjects, χ2(2)=13.6, p = 0.001).
Subjects were recruited using advertisements in local newspapers, referrals from local drug addiction treatment centers, and by word-of mouth. Subjects were initially screened by phone. If eligible, subjects were scheduled for a subsequent on site evaluation that included a full physical and neurological examination by a neurologist. In addition, a clinical psychologist conducted an in-depth, 1–3 hour, diagnostic interview which included the Structured Clinical Interview for DSM-IV Axis I Disorders [research version (First et al., 1996; Ventura et al., 1998)] – Nonpatient Edition or Patient Edition for control subjects or cocaine addicted subjects, respectively. This interview assessed diagnostic and inclusion/exclusion criteria. Other measures used were: the Addiction Severity Index (McLellan et al., 1992), a semistructured interview that collects data in seven problem areas (medical, employment, legal, alcohol, other drug use, family-social functioning, and psychological status) to provide an estimate of the severity of the drug abuse problems and a detailed assessment for recent and lifetime history of use of various drugs including alcohol; the Cocaine Selective Severity Assessment Scale (Kampman et al., 1998) conducted to evaluate cocaine abstinence/withdrawal signs and symptoms (i.e., sleep impairment, anxiety, energy levels, craving and depressive symptoms) 24 hours within time of interview; and the Cocaine Craving Questionnaire (Tiffany et al., 1993).
All cocaine abusers were free of illnesses that required hospitalization or regular monitoring. In addition, they all met DSM-IV criteria for Cocaine Dependence (N=15) or Abuse (N=1). Mean age of onset of cocaine use was 24.8 years (SD=8) and mean duration of cocaine use was 17.6 years (SD=7). There were nine cocaine abusers who reported using cocaine the night before the study; their urine was indeed positive for cocaine, indicating that they had used the drug recently (within 72 hours of testing, which is the maximum resolution provided by the urine screen). For the other seven cocaine abusers, self-reported time since last use ranged from 5 to 90 days. Four of the cocaine addicted subjects also reported current alcohol dependence (N=3, two of them in early remission) or abuse (N=1). Current use or dependence on other drugs (marijuana, barbiturates, amphetamines, or opiates) was denied and corroborated by pre-scan urine tests in all subjects. Inclusion/exclusion criteria were the same for the control subjects, except history of drug dependence or positive urine screens for any drug were exclusionary. Subjects were fully informed of all study procedures and risks associated with the magnetic resonance imaging (MRI) and provided written consent for their involvement in this study in accordance with the local Institutional Review Board. The possible confounding effects on results of age, cigarette smoking, and cocaine urine status were examined as described in Analyses and Results.
Task
Subjects performed a sustained attention task, in a blocked design format. There were two consecutive repetitions of two identical blocks (Novel: N; and Practice: P) (Figure 1A). Each block was comprised of three separate runs, each run was comprised of three different monetary feedback conditions (0, 1, and 45 cents; each of 63 sec duration, preceded by a 35 sec fixation cross to preclude carry over effects), and each condition was comprised of 9 press and 9 no press pseudorandomized trials, for a total of 81 go and 81 no go trials per block. Within these trials, subjects either responded (pressed a button) or refrained from responding during a trigger (red square), depending on one of two preceding instruction stimuli (two fractal images adapted from (Thut et al., 1997)) (Figure 1B). Each trial was of 3.5 sec fixed duration (1000 msec fixation cross + 500 msec for one of two fractal images at screen center + 1000 msec fixed delay + 500 msec for the trigger stimulus at screen center + 500 msec feedback slide) (Figure 1B).
Figure 1.
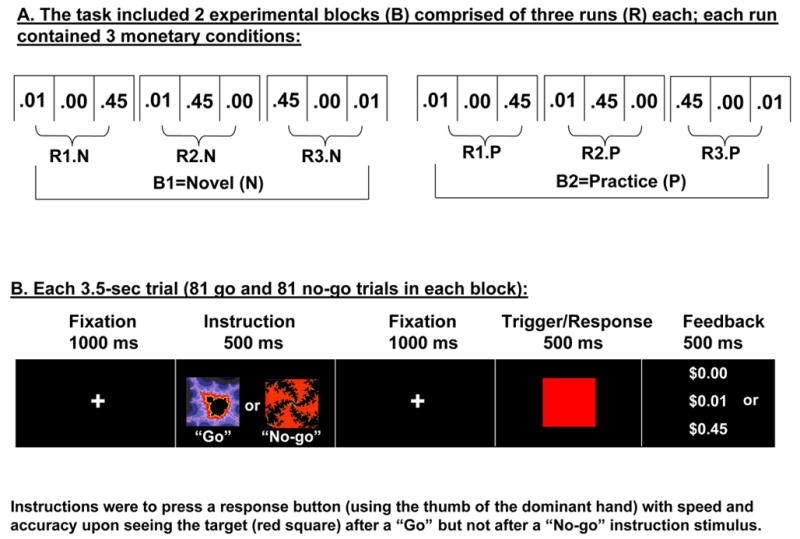
Experimental paradigm. Overall design and experimental conditions are depicted at the top (A); at each monetary condition onset (conditions were separated by 35 sec), a 3.5 sec screen (not depicted) displayed the monetary reward ($0.00, $0.01, or $0.45). Together with the feedback delivered at the end of each trial (B), this 3.5 sec screen (similar in appearance to the feedback screen) guaranteed the subjects were continuously aware of the reward contingencies. Both task repetitions (Novel and Practice) were identical in monetary reward contingencies.
The randomization of the monetary reward conditions in the first block of runs (the first three runs) was novel to the subjects; the behavioral and fMRI data from these first three runs was averaged to represent the novel task session (N) (Figure 1A). Similarly to our previous fMRI study of practice effects (Tomasi et al., 2004), these three runs were then repeated and data averaged to represent the second/practiced task session (P). Note that there were no differences between N and P in any of the task related parameters, and that P was the exact repetition of N (i.e., both N and P blocks contained all three monetary conditions, each repeated for a total of three times). This complete task was administered in a single fMRI session; total duration was 35 min (5 min per each of the 6 runs, with less than a minute separation between the runs for data storage). The task was presented via MRI compatible goggles. Reaction time and accuracy data were collected across all trials. In all subjects, an identical amount (30% of actual task) of training preceded task onset. The training runs were the same as the experimental runs except for different trial and condition randomization; in addition, subjects were not paid for these training runs.
Subjects were rewarded for correct performance with 0, 1, or 45 cents depending on the monetary condition, receiving up to $50 for this task; subjects saw a numeral designating the reward contingencies before each monetary condition and immediately after each trial (in cases of an error, which happened in less than 2.5% of trials across all subjects as further described in Results, subjects saw an “X” instead of the monetary reward symbol: “$0.00”, “$0.01”, or “$0.45”). Subjects were therefore aware of the reward contingencies throughout the task. Upon task completion, subjects rated their interest and excitement in the three monetary conditions on two visual analogue scales (range: 0 to 7, boring to interesting and dull to exciting, respectively).
