Abstract
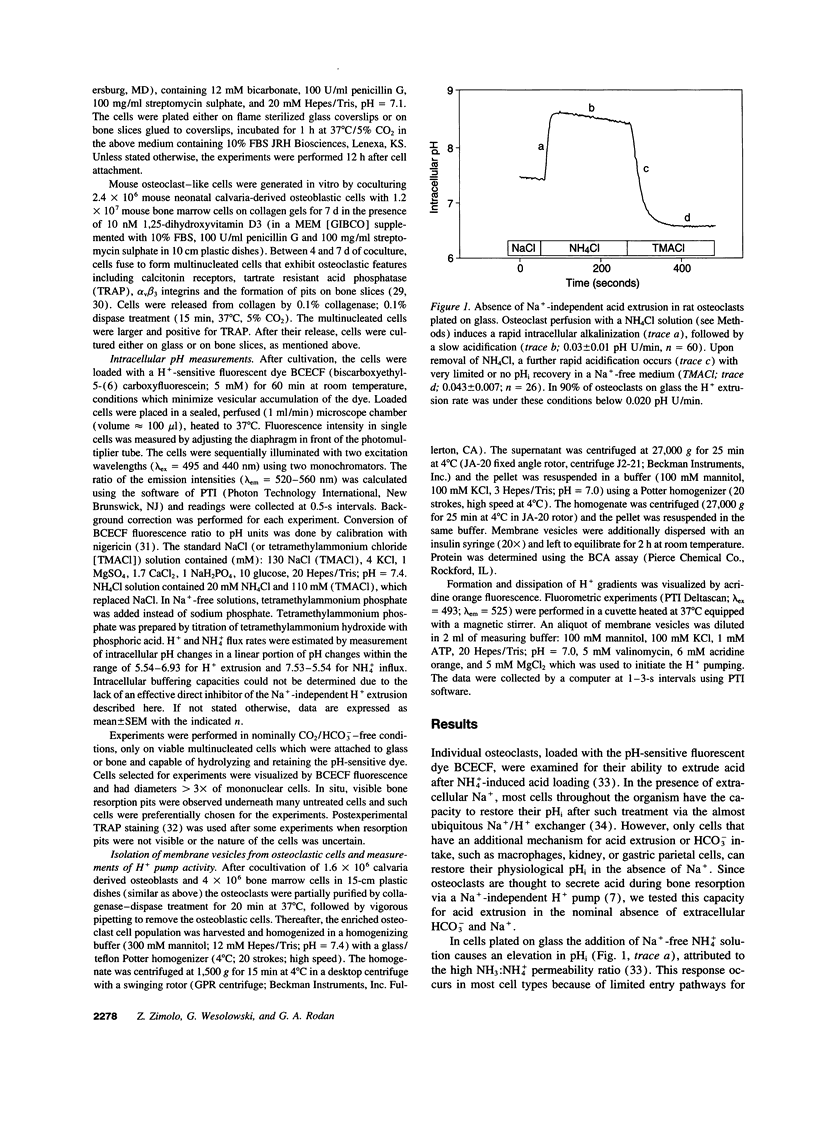
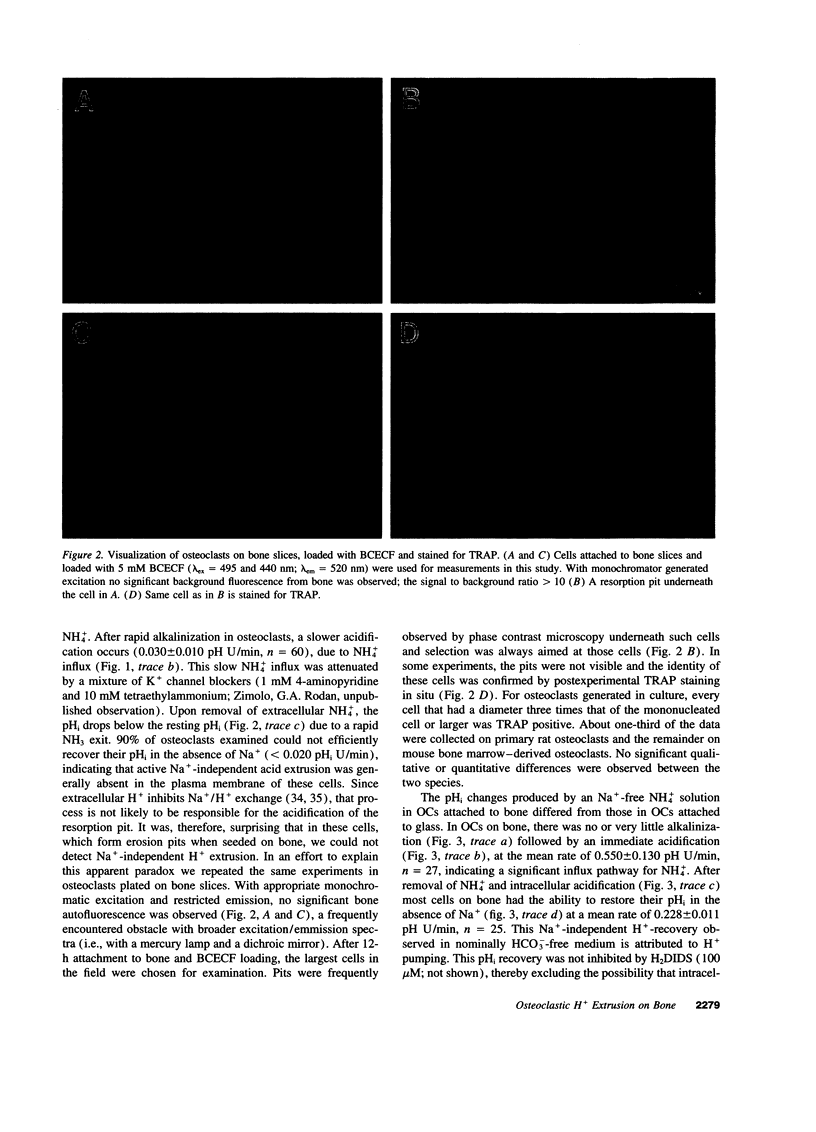
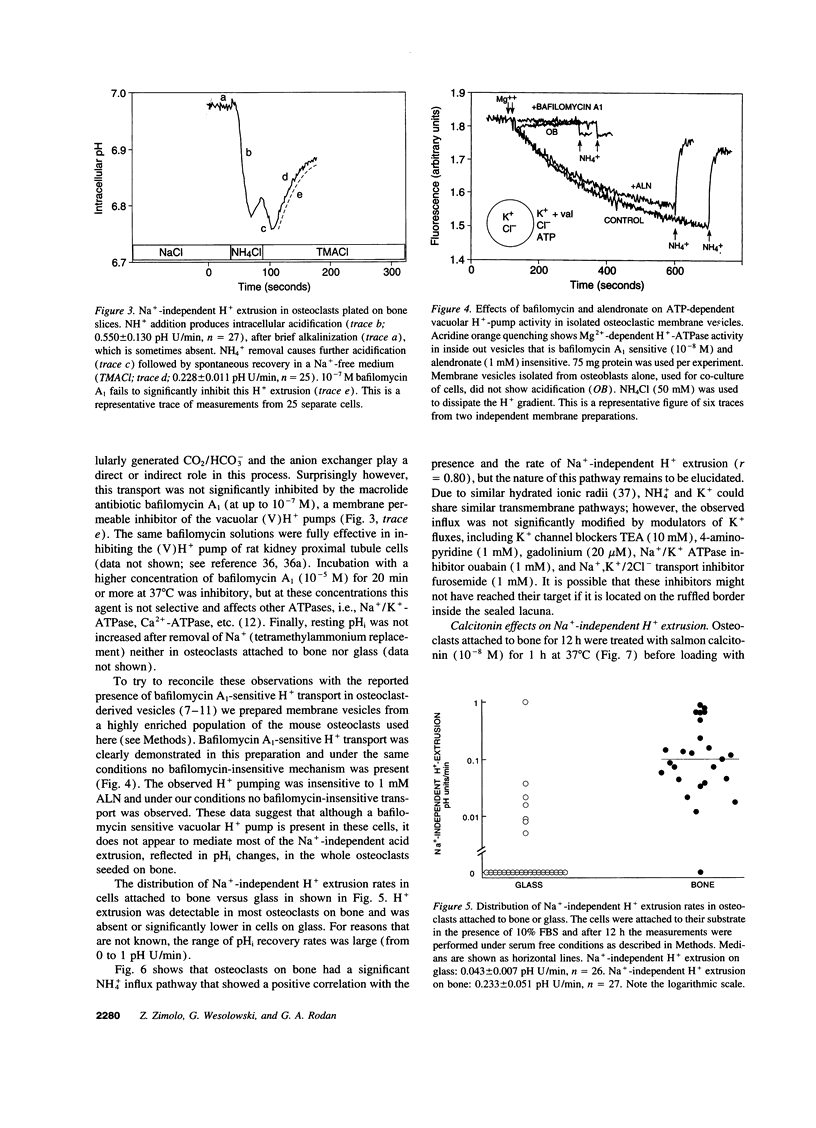
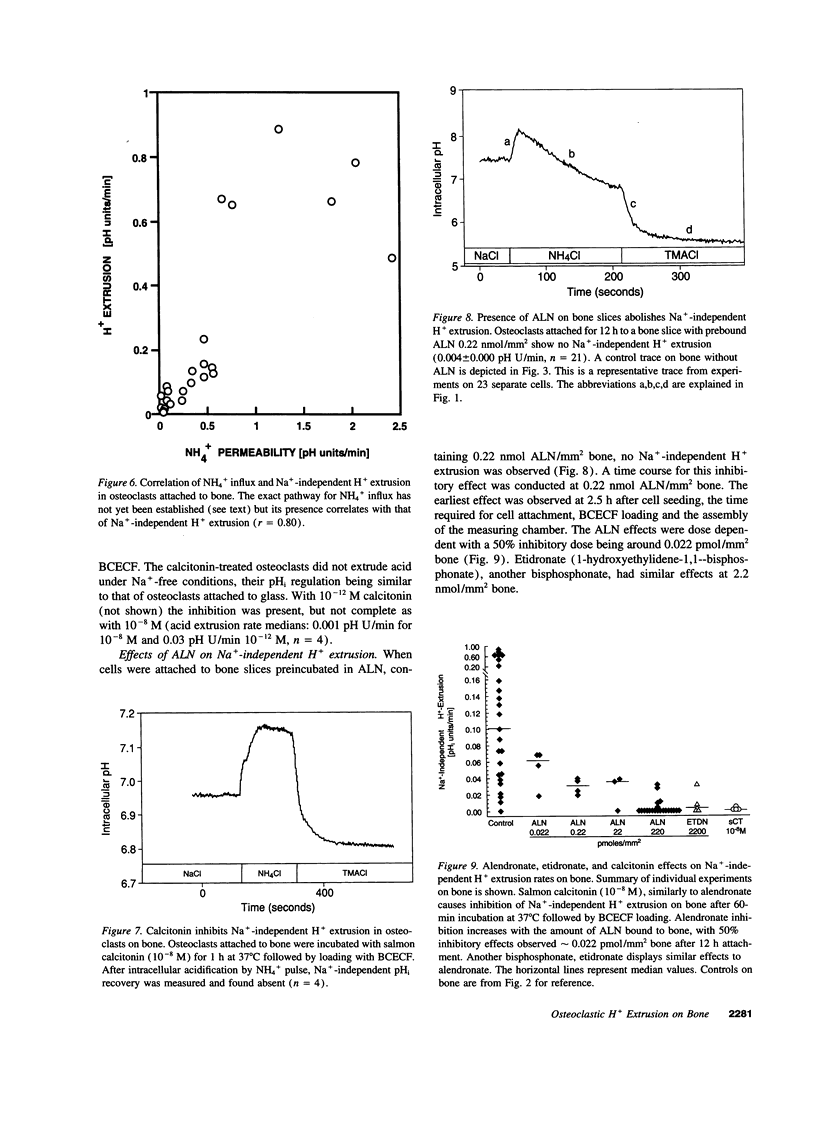
Acid extrusion is essential for osteoclast (OC) activity. We examined Na+ and HCO3(-)-independent H+ extrusion in rat- and mouse OCs by measuring intracellular pH (pHi) changes, with the pHi indicator BCECF (biscarboxyethyl-5-(6) carboxyfluorescein) after H+ loading with an ammonium pulse. 90% of OCs attached to glass do not possess HCO3- and Na(+)-independent H(+)-extrusion (rate of pHi recovery = 0.043 +/- 0.007 (SEM) pH U/min, n = 26). In contrast, in OCs attached to bone, the pHi recovery rate is 0.228 +/- 0.011 pHi U/min, n = 25. OCs on bone also possess a NH(4+)-permeable pathway not seen on glass. The bone-induced H+ extrusion was inhibited by salmon calcitonin (10(-8) M, for 2 h), and was not present after pretreating the bone slices with the aminobisphosphonate alendronate (ALN). At ALN levels of 0.22 nmol/mm2 bone, H+ extrusion was virtually absent 12 h after cell seeding (0.004 +/- 0.002 pH U/min) and approximately 50% inhibition was observed at 0.022 pmol ALN/mm2 bone. The Na(+)-independent H+ extrusion was not inhibited by bafilomycin A1 (up to 10(-7) M), although a bafilomycin A1 (10(-8) M)-sensitive H+ pump was present in membrane vesicles isolated from these osteoclasts. These findings indicate that Na(+)-independent acid extrusion is stimulated by osteoclast attachment to bone and is virtually absent when bone is preincubated with ALN, or when osteoclasts are treated with salmon calcitonin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990 Jan;70(1):79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- Arkett S. A., Dixon S. J., Sims S. M. Substrate influences rat osteoclast morphology and expression of potassium conductances. J Physiol. 1992 Dec;458:633–653. doi: 10.1113/jphysiol.1992.sp019438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balena R., Toolan B. C., Shea M., Markatos A., Myers E. R., Lee S. C., Opas E. E., Seedor J. G., Klein H., Frankenfield D. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993 Dec;92(6):2577–2586. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker P. J., Gay C. V. Biochemical characterization of an electrogenic vacuolar proton pump in purified chicken osteoclast plasma membrane vesicles. J Bone Miner Res. 1990 Jun;5(6):569–579. doi: 10.1002/jbmr.5650050606. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Boonekamp P. M., van der Wee-Pals L. J., van Wijk-van Lennep M. M., Thesing C. W., Bijvoet O. L. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner. 1986 Feb;1(1):27–39. [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury N. A., Bridges R. J. Role of membrane trafficking in plasma membrane solute transport. Am J Physiol. 1994 Jul;267(1 Pt 1):C1–24. doi: 10.1152/ajpcell.1994.267.1.C1. [DOI] [PubMed] [Google Scholar]
- Carano A., Teitelbaum S. L., Konsek J. D., Schlesinger P. H., Blair H. C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990 Feb;85(2):456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini M. G., Felix R., Fleisch H., Cooper P. H. Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J Bone Miner Res. 1987 Apr;2(2):135–142. doi: 10.1002/jbmr.5650020209. [DOI] [PubMed] [Google Scholar]
- Cecchini M. G., Fleisch H. Bisphosphonates in vitro specifically inhibit, among the hematopoietic series, the development of the mouse mononuclear phagocyte lineage. J Bone Miner Res. 1990 Oct;5(10):1019–1027. doi: 10.1002/jbmr.5650051005. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Chakraborty M., Leit M., Neff L., Jamsa-Kellokumpu S., Fuchs R., Baron R. Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton pump from other vacuolar H+ ATPases. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6257–6261. doi: 10.1073/pnas.89.14.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan A. M., Chambers T. J. Dichloromethylenebisphosphonate (Cl2MBP) inhibits bone resorption through injury to osteoclasts that resorb Cl2MBP-coated bone. Bone Miner. 1989 Apr;6(1):33–43. doi: 10.1016/0169-6009(89)90021-4. [DOI] [PubMed] [Google Scholar]
