Abstract
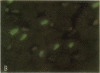
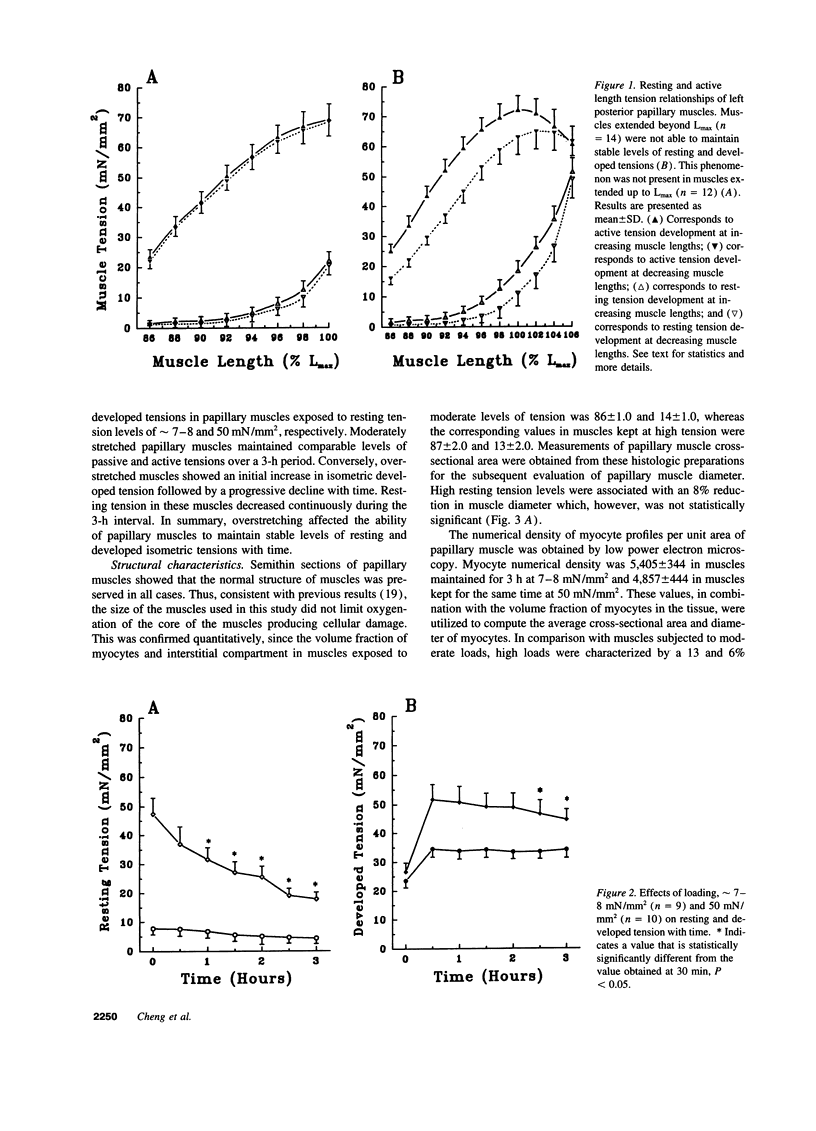
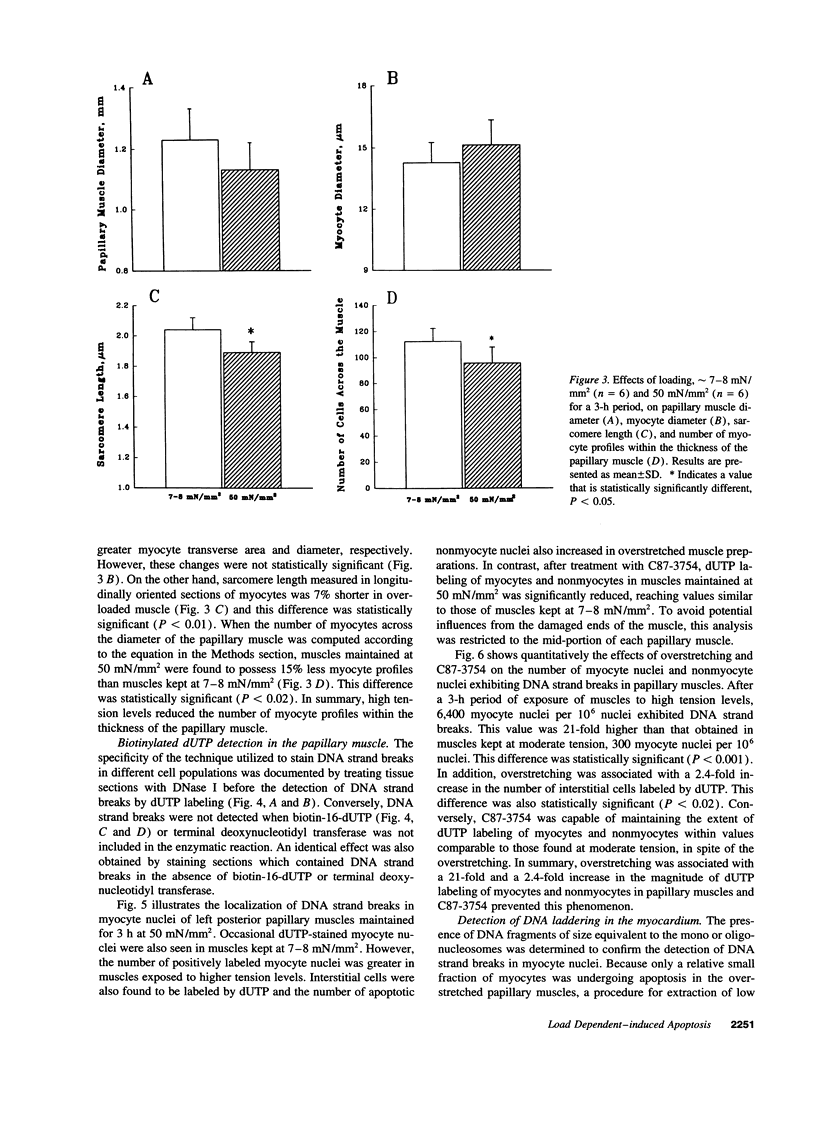
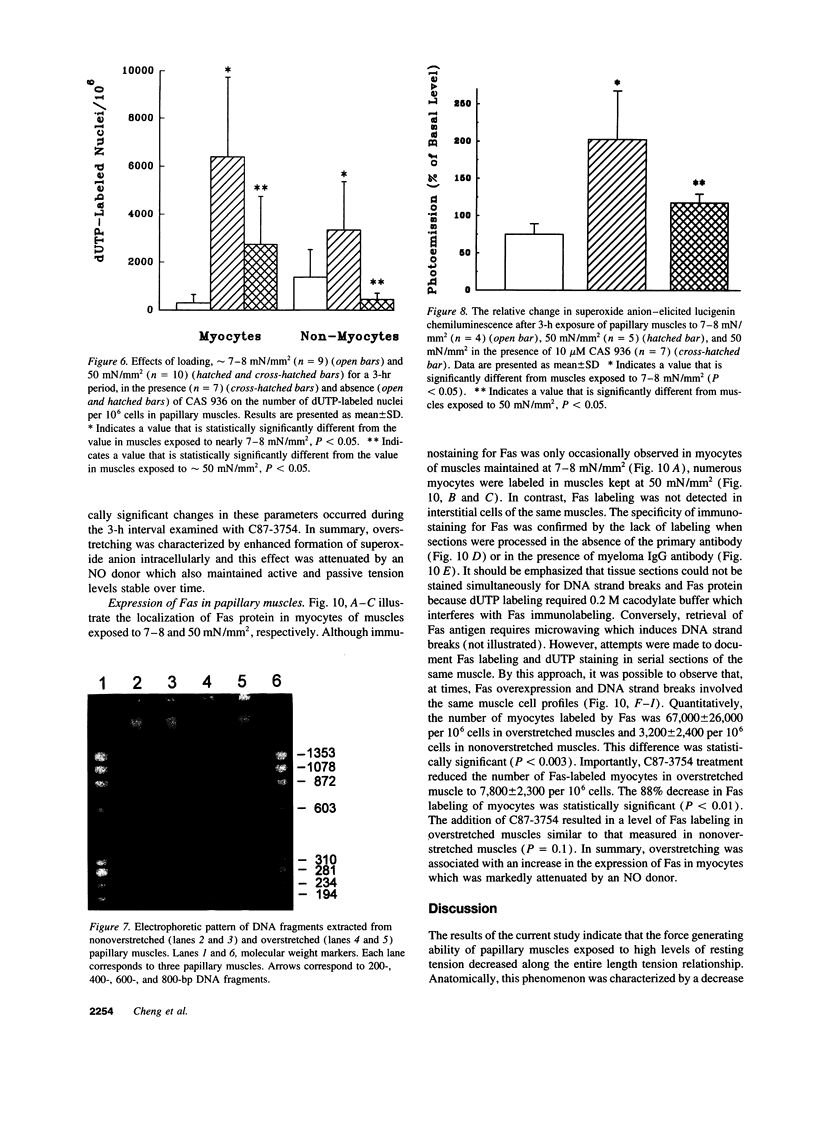
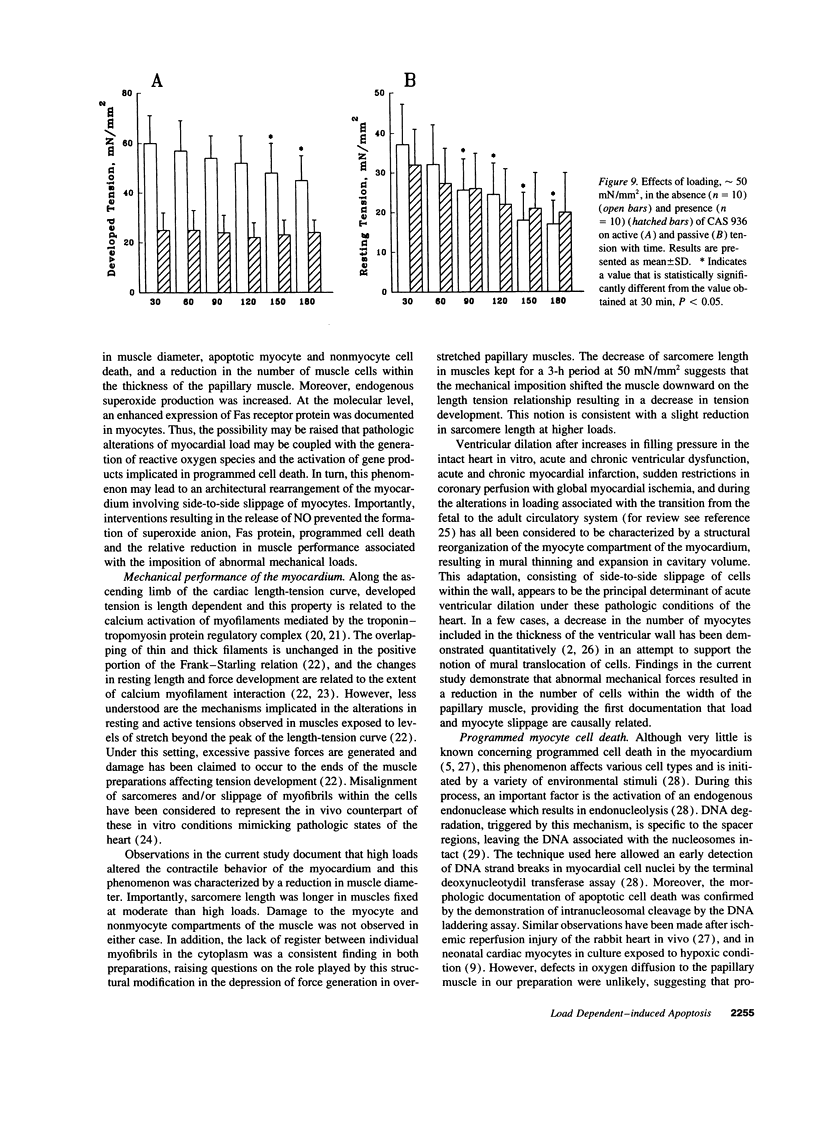
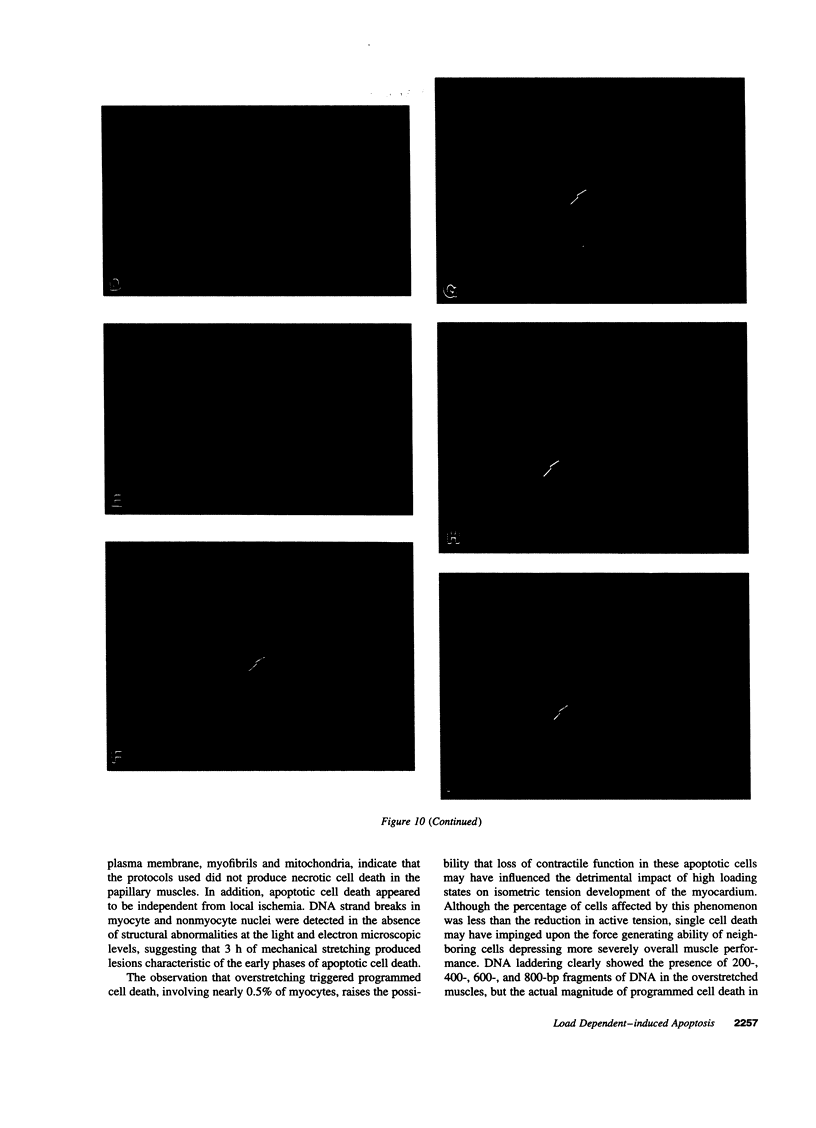
To determine the effects of loading on active and passive tensions, programmed cell death, superoxide anion formation, the expression of Fas on myocytes, and side-to-side slippage of myocytes, papillary muscles were exposed to 7-8 and 50 mN/mm2 and these parameters were measured over a 3-h period. Overstretching produced a 21- and a 2.4-fold increase in apoptotic myocyte and nonmyocyte cell death, respectively. Concurrently, the generation of reactive oxygen species increased 2.4-fold and the number of myocytes labeled by Fas protein 21-fold. Moreover, a 15% decrease in the number of myocytes included in the thickness of the papillary muscle was found in combination with a 7% decrease in sarcomere length and the inability of muscles to maintain stable levels of passive and active tensions. The addition of the NO-releasing drug, C87-3754, prevented superoxide anion formation, programmed cell death, and the alterations in active and passive tensions with time of overloaded papillary muscles. In conclusion, overstretching appears to be coupled with oxidant stress, expression of