MRI acquisition and processing
Subjects underwent MRI in a 4 Tesla whole-body Varian/Siemens MRI scanner, equipped with a self-shielded whole-body SONATA gradient set. A T2*-weighted single-shot gradient-echo planar imaging pulse sequence (TE/TR=20/3500 ms, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64×64 matrix size, 3.1 × 3.1 mm in-plane resolution, 90°-flip angle, 91 time points) was used to collect the fMRI datasets. Padding was used to minimize motion. Anatomical brain images were acquired using a T1-weighted 3D-MDEFT sequence (Lee et al., 1995) and a modified T2-weighted Hyperecho sequence (Hennig and Scheffler, 2001) to rule out gross morphological abnormalities; all structural scans were reviewed by a neurologist.
The first four volumes in the time series were discarded to avoid non-equilibrium effects in the fMRI signal. Subsequent analyses were performed with the SPM99 package (Welcome Department of Cognitive Neurology, London UK). The images were realigned to the first volume using a six-parameter rigid body transformation, to correct for head motion. Head motion was less than 1.5-mm translation and 1.5°-rotation for all fMRI runs, as determined immediately after each run (Caparelli et al., 2003). The realigned datasets were then normalized to the Talairach frame using a voxel size of 3×3×3 mm3, and smoothed with an 8-mm full-width-half-maximum Gaussian kernel. A general linear model was used to estimate the blood oxygenation level dependent (BOLD) signal amplitude (Friston et al., 1995). We used a blocked analysis based on a box-car design convolved with the canonical hemodynamic response function (as low pass filter); a high-pass filter (cut-off: 1/750 sec) was applied to minimize baseline fluctuations.
Statistical analyses
A voxel based (whole brain) statistical analysis with two positive contrasts (N and P: the average of the BOLD signal maps corresponding to the first and last three fMRI runs, respectively) was applied for each subject across the monetary incentive conditions (i.e., the same identical monetary conditions were averaged within the N and P blocks, and the effect of money was therefore rendered moot). Thus, for each subject for each of the 6 runs, the BOLD amplitudes were estimated for each monetary condition, and these maps of BOLD signals were then averaged (with IDL: Research Systems, Boulder, Colo.) across the respective three runs to represent the two blocks. Group analyses were then carried out using random effects SPM analyses; specifically, a voxel-by-voxel two-way repeated measures analysis of variance (ANOVA) model was used with performance accuracy as a nuisance variable, to examine the effects of practice (N and P vs. fixation baseline), group (cocaine vs. control), and their interaction. To balance the potential effect of the differential number of subjects in the groups (16 vs. 12), we adjusted the contrast weight vectors when performing the direct group comparisons in SPM; this eliminates spurious main effects associated with sample size differences. Statistical thresholds were: p<0.01 corrected for multiple comparisons for the main activations and deactivations (N or P vs. baseline; first-order analyses); p<0.05 corrected for multiple comparisons for the practice (N vs. P) or group (control vs. cocaine) effects (second-order analyses: main effects of practice or group); and p<0.005 uncorrected for the practice by group interaction (third-order analyses: interaction and simple effects). Note that for all analyses, all the possible comparisons were inspected (e.g., N>P, N<P, N or P > or < fixation baseline, etc.); these relative signal increases or decreases are indicated with different colors (red or blue) throughout the figures and with different symbols (‘+’ or ‘−’) throughout the tables. Minimum cluster size was 15 contiguous voxels (0.41 cc) for all analyses. Additional linear regression analyses between practice-related changes of BOLD responses and the respective practice-related changes of behavioral measures were conducted separately for each group in SPM to identify brain regions whose change in activity was associated with change in task performance. Threshold for these correlation analyses was p<0.005 uncorrected, 15 voxels minimum, masked with the general task activations at p<0.05.
Region-of-interest (ROI) analyses
Finally, functional ROIs with a volume of 0.73 cc (cubic, 27 voxels) were defined at the peak of the clusters that showed a significant practice by group interaction in the SPM analyses; the average BOLD signal was extracted using a customized program written in IDL (Research Systems, Boulder, CO). Clarification of anatomical specificity of all ROIs was corroborated with a co-planar stereotaxic atlas of the human brain (Talairach and Tournoux, 1988). These ROIs were used to complement the SPM analyses; two (group) by two (practice) repeated measures ANOVAs, and independent (group differences) or paired (practice differences) t-tests were performed for all ROIs. The possibly confounding variables were inspected with correlations (for age) or t-tests (for smoking history and urine status), and used as covariates when necessary (i.e., when associations with the ROIs were significant at the p<0.05 level). The same procedure was utilized to confirm the SPM linear regression results.
Results
Behavioral performance
Consistent with the desired effect of training, all subjects reached stable levels of performance. In addition, reaction times did not display main effects of practice, group, or an interaction between practice and group (all p>0.5). The group main effect reached significance for performance accuracy (p=0.01): a lower percent of correct responses was observed for the cocaine abusers across both repetitions (cocaine<control: N, 96.9±2.4<98.7±1.4, p<0.05; P: 96.6±2.9<99.0±1.1, p<0.01; mean±SD). Accuracy was therefore entered as a nuisance variable in the SPM ANOVA. Because the practice main effect did not reach significance for any of the study groups (i.e., there were no differences between N and P in performance), and accuracy was further treated as a nuisance variable in the subsequent SPM ANOVA, the fMRI results described below and the group differences in neural habituation to practice cannot be attributed to performance differences between the cocaine addicted and control subjects. Note that the ratings of task interest (45¢>1¢>0¢) and excitement (45¢>1¢=0¢) demonstrated the expected effect of monetary value but did not differ between the study groups, as previously reported (Goldstein et al., 2007).
To further understand the potential impact on results of drug use variables, we conducted correlations between speed and accuracy on the task with numerous (>10) cocaine and alcohol use variables (including age of onset, duration of use, length of abstinence, craving and withdrawal symptoms at study day, amount and frequency of use). Here, to protect against Type I error, significance threshold was set to p<.01. One correlation reached significance: the higher the reaction time at second time repetition (P), the more frequent was cocaine use (days/week) during the year preceding the study (r=.63, p=.01). There were no other correlations that reached this nominal significance level.
Task-related fMRI BOLD signal changes from baseline (Figures 2–3 and Table 1)
Figure 2.