- Fleisch H., Russell R. G., Francis M. D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969 Sep 19;165(3899):1262–1264. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Hanada H., Moriyama Y., Maeda M., Futai M. Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990 Jul 31;170(2):873–878. doi: 10.1016/0006-291x(90)92172-v. [DOI] [PubMed] [Google Scholar]
- Hughes D. E., MacDonald B. R., Russell R. G., Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989 Jun;83(6):1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio D. M., Garant P. R., Minkin C. Ultrastructural effects of calcitonin on osteoclasts in tissue culture. J Ultrastruct Res. 1972 May;39(3):205–216. doi: 10.1016/s0022-5320(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Kanehisa J., Heersche J. N. Osteoclastic bone resorption: in vitro analysis of the rate of resorption and migration of individual osteoclasts. Bone. 1988;9(2):73–79. doi: 10.1016/8756-3282(88)90106-8. [DOI] [PubMed] [Google Scholar]
- Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest. 1987 Oct;80(4):928–935. doi: 10.1172/JCI113184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitala T., Vänänen H. K. Inhibition of bone resorption in vitro by antisense RNA and DNA molecules targeted against carbonic anhydrase II or two subunits of vacuolar H(+)-ATPase. J Clin Invest. 1994 Jun;93(6):2311–2318. doi: 10.1172/JCI117235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs G. L., Kapus A., Nanda A., Romanek R., Grinstein S. Proton conductance of the plasma membrane: properties, regulation, and functional role. Am J Physiol. 1993 Jul;265(1 Pt 1):C3–14. doi: 10.1152/ajpcell.1993.265.1.C3. [DOI] [PubMed] [Google Scholar]
- Löwik C. W., van der Pluijm G., van der Wee-Pals L. J., van Treslong-De Groot H. B., Bijvoet O. L. Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. J Bone Miner Res. 1988 Apr;3(2):185–192. doi: 10.1002/jbmr.5650030210. [DOI] [PubMed] [Google Scholar]
