Abstract
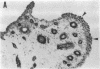
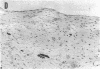
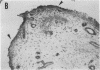
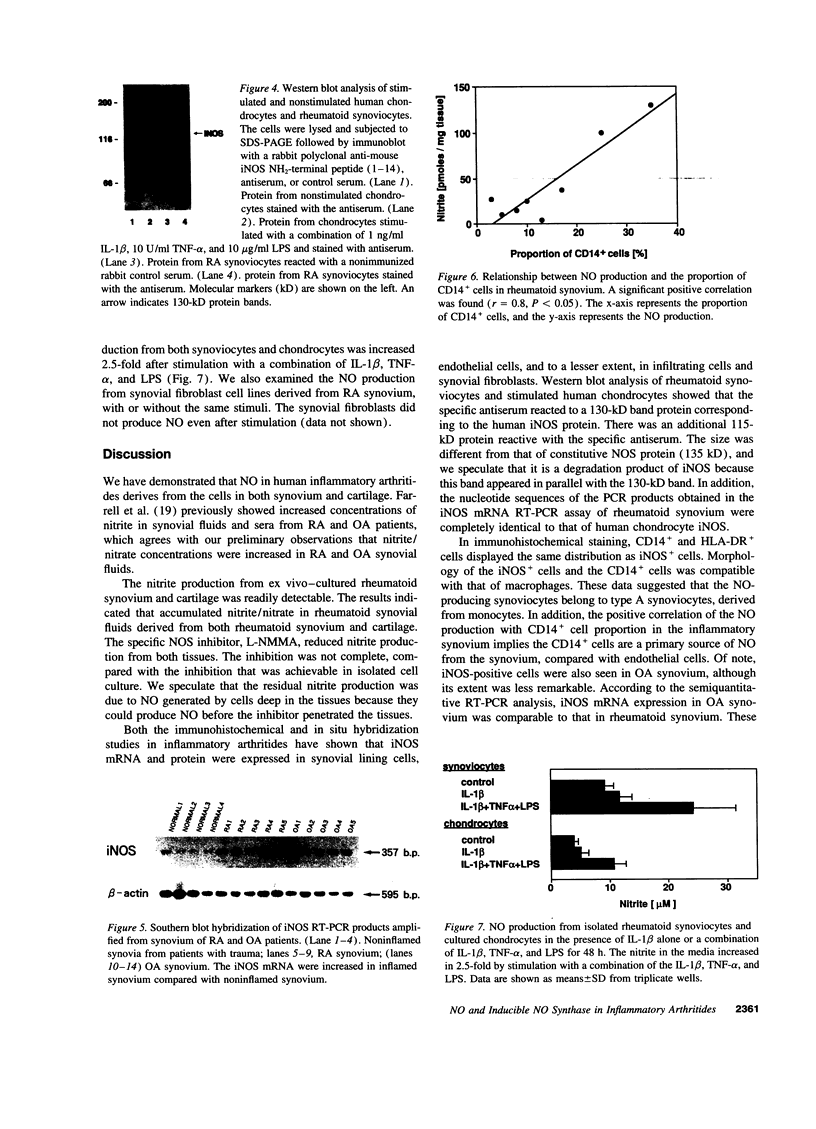
In this study, we have identified the source of nitric oxide (NO) produced in the human inflammatory joints by analyzing expression of inducible NO synthase. In ex vivo organ cultures, both inflammatory synovium and cartilage from patients with rheumatoid arthritis produced NO. The NO production was suppressed by NG-monomethyl-L-arginine, an inhibitor of NO synthase. The amount of NO produced by the synovium correlated with the proportion of CD14+ cells in the corresponding tissue (r = 0.8, P < 0.05). Immunohistochemical analysis as well as in situ hybridization showed that inducible NO synthase was predominantly expressed in synovial lining cells, endothelial cells, chondrocytes, and to a lesser extent, in infiltrating mononuclear cells and synovial fibroblasts. The synovial lining cells and the infiltrating cells expressing inducible NO synthase were identified where CD14+ cells were located. Together with morphological features, this suggests that they are type A synoviocytes. NO production from freshly isolated synoviocytes and chondrocytes was up-regulated by in vitro stimulation with a combination of IL-TNF-beta, TNF-alpha, and LPS. In summary, the present results suggest that NO is produced primarily by CD14+ synoviocytes, chondrocytes, and endothelial cells in inflammatory joints of arthritides. NO production can be upregulated by cytokines present in inflamed joints. The increased NO production may thus contribute to the pathological features in inflammatory arthritides.
Full text
PDF