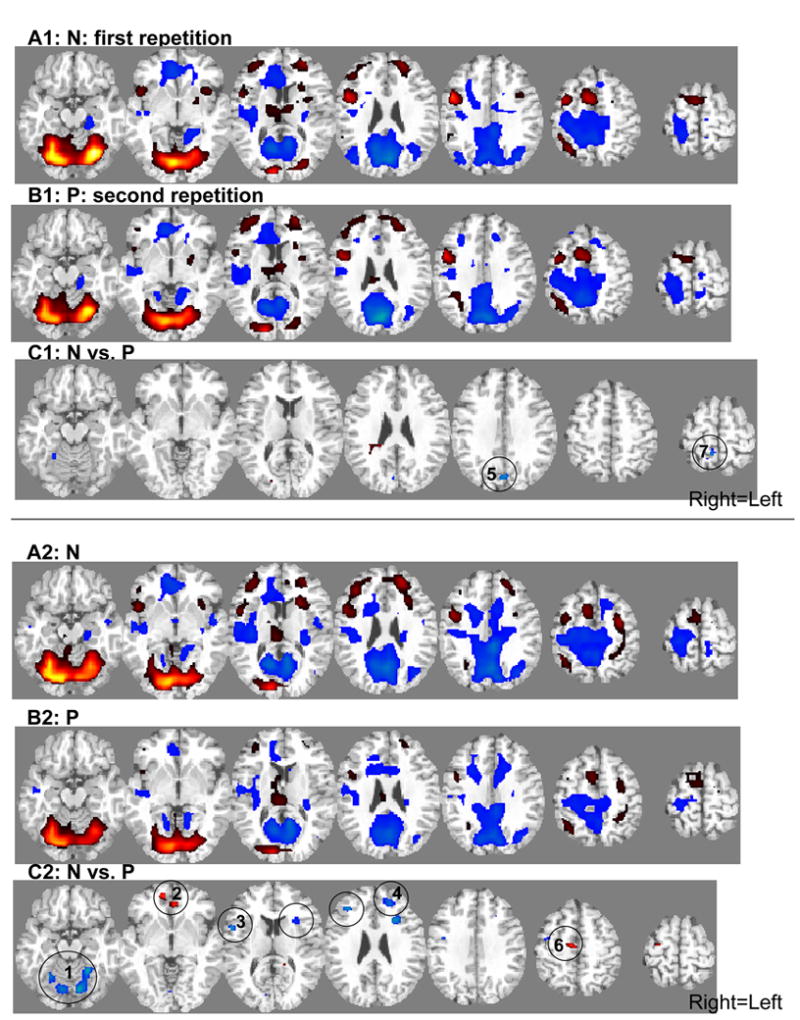
SPM results of the task main effect during the first (A: N) and second (B: P) repetition and SPM results of the practice effect (C: N vs. P) in 12 control subjects (1) and 16 cocaine abusers (2). Statistical thresholds were p<0.01 corrected for multiple comparisons for A and B and p<0.05 corrected for C, minimum cluster size 15 voxels (0.41 cc). For C, numbers denote respective regions in Table 2 and Figure 4. Red: activations (vs. baseline) or relative BOLD signal increases (e.g., a decrease in intensity of deactivation from N to P, while still a deactivation, will be denoted in red=P minus N); Blue: deactivations (vs. baseline) or relative BOLD signal decreases (N minus P) (see Table 2 for arrows). For A and B: T-score for display purposes is 5-40. For C: T-score=4–7. Right=Left.
Figure 3.
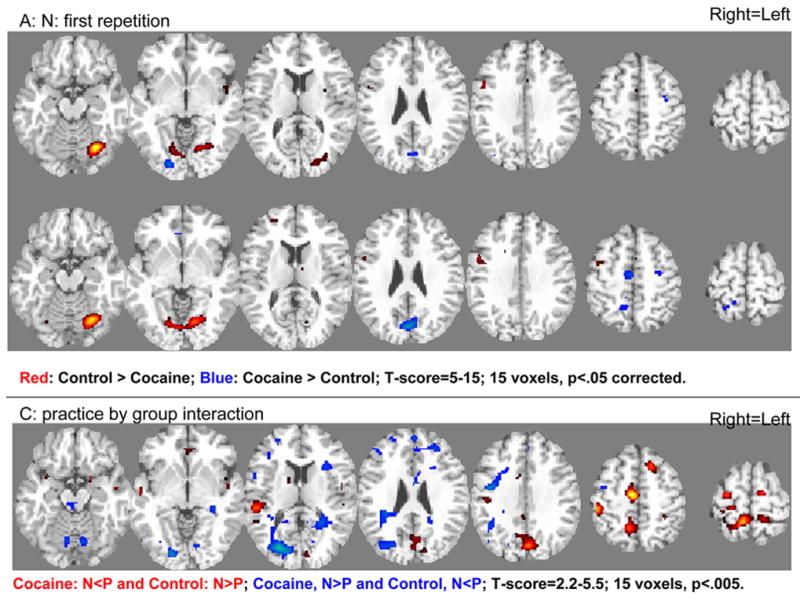
SPM results of direct group comparisons (12 control subjects vs. 16 cocaine abusers) during the first (A: N) and second (B: P) repetition and for the practice by group interaction (C: N vs. P). Statistical thresholds were p<0.05 corrected for multiple comparisons for A and B and p<0.005 uncorrected for C, minimum cluster size 15 voxels (0.41 cc). For A and B: Red: Control>Cocaine; Blue: Cocaine>Control; For C: Red: Cocaine, N<P and/or Control, N>P; Blue: Cocaine, N>P and/or Control, N<P (see Figure 4 and Table 2). For A and B: T-score=5–15 (for display purposes). For C: T-score=2.2–5.5 (for display purposes). Right=Left.
Table 1.
Main task activations: location of center of major areas of brain activations (+) and deactivations (−) from a fixation baseline in the Talairach frame of reference, and statistical significance (T-score value) of BOLD responses in these regions.