Fas, programmed cell death, architectural rearrangement of myocytes, and impairment in force development of the myocardium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Anversa P., Li P., Zhang X., Olivetti G., Capasso J. M. Ischaemic myocardial injury and ventricular remodelling. Cardiovasc Res. 1993 Feb;27(2):145–157. doi: 10.1093/cvr/27.2.145. [DOI] [PubMed] [Google Scholar]
- Anversa P., Puntillo E., Nikitin P., Olivetti G., Capasso J. M., Sonnenblick E. H. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol. 1989 May;256(5 Pt 2):H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu D., Kelly R. A., Kobzik L., Pimental D., Michel T., Smith T. W. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993 May;91(5):2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn H., Beyerle R., Martorana P. A., Schönafinger K. CAS 936, a novel syndnonimine with direct vasodilating and nitric oxide-donating properties: effects on isolated blood vessels. J Cardiovasc Pharmacol. 1991 Oct;18(4):522–527. doi: 10.1097/00005344-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Bolli R., Jeroudi M. O., Patel B. S., Aruoma O. I., Halliwell B., Lai E. K., McCay P. B. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial "stunning" is a manifestation of reperfusion injury. Circ Res. 1989 Sep;65(3):607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Warren J. B., Poole-Wilson P. A., Williams T. J., Harding S. E. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1993 Jul;265(1 Pt 2):H176–H182. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Jeanty M. W., Palackal T., Olivetti G., Anversa P. Ventricular remodeling induced by acute nonocclusive constriction of coronary artery in rats. Am J Physiol. 1989 Dec;257(6 Pt 2):H1983–H1993. doi: 10.1152/ajpheart.1989.257.6.H1983. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Gong J., Traganos F., Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994 May 1;218(2):314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994 Oct;94(4):1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Sonnenblick E., Babu A. The role of troponin C in the length dependence of Ca(2+)-sensitive force of mammalian skeletal and cardiac muscles. J Physiol. 1991 Sep;441:305–324. doi: 10.1113/jphysiol.1991.sp018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. M., Keaney J. F., Jr, Balligand J. L., Loscalzo J., Smith T. W., Colucci W. S. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995 Jan;95(1):360–366. doi: 10.1172/JCI117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kajstura J., Mansukhani M., Cheng W., Reiss K., Krajewski S., Reed J. C., Quaini F., Sonnenblick E. H., Anversa P. Programmed cell death and expression of the protooncogene bcl-2 in myocytes during postnatal maturation of the heart. Exp Cell Res. 1995 Jul;219(1):110–121. doi: 10.1006/excr.1995.1211. [DOI] [PubMed] [Google Scholar]