- Mattsson J. P., Lorentzon P., Wallmark B., Keeling D. J. Characterization of proton transport in bone-derived membrane vesicles. Biochim Biophys Acta. 1993 Feb 23;1146(1):106–112. doi: 10.1016/0005-2736(93)90344-y. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Jee W. S. The effect of dichloromethylene diphosphonate, a pyrophosphate analog, on bone and bone cell structure in the growing rat. Anat Rec. 1979 Mar;193(3):439–462. doi: 10.1002/ar.1091930309. [DOI] [PubMed] [Google Scholar]
- Nordström T., Rotstein O. D., Romanek R., Asotra S., Heersche J. N., Manolson M. F., Brisseau G. F., Grinstein S. Regulation of cytoplasmic pH in osteoclasts. Contribution of proton pumps and a proton-selective conductance. J Biol Chem. 1995 Feb 3;270(5):2203–2212. doi: 10.1074/jbc.270.5.2203. [DOI] [PubMed] [Google Scholar]
- Palokangas H., Metsikkö K., Vänänen K. Active vacuolar H+ATPase is required for both endocytic and exocytic processes during viral infection of BHK-21 cells. J Biol Chem. 1994 Jul 1;269(26):17577–17585. [PubMed] [Google Scholar]
- Papapoulos S. E., Hoekman K., Löwik C. W., Vermeij P., Bijvoet O. L. Application of an in vitro model and a clinical protocol in the assessment of the potency of a new bisphosphonate. J Bone Miner Res. 1989 Oct;4(5):775–781. doi: 10.1002/jbmr.5650040518. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Au W. Y., Friedman J., Niemann I. Thyrocalcitonin and bone resorption. Studies employing a tissue culture bioassay. Am J Med. 1967 Nov;43(5):684–690. doi: 10.1016/0002-9343(67)90110-6. [DOI] [PubMed] [Google Scholar]
- Rautiala T. J., Koskinen A. M., Vänänen H. K. Purification of vacuolar ATPase with bafilomycin C1 affinity chromatography. Biochem Biophys Res Commun. 1993 Jul 15;194(1):50–56. doi: 10.1006/bbrc.1993.1783. [DOI] [PubMed] [Google Scholar]