| Coordinates [mm] | T-scores Main Activations: N | T-scores Main Activations: P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Side | x | y | z | Control‡ (N=12) | Cocaine‡ (N=16) | Control >(+) or < (−) Cocaine† | Control‡ (N=12) | Cocaine‡ (N=16) | Control >(+) or < (−) Cocaine† |
| Cerebellum / ventral occipital (fusiform, lingual gyrus) (BA 18,19) | R | 24 | −57 | −15 | +27.8 | +27.3 | +3.3* | +22.2 | +18.9 | +4.3* |
| R | 9 | −72 | −15 | +22.2 | +25.2 | ns | +20.2 | +17.4 | +3.8* | |
| R | 6 | −72 | −18 | +14.3 | +20.3 | ns | +14.4 | +13.1 | ns | |
| L | −30 | −48 | −18 | +16.0 | +16.8 | ns | +11.9 | +7.8 | +3.8* | |
| L | −18 | −75 | −15 | +23.7 | +24.5 | ns | +21.2 | +15.6 | +5.7 | |
| L | −15 | −72 | −15 | +17.1 | +20.4 | ns | +16.2 | +12.0 | +4.3* | |
| ACC (BA 24, 32) / vmrPFC (BA 10) | R | 9 | 30 | 0 | −11.3 | −11.7 | ns | −10.9 | ns | −5.8 |
| R | 9 | 54 | −3 | −9.5 | −13.2 | ns | ns | −5.2 | ns | |
| R | 6 | 27 | 0 | −10.9 | −11.6 | ns | −10.8 | ns | −5.4 | |
| L | −6 | 33 | 3 | −12.0 | −12.8 | ns | −10.6 | ns | −4.9 | |
| L | −6 | 30 | 3 | −10.8 | −12.1 | ns | −10.1 | ns | −4.7 | |
| L | −3 | 30 | 3 | −11.7 | −13.1 | ns | −10.8 | −5.1 | −4.7 | |
| L | −12 | 39 | 0 | −11.1 | −11.2 | ns | −9.2 | ns | −4.7 | |
| L | −12 | 36 | 0 | −11.4 | −11.3 | ns | −10.4 | ns | −5.4 | |
| IFG (BA 44, 45) | R | 54 | 9 | 15 | +8.9 | +7.5 | ns | +6.3 | ns | +5.4 |
| MFG/SFG (BA 9,10) | R | 33 | 36 | 24 | ns | +12.4 | −4.6* | ns | ns | ns |
| R | 24 | 51 | 15 | +7.2 | +6.9 | ns | +8.7 | ns | +5.1 | |
| L | −24 | 42 | 27 | +9.1 | +12.0 | ns | +7.5 | ns | +2.7* | |
| L | −30 | 42 | 27 | +9.0 | +11.5 | ns | +8.9 | ns | +3.7* | |
| L | −30 | 54 | 21 | +8.0 | +9.6 | ns | +10.6 | +6.1 | +3.9* | |
| L | −12 | 39 | 21 | ns | ns | ns | ns | ns | ns | |
| L | −6 | 57 | 30 | ns | ns | ns | +6.9 | ns | +4.8 | |
| L | −24 | 60 | 18 | +5.3 | +5.4 | ns | +9.0 | ns | +4.6* | |
| Occipital: CUN (BA 17-19) | R | 3 | −78 | 42 | ns | ns | +3.7* | −6.3 | −7.3 | ns |
| R | 24 | −78 | 15 | ns | ns | −3.9* | ns | ns | +3.0* | |
| R | 15 | −87 | −3 | +11.2 | +26.2 | −8.6 | ns | +22.6 | −2.7* | |
| R | 30 | −60 | 6 | ns | ns | ns | ns | ns | ns | |
| L | −3 | −78 | 33 | −10.7 | −10.3 | ns | −18.8 | −11.0 | −6.9 | |
| L | −6 | −78 | 33 | −12.7 | −12.9 | ns | −20.3 | −12.6 | −7.0 | |
| L | −18 | −81 | 30 | −9.8 | −10.9 | ns | −11.8 | −6.8 | −4.4* | |
| MedFG (BA 6) | R | 6 | −15 | 54 | −7.7 | −9.3 | ns | −10.4 | ns | −6.8 |
| R | 9 | −24 | 42 | ns | −10.5 | +5.6 | −5.3 | −6.3 | ns | |
| R | 9 | −3 | 42 | −5.7 | −7.4 | ns | −9.0 | ns | −4.6* | |
| R | 3 | 9 | 36 | ns | ns | +4.4* | ns | ns | ns | |
| Parietal: preCUN (BA 7) | R | 6 | −48 | 66 | ns | ns | ns | ns | ns | −4.6* |
| R | 9 | −51 | 63 | ns | ns | ns | −8.2 | ns | −5.2 | |
|
| ||||||||||
| Other regions: | ||||||||||
|
| ||||||||||
| Parahippocampal gyrus, uncus (BA 28) | L | −24 | −24 | −18 | −9.8 | −9.3 | ns | −8.9 | −7.0 | ns |
| R | 21 | −21 | −18 | −6.7 | −9.0 | ns | −7.3 | ns | ns | |
| Midbrain: substantia nigra | R | 6 | −21 | −12 | ns | +7.6 | ns | ns | ns | ns |
| Lateral orbitofrontal cortex (BA 47,11) | L | −27 | 24 | −12 | −5.2 | ns | ns | −6.0 | ns | ns |
| R | 45 | 33 | −12 | ns | +8.1 | ns | +5.8 | +5.5 | ns | |
| Putamen | L | −33 | 3 | 6 | +8.0 | ns | +6.9 | +7.8 | ns | +6.5 |
| L | −27 | 0 | −6 | +7.7 | ns | ns | +7.5 | ns | ns | |
| Superior temporal gyrus (BA 22) | L | −54 | −3 | −6 | ns | −12.0 | +5.2 | −6.4 | −9.3 | +5.0 |
| Anterior insula (BA 13,44) | R | 45 | 12 | 3 | +13.0 | +12.9 | ns | +10.0 | +7.9 | +5.4 |
| L | −39 | 15 | 0 | +11.4 | +12.6 | ns | +8.1 | +6.2 | ns | |
| Caudate | R | 9 | 18 | 3 | −7.5 | −6.9 | ns | −8.7 | ns | ns |
| L | −9 | 18 | 9 | −5.4 | −7.3 | ns | −5.8 | −5.7 | ns | |
| Thalamus | R | 9 | −12 | 6 | +9.3 | +9.5 | ns | +9.2 | +9.6 | ns |
| L | −9 | −12 | 9 | +9.3 | +7.9 | +6.1 | +9.7 | +8.4 | +6.6 | |
| Posterior insula (BA 13) | R | 39 | −15 | 18 | −10.9 | −14.0 | ns | −7.8 | −8.9 | ns |
| L | −30 | −18 | 18 | −8.4 | −10.2 | ns | −5.6 | −9.0 | ns | |
| Precentral/postcentral gyrus (BA 3,4) | R | 36 | −18 | 42 | −10.1 | −8.0 | −5.2 | −11.7 | −6.8 | −6.0 |
| L | −36 | −9 | 48 | ns | ns | −7.9 | ns | +9.2 | −8.3 | |
| Dorsomedial PFC (BA 8) | L | −18 | 27 | 45 | −9.5 | −14.1 | ns | −13.4 | −12.6 | −6.6 |
| R | 27 | 21 | 42 | −8.7 | −11.4 | ns | −9.2 | −11.4 | ns | |
| Posterior cingulate(BA 24) | B | 0 | −9 | 42 | −8.8 | −10.9 | ns | −11.1 | −8.3 | −8.1 |
| Dorso-caudal ACC (BA 6, 8, 32) | R | 9 | 15 | 45 | +17.2 | +12.2 | +5.8 | +15.5 | +7.0 | +8.2 |
| R | 6 | 3 | 57 | +16.3 | +9.8 | +5.7 | +12.6 | +9.5 | ns | |
| L | −3 | 12 | 48 | +11.3 | ns | +5.0 | +12.8 | ns | +7.5 | |
Random-effects analyses. Bonferroni corrections:
p<0.01 corrected;
p<0.05 corrected;
cluster-level corrected p<0.005; all non-significant (ns) results are noted; ‘+’ designates activations (task > baseline) and ‘−’ designates deactivations (task < baseline); in the group comparisons, separately for N and P, ‘+’ designates task-related BOLD that is higher in the control subjects while ‘−’ designates task-related BOLD that is higher in the cocaine abusers. Minimum cluster = 15 voxels.
Similar patterns of activations and deactivations were observed for the control subjects (Figure 2A.1 and B.1) as compared to the cocaine abusers (Figure 2A.2 and B.2) during both the first (N) and second (P) task repetitions. Direct group comparisons in SPM revealed several differences between the groups for both N and P (Figure 3A–B). These results are listed in Table 1.
Practice effects (Figures 2–4 and Table 2)
Figure 4.