- Kaminski P. M., Wolin M. S. Hypoxia increases superoxide anion production from bovine coronary microvessels, but not cardiac myocytes, via increased xanthine oxidase. Microcirculation. 1994 Dec;1(4):231–236. doi: 10.3109/10739689409146750. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Kiuchi K., Sato N., Shannon R. P., Vatner D. E., Morgan K., Vatner S. F. Depressed beta-adrenergic receptor- and endothelium-mediated vasodilation in conscious dogs with heart failure. Circ Res. 1993 Dec;73(6):1013–1023. doi: 10.1161/01.res.73.6.1013. [DOI] [PubMed] [Google Scholar]
- Li P., Sonnenblick E. H., Anversa P., Capasso J. M. Length-dependent modulation of ANG II inotropism in rat myocardium: effects of myocardial infarction. Am J Physiol. 1994 Feb;266(2 Pt 2):H779–H786. doi: 10.1152/ajpheart.1994.266.2.H779. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Mannick J. B., Asano K., Izumi K., Kieff E., Stamler J. S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994 Dec 30;79(7):1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mohazzab K. M., Wolin M. S. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol. 1994 Dec;267(6 Pt 1):L823–L831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- Niu X. F., Smith C. W., Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res. 1994 Jun;74(6):1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Capasso J. M., Meggs L. G., Sonnenblick E. H., Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991 Mar;68(3):856–869. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Capasso J. M., Sonnenblick E. H., Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res. 1990 Jul;67(1):23–34. doi: 10.1161/01.res.67.1.23. [DOI] [PubMed] [Google Scholar]
- Puceat M., Clement O., Lechene P., Pelosin J. M., Ventura-Clapier R., Vassort G. Neurohormonal control of calcium sensitivity of myofilaments in rat single heart cells. Circ Res. 1990 Aug;67(2):517–524. doi: 10.1161/01.res.67.2.517. [DOI] [PubMed] [Google Scholar]
- Ross J., Jr, Sonnenblick E. H., Taylor R. R., Spotnitz H. M., Covell J. W. Diastolic geometry and sarcomere lengths in the chronically dilated canine left ventricle. Circ Res. 1971 Jan;28(1):49–61. doi: 10.1161/01.res.28.1.49. [DOI] [PubMed] [Google Scholar]
- Sandstrom P. A., Mannie M. D., Buttke T. M. Inhibition of activation-induced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leukoc Biol. 1994 Feb;55(2):221–226. doi: 10.1002/jlb.55.2.221. [DOI] [PubMed] [Google Scholar]