- Sahni M., Guenther H. L., Fleisch H., Collin P., Martin T. J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993 May;91(5):2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Grasser W., Endo N., Akins R., Simmons H., Thompson D. D., Golub E., Rodan G. A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991 Dec;88(6):2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sundquist K., Lakkakorpi P., Wallmark B., Vänänen K. Inhibition of osteoclast proton transport by bafilomycin A1 abolishes bone resorption. Biochem Biophys Res Commun. 1990 Apr 16;168(1):309–313. doi: 10.1016/0006-291x(90)91709-2. [DOI] [PubMed] [Google Scholar]
- Swallow C. J., Grinstein S., Rotstein O. D. A vacuolar type H(+)-ATPase regulates cytoplasmic pH in murine macrophages. J Biol Chem. 1990 May 5;265(13):7645–7654. [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J. M., Martin T. J., Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988 Nov;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Sasaki T., Yamaguchi A., Kodama H., Martin T. J., Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989 Oct;125(4):1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- Vänänen H. K., Karhukorpi E. K., Sundquist K., Wallmark B., Roininen I., Hentunen T., Tuukkanen J., Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 1990 Sep;111(3):1305–1311. doi: 10.1083/jcb.111.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S., Feick P., Zimmermann P., Haase W., Kahn R. A., Schulz I. Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6619–6623. doi: 10.1073/pnas.89.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S., Zimmermann P., Schulz I. Association of a 19- and a 21-kDa GTP-binding protein to pancreatic microsomal vesicles is regulated by the intravesicular pH established by a vacuolar-type H(+)-ATPase. J Membr Biol. 1992 Feb;125(3):231–241. doi: 10.1007/BF00236436. [DOI] [PubMed] [Google Scholar]
- Zimolo Z., Montrose M. H., Murer H. H+ extrusion by an apical vacuolar-type H(+)-ATPase in rat renal proximal tubules. J Membr Biol. 1992 Feb;126(1):19–26. doi: 10.1007/BF00233457. [DOI] [PubMed] [Google Scholar]
- van Hille B., Richener H., Evans D. B., Green J. R., Bilbe G. Identification of two subunit A isoforms of the vacuolar H(+)-ATPase in human osteoclastoma. J Biol Chem. 1993 Apr 5;268(10):7075–7080. [PubMed] [Google Scholar]