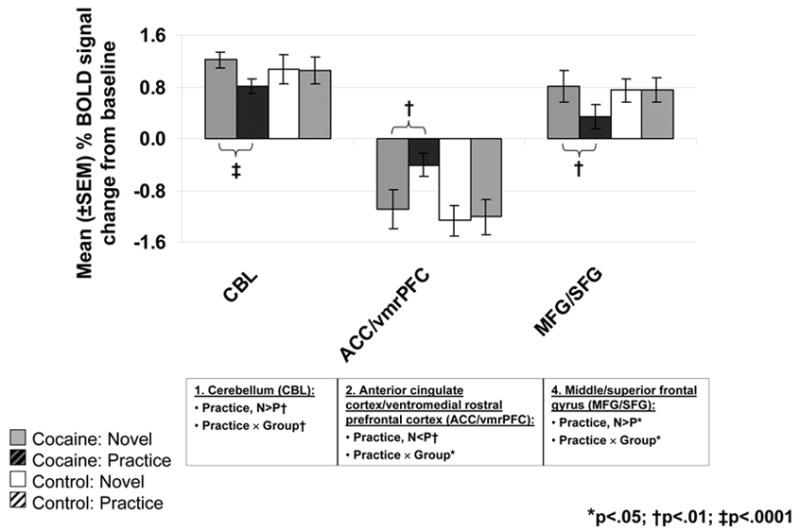
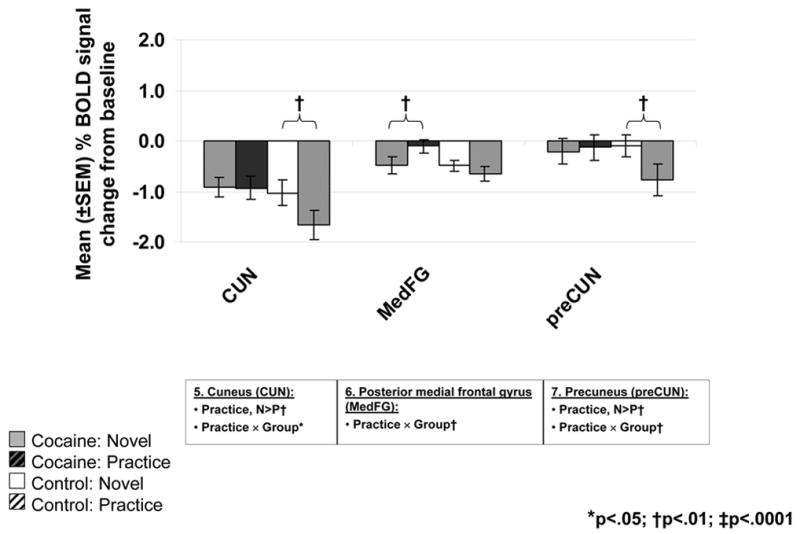
BOLD signals in the ROIs located at the cerebellum (CBL), anterior cingulate cortex/ventromedial rostral prefrontal cortex (ACC/vmrPFC), middle/superior frontal gyrus (MFG/SFG) (A), cuneus (CUN), posterior medial frontal gyrus (MedFG, BA 6), and precuneus (preCUN) (B) as a function of practice (solid = N; diagonal line = P) and diagnostic group (left/gray: 16 cocaine abusers; right/white: 12 comparison subjects) (see Figure 2C and Table 2, numbers in Figure 4 designate the clusters in the left column, ROIs correspond to the regions marked in boldface in the right-most column). Bar graphs represent mean % signal change from baseline ± SEM. Results of significant ANOVA effects are noted below each ROI. Results of significant paired t-tests are marked inside the figures.
Table 2.
Practice effects: location of major areas of relative brain activations (+) and deactivations (−) in the Talairach frame of reference, and statistical significance (T-score value) of BOLD responses in these regions.
| Coordinates [mm] | T-scores Practice Effect (N > P: −; or N < P: +) | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Side | Size1 | x | y | z | Control‡ (N=12) | Cocaine‡ (N=16) | Control > or < Cocaine† |
| 1. Cerebellum / ventral occipital (fusiform, lingual gyrus) (BA 18,19) | R | 79 | 24 | −57 | −15 | ns | −5.7: ↑↑:↑ | ns |
| R | 9 | −72 | −15 | ns | −5.4: ↑↑:↑ | ns | ||
| R | 6 | −72 | −18 | ns | −5.0: ↑↑:↑ | −3.4* | ||
| L | 141 | −30 | −48 | −18 | ns | −6.3: ↑↑:↑ | ns | |
| L | −18 | −75 | −15 | ns | −6.1: ↑↑:↑ | −2.7* | ||
| L | −15 | −72 | −15 | ns | −5.8: ↑↑:↑ | −3.3* | ||
| 2. ACC (BA 24, 32) / vmrPFC (BA 10) | R | 15 | 9 | 30 | 0 | ns | +5.7: ↓↓:↓ | +3.5* |
| R | 9 | 54 | −3 | ns | +5.6: ↓↓:↓ | ns | ||
| R | 6 | 27 | 0 | ns | +5.2: ↓↓:↓ | +3.4* | ||
| L | 94 | −6 | 33 | 3 | ns | +5.7: ↓↓:↓ | +3.0* | |
| L | −6 | 30 | 3 | ns | +5.5: ↓↓:↓ | +3.2* | ||
| L | −3 | 30 | 3 | ns | +5.6: ↓↓:↓ | +3.1* | ||
| L | −12 | 39 | 0 | ns | +5.5: ↓↓:↓ | ns | ||
| L | −12 | 36 | 0 | ns | +5.4: ↓↓:↓ | +3.0* | ||
| 3. IFG (BA 44, 45) | R | 44 | 54 | 9 | 15 | ns | −6.1: ↑:↓ | ns |
| 4. MFG/SFG (BA 9,10) | R | 30 | 33 | 36 | 24 | ns | −5.9: ↑↑:↑ | ns |
| R | 24 | 51 | 15 | ns | ns | −3.0* | ||
| L | 40 | −24 | 42 | 27 | ns | −5.3: ↑↑:↑ | ns | |
| L | −30 | 42 | 27 | ns | −4.8: ↑↑:↑ | −3.1* | ||
| L | −30 | 54 | 21 | ns | ns | −3.0* | ||
| L | −12 | 39 | 21 | ns | ns | −3.0* | ||
| L | −6 | 57 | 30 | ns | ns | −3.4* | ||
| L | −24 | 60 | 18 | ns | ns | −3.0* | ||
| 5. Occipital: CUN (BA 17-19) | R | 24 | 3 | −78 | 42 | −5.5: ↑:↓ | ns | ns |
| R | 24 | −78 | 15 | ns | ns | +4.9 | ||
| R | 15 | −87 | −3 | ns | ns | +4.2 | ||
| R | 30 | −60 | 6 | ns | ns | +3.0 | ||
| L | 24 | −3 | −78 | 33 | −5.8: ↓:↓↓ | ns | +4.0 | |
| L | −6 | −78 | 33 | −5.4: ↓:↓↓ | ns | +4.2 | ||
| L | −18 | −81 | 30 | ns | ns | +3.0 | ||
| 6. MedFG (BA 6) | R | 17 | 6 | −15 | 54 | ns | +5.5: ↓↓:↓ | +5.1 |
| R | 9 | −24 | 42 | ns | ns | +3.9 | ||
| R | 9 | −3 | 42 | ns | ns | +3.7 | ||
| R | 3 | 9 | 36 | ns | ns | +3.0 | ||
| 7. Parietal: preCUN (BA 7) | R | 18 | 6 | −48 | 66 | −5.5: ↑:↓ | ns | +4.2 |
| R | 9 | −51 | 63 | −5.2: ↓:↓↓ | ns | +4.4 | ||
Random-effects analyses.