- Shen W., Xu X., Ochoa M., Zhao G., Wolin M. S., Hintze T. H. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994 Dec;75(6):1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano M., Totsune-Nakano H., Minakami H., Tero-Kubota S., Ikegami Y. Mechanism of O2- generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochim Biophys Acta. 1988 Dec 7;936(3):377–385. doi: 10.1016/0005-2728(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ito H., Adachi S., Akimoto H., Nishikawa T., Kasajima T., Marumo F., Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 1994 Sep;75(3):426–433. doi: 10.1161/01.res.75.3.426. [DOI] [PubMed] [Google Scholar]
- Turner M. J., 3rd, Fields C. E., Everman D. B. Evidence for superoxide formation during hepatic metabolism of tamoxifen. Biochem Pharmacol. 1991 Jun 1;41(11):1701–1705. doi: 10.1016/0006-2952(91)90172-2. [DOI] [PubMed] [Google Scholar]
- Vitali-Mazza L., Anversa P., Tedeschi F., Mastandrea R., Mavilla V., Visioli O. Ultrastructural basis of acute left ventricular failure from severe acute aortic stenosis in the rabbit. J Mol Cell Cardiol. 1972 Dec;4(6):661–671. doi: 10.1016/0022-2828(72)90119-8. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Yoran C., Covell J. W., Ross J., Jr Structural basis for the ascending limb of left ventricular function. Circ Res. 1973 Feb;32(2):297–303. doi: 10.1161/01.res.32.2.297. [DOI] [PubMed] [Google Scholar]