Number of voxels for the practice main effect in the cocaine and control groups, respectively. Bonferroni corrections:
p<0.05 corrected;
cluster-level corrected p<0.005;
SVC (15 mm); all non-significant (ns) results are noted; ‘+’ designates BOLD signal increases (red in Figure 2C) and ‘−’ designates BOLD signal decreases (blue in Figure 2C) from N to P (absolute values and SPM analyses); the last column presents SPM results of the practice by group interaction: ‘+’ designates N<P in the cocaine subjects and N>P in the control subjects (red in Figure 3C) while ‘−’ designates N>P in the cocaine subjects and N<P in the control subjects (blue in Figure 3C); T-scores in boldface designate the ROIs presented in Figure 4; arrow pointing up indicates activation (BOLD signal increase from baseline), arrow pointing down indicates deactivation (BOLD signal decrease from baseline), two arrows vs. one arrow indicate larger vs. smaller fMRI BOLD signal change for first vs. second repetition (N:P). Some of the arrows indicate activations/deactivations at levels that in Table 1 did not reach nominal (p<0.01 corrected) level. Minimum cluster = 15 voxels.
Most pertinent to the goals of the current study, significant practice-related fMRI BOLD signal changes were noted mostly in the cocaine addicted individuals. In the comparison group, the only changes were in two limited clusters in the cuneus (CUN) (the cluster also included the lingual and middle/superior occipital gyrus) and precuneus (preCUN) (the cluster also included the superior parietal lobule) (Table 2 and Figure 2C.1, numbers in circles correspond to the clusters in Table 2; note that the high significance threshold level that was chosen for these analyses rendered the clusters’ appearance as limited in size in Figure 2C; however, all circled clusters were significant. Also, all were individually inspected for fMRI BOLD signal increases and decreases as noted by arrows in Table 2). In contrast, in the cocaine abusers, significant practice-related differences were more widespread and noted in the cerebellum, anterior cingulate cortex/ventromedial rostral PFC (ACC/vmrPFC), inferior frontal gyrus (IFG), middle/superior frontal gyrus (MFG/SFG), and medial frontal gyrus (MedFG) (Table 2 and Figure 2C.2). Direct group comparisons in SPM revealed that these practice-related group differences (i.e., the interaction between practice and group) were significant in all areas except the IFG (Table 2, last column, and Figure 3C). These SPM analyses therefore showed greater practice effects in the cocaine abusers than control subjects in the cerebellum, ACC/vmrPFC, and MFG/SFG, and also more posteriorly in the MedFG and in the control subjects than the cocaine abusers in the posterior CUN and preCUN. Results of the ROI analyses confirmed the significant practice by group interaction in all these regions (Figure 4, box below each ROI designates results of significant analyses). Note that group differences in these practice-related (N vs. P) regions were mostly distinct from the group differences in task-related (N or P vs. baseline) activations and deactivations (compare Figure 3C to 3A and 3B); nevertheless, the effect of these task-related differences between the groups (Figure 3A–B and Table 1) needs to be considered when interpreting results.
There were no significant correlations between any of these ROIs with age. Similarly, there were no differences in any of these ROIs between the urine status or cigarette smoking subgroups; age, urine status, and smoking were therefore not used as covariates (note that a significant association between a dependent variable and a possibly confounding variable is a prerequisite for a covariate analysis, (Stevens, 1992)).
Again, we conducted correlations between these ROIs with numerous drug use variables as described above. Here, the cerebellum (at both N: r=−.66, p<.01; and P: r=−.72, p<.01) correlated with cocaine craving such that the more the cerebellar activation, the lower the reported craving at study day. The CUN correlated with the time period of the heaviest use of cocaine: the more the deactivation, again especially at second task repetition, the less was the frequency of cocaine use (days/week; N: r=.62, p=.02, P: r=.70, p<.01). Finally, the MedFG correlated with the length of last voluntary abstinence: the more the deactivation, the longer the abstinence (at N: r=−.70, p<.01).
Practice-related correlations between neural responses with performance (Figure 5)
Figure 5.
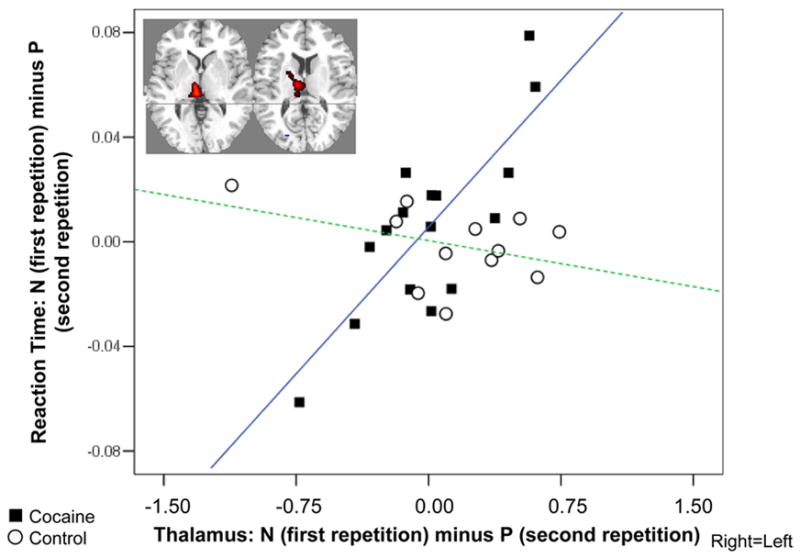
Correlation between practice-related thalamic BOLD signal decreases and reaction time decreases. Scatterplot shows association between the BOLD signal change for the first as compared to the second repetition (N minus P) in the right thalamus (average of x=6, y=−12, z=6 and x=3, y=−27, z=3; 239 voxels, T=6.9, p<0.0001 cluster level corrected) with a respective change in performance speed in the cocaine (black squares, r=0.80, p<0.0001) and control (white circles, r=−0.40, p>0.1) subjects. The inserted statistical map of brain activation depicts the cluster location corresponding to the significant correlation. Thresholded at p<0.005 uncorrected, minimum cluster size 15 voxels (0.41 cc). Right=Left.
The average practice-related decreases of BOLD responses in the right thalamus correlated positively with practice-related decreases in reaction time in the cocaine abusers only (Figure 5). That is, lower activation was associated with faster reaction time at second task repetition (P). Note that some subjects had longer reaction times at P and in these subjects there was an increased activation of the thalamus (this in turn explains why there were no differences between N and P in the thalamus or in reaction times, since some subjects improved/decreased activations but others worsened/increased activations upon repetition). This significant correlation was unchanged when controlling for age, urine status, or cigarette smoking (with partial correlations). There were no significance correlations between these differential variables (N minus P for reaction time or thalamus) with the inspected drug use variables. There were no significant correlations with practice-related changes in performance accuracy.
Discussion
Consistent with our a priori hypothesis, results point to a change in the neural habituation responses to practice on an incentive sustained attention task as a function of cocaine addiction. As predicted, this change encompassed the PFC. Specifically, cocaine abusers demonstrated significant BOLD signal attenuations from novelty to practice in the ACC/vmrPFC and MedFG (deactivations returned towards baseline), and in the dorsolateral PFC (MFG/SFG) and also the cerebellum (activations returned towards baseline) (Figures 2C.2 and Figure 4A and B). This pattern in the cocaine subjects was associated with more severe cocaine use (encompassing craving, frequency of use and length of abstinence). In contrast, control subjects demonstrated significant BOLD signal amplifications (i.e., an increase in deactivations further away from baseline) in posterior areas (CUN and preCUN) (Figure 2C.1 and Figure 4B). Moreover, practice-related BOLD signal decreases in the thalamus correlated with practice-related reaction time decreases, in the cocaine abusers but not control subjects (Figure 5). Thus, although we report secondary analyses of separately published data, which renders the task not optimized for the assessment of neural responses to habituation, our task design (exact repetition of the same block of events) allowed us to add new information about the effect of psychopathology on neural habituation to repeated stimuli (see one prior fMRI study with auditory stimulation in 11 patients with major depressive disorder, (Michael et al., 2004)).
In healthy human volunteers, habituation after prolonged or repeated exposure to a certain stimulus or task is frequently associated with fMRI BOLD signal changes in a distributed network spanning the PFC (ACC/vmrPFC and dorsolateral PFC), insula, parietal and occipito-temporal regions (including the CUN and preCUN) (Chein and Schneider, 2005) as well as subcortical regions (putamen) and the cerebellum (Puttemans et al., 2005). Specifically, two major patterns have been identified: (1) BOLD signal attenuations (i.e., decreases towards baseline): these may reflect the more precise task-related functional map (where processes specific to novelty are removed) (Garavan et al., 2000) that accompanies greater stimulus familiarity and task exposure (Asaad et al., 1998); and (2) BOLD signal amplifications (i.e., increases away from baseline): these may instead reflect an expanded cortical representation of the task-relevant information (Karni et al., 1995) or indicate a regional role in long-term memory formation that accompanies the automatization or overlearning of a response (Puttemans et al., 2005).
The former pattern of fMRI BOLD signal attenuation with practice has frequently been observed in the PFC. The PFC is the main area considered to perform a ‘scaffolding’ role; that is, it copes with novel demands during unskilled and effortful performance. After practice, processes or associations are more efficiently stored and accessed and the scaffolding network falls away, evidenced by decreased signal (Petersen et al., 1998). The latter pattern of fMRI BOLD signal amplification with practice has frequently been observed in the deactivations that characterize the more posterior regions (including the CUN and preCUN); it has been interpreted to reflect increased neural efficiency in the brain networks underlying task performance (Kelly and Garavan, 2005), stronger representation of task demands and guidance of task output (Kirschen et al., 2005), or an amplification of neural activity within task-relevant processing systems (Milham et al., 2003). Note also that deactivations in the preCUN and adjacent posteromedial cortical regions have been implicated in altered states of consciousness such as sleep (reviewed in (Cavanna and Trimble, 2006)); therefore these regions may be associated with maintaining alertness which one would expect to decrease with practice and habituation to novelty. Together, both patterns are interpreted as a learning-related transition from attention/control-demanding and declarative mechanisms to more automatic/procedural processes (reviewed in (Kelly and Garavan, 2005)).
The first pattern (fMRI signal attenuation with practice) was not significant in any of the inspected regions in the control subjects. In contrast, in the cocaine abusers, attenuations to practice were significant in the ACC/vmrPFC and MedFG BA 6 (deactivations returned/decreased towards baseline) and the MFG/SFG (dorsolateral PFC) and cerebellum (activations returned/decreased towards baseline). Recent fMRI studies documented decreases in the rostral ACC (BA 24/32) and MedFG (BA 6) to repeated presentations of emotionally salient stimuli in healthy subjects (Feinstein et al., 2002; Phan et al., 2003). Similarly, roles for the dorsolateral PFC (Chein and Schneider, 2005) and cerebellum (Puttemans et al., 2005) in intact practice-related processing were previously described (note that although we had no a priori hypotheses regarding the cerebellum, the cerebellum forms an integral part of the cortical-subcortical network that subserves higher cognitive function (Ramnani, 2006)). Therefore, lack of similar results in the healthy control subjects in the current study may indicate that the control group continued responding to our incentive manipulation longer than did the cocaine group; this lingering responsivity to monetary reward may indeed be related to the control subjects’ increased ability to maintain the alert state as suggested below. Thus, although all study subjects provided higher interest and excitement ratings to the higher than lower monetary conditions, we cannot rule out the possibility that there may have been differences between the groups in their conscious awareness of the emotional saliency of the stimulus; indeed a failure to see differences in these self-reports between the groups could reflect disrupted interoception in the cocaine abusers as we suggested separately (Goldstein et al., 2007). The use of on-line (and not post-task) self-reports may be more sensitive to these group differences.
The second pattern (fMRI signal amplification with practice) was observed only in the control and not cocaine subjects and encompassed the CUN and preCUN, consistent with an increasing reliance as practice progresses on performance-related areas located posteriorly in the brain (reviewed in (Kelly and Garavan, 2005)). Recall that our task was simple and average performance was very high in all subjects (>96% accuracy). Therefore, this second pattern in the control subjects may also be indicative of a relative decrease in goal-directed processes (recruited during N) and a return to resting states (at P); these resting states are devoted to general information gathering and evaluation (Gusnard and Raichle, 2001) possibly in the service of maintaining an alert consciousness (Cavanna and Trimble, 2006) especially when the novelty elements from the task decrease with repetition. The absence of similar results (BOLD signal amplifications/increases) in the cocaine abusers cannot be entirely attributed to the group differences in task difficulty level, because performance accuracy did not differ between N and P and it was also treated as a nuisance variable in all SPM analyses. Our results therefore suggest that in cocaine addiction, a practice-dependent development of efficient, automatic, and procedural neural processes or the regulation of return to a relatively task-disengaged but alert resting state may be impaired.
Finally, in the current study where we used secondary analyses of previously published data, the fMRI BOLD signal change in the thalamus was correlated with a behavioral change to practice, such that the more the activation decreased, the faster was the behavioral response in the current sample of cocaine addicted subjects. These results are consistent with a recent implication of the thalamus in goal-directed behavior (e.g., abolishing bias for large-reward option of action and pursuing a requested small-reward action instead (Minamimoto et al., 2005)). Overall, both the correlation between the thalamus and speed of response (Figure 5) and the PFC practice effects (Figure 4) observed in the cocaine abusers but not control subjects in the current study may be indicative of dopaminergic influences on habituation in the former group (for dopaminergic thalamic contributions see (Sanchez-Gonzalez et al., 2005); for similar PFC associations see (Volkow et al., 2005)). Nevertheless, our results do not negate the potential contribution from other neurotransmitter systems (such as glutamate, GABA, norepinephrine and serotonin) that are also altered in cocaine addiction (e.g., (Cornish and Kalivas, 2000)).
Study limitations
Limitations mostly pertain to the secondary analysis nature of this study, consequently not allowing us to optimize the task or the sample size for the current purposes. The following limitations are of particular mention. First, similarly to other studies (e.g., (Landau et al., 2004)), in our study changes in activation occurred in the absence of performance changes, i.e., the neural effects of practice were independent of evidence of changes in the behavioral data; we attribute this to the pre-task training which achieved an asymptotic behavior in all subjects and to the decreased performance variability induced by the restricted level of difficulty on the current simple task. We were therefore not able to directly attribute behavior on the task to its neural changes; use of a more difficult task could allow documentation of behavioral changes in performance during the novel block, with asymptotic levels of performance reached by the second block, and may also render the fMRI analyses more sensitive to neural habituation. Nevertheless direct brain-behavior correlations suggested that such neural habituation-related changes were behaviorally meaningful, at least for the cocaine abusers, even in the absence of overt behavioral differences between novelty and practice. We were also not able to examine error-related signal changes, and this finer analysis remains to be conducted in future studies; for example, commission errors may be indicative of increased impulsivity while non-commission errors may instead be indicative of diminished ability to maintain interest or salience attribution to the monetary reward in the cocaine addicted individuals (see for example (Garavan et al., 2003)).
Second, because this was one of the first fMRI studies to target habituation effects in psychopathology, power considerations led us to model responses over as many trials as possible; consequently, we may have missed habituation effects that occur rapidly, i.e., within few trials. Moreover, since this study used a block design, practice may have influenced not only sustained attention but also any one or all of the other processes involved in the task including other attention, sensory and memory processes, motor preparation, motor response and incentive motivation. Future studies using event-related designs on a trial-by-trial basis would be needed to allow a finer-grained characterization of such psychopathology-driven habituation dynamics.
Third, although activations/deactivations in the selected ROIs were not associated with age, urine status for cocaine (i.e., acute withdrawal), or cigarette smoking, the effect of these variables on practice-related neural habituation remains to be more systematically explored in larger and more homogeneous samples with drug addiction or other psychopathology. The potential effects of task-related differences (Table 1) and other factors (e.g., measures of premorbid functioning) that commonly differ between subjects with an identifiable psychopathology and healthy control subjects should also be considered. In such future efforts, results need to be replicated in subgroups equated for size. In the current study group sizes were unequal; although we adjusted for this sample size difference in our analyses, we consequently also restricted the possibility of a residual Type I error or unreliable results by using stringent Bonferroni corrections. Thus, important results may have been missed.
Summary
We report habituation-related neural adaptation to a sustained attention task in frontal brain regions, encompassing the ACC/vmrPFC, dorsolateral PFC, and MedFG, and also in the cerebellum in cocaine abusers. In contrast, in control subjects, neural habituation encompassed posterior brain regions (including the CUN and preCUN). Increased response speed with practice was correlated with a respective decreased activation in the thalamus only in the cocaine abusers. The practice-related habituation of the PFC and the thalamic correlation with behavioral change in the cocaine abusers and not control subjects possibly reflect the disruption of thalamo-frontal circuits in drug addiction; structural (e.g., decreased gray or increased white matter) and functional (e.g., decreased responsiveness to neuropsychological tasks) abnormalities in this circuit have been frequently demonstrated in drug addicted individuals as previously reviewed. It is further intriguing to note that the practice-related changes in this circuit in the current study were correlated with severity of drug use (encompassing cocaine craving, frequency of cocaine use, and abstinence length). Overall, we conclude that cocaine abusers habituate faster to the environmental conditions linked with the task; this may reflect disruption of the physiological mechanisms (including dopamine and norepinephrine) involved with maintenance of interest and alertness not mitigated by a compromised PFC ability to attribute salience to a reward.
Of final note is the fact that the current study focused on the practice main effect while our separately reported analyses (Goldstein et al., 2007) focused on the monetary main effect on this task in subjects with cocaine use disorders. Future studies using event-related designs need to employ different levels of both reward and habituation/practice, studied orthogonally, to allow for the inspection of these variables’ main and potential interaction effects on neural and behavioral responses to a sustained attention and other cognitive tasks in drug addiction.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to DT: R03 DA 017070-01, and to RZG: 1K23 DA15517-01); Laboratory Directed Research and Development from U.S. Department of Energy (OBER); NARSAD Young Investigator Award (to RZG); National Institute on Alcohol Abuse and Alcoholism (AA/ODO9481-04 to NDV); and General Clinical Research Center (5-MO1-RR-10710). We are also grateful to the anonymous reviewers’ constructive and thought-provoking comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Oberling P, Di Scala G, Sandner G. Amphetamine-induced conditioned activity does not result from a failure of rats to habituate to novelty. Psychopharmacology (Berl) 1996;123:325–332. doi: 10.1007/BF02246642. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Bechara AB, Dolan S, Andrea H. Decision-Making and addiction (part II): myopia for the future or hypersensitivity to reward. Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94:47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Cocaine-conditioned behavioral effects: a role for habituation processes. Pharmacol Biochem Behav. 2003;74:701–712. doi: 10.1016/s0091-3057(02)01072-9. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Biol Psychiatry. 2006. Reflection Impulsivity in Current and Former Substance Users. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Scinto LF, Weitzman AM, Faust R, Rentz DM, Budson AE, Holcomb PJ. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J Cogn Neurosci. 2003;15:294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biol Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Goldin PR, Stein MB, Brown GG, Paulus MP. Habituation of attentional networks during emotion processing. Neuroreport. 2002;13:1255–1258. doi: 10.1097/00001756-200207190-00007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. In: Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0) Williams J, editor. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Franckowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Cottone LA, Volkow ND, editors. The Orbitofrontal Cortex in Drug Addiction. Oxford University Press; 2006. [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:1–9. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magnetic Resonance in Medicine. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D’Esposito M. A functional MRI study of the influence of practice on component processes of working memory. Neuroimage. 2004;22:211–221. doi: 10.1016/j.neuroimage.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magnetic Resonance in Medicine. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Michael N, Ostermann J, Soros P, Schwindt W, Pfleiderer B. Altered habituation in the auditory cortex in a subgroup of depressed patients by functional magnetic resonance imaging. Neuropsychobiology. 2004;49:5–9. doi: 10.1159/000075331. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug Alcohol Depend. 2003;71:133–141. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28:1344–1350. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005;25:4270–4278. doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. 2. Lawrence Erlbaum Associates; NewJersey: 1992. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Geusau NA, Stalenhoef P, Huizinga M, Snel J, Ridderinkhof KR. Impaired executive function in male MDMA (“ecstasy”) users. Psychopharmacology (Berl) 2004;175:331–341. doi: 10.1007/s00213-004-1832-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The Wide-Range Achievement Test 3- Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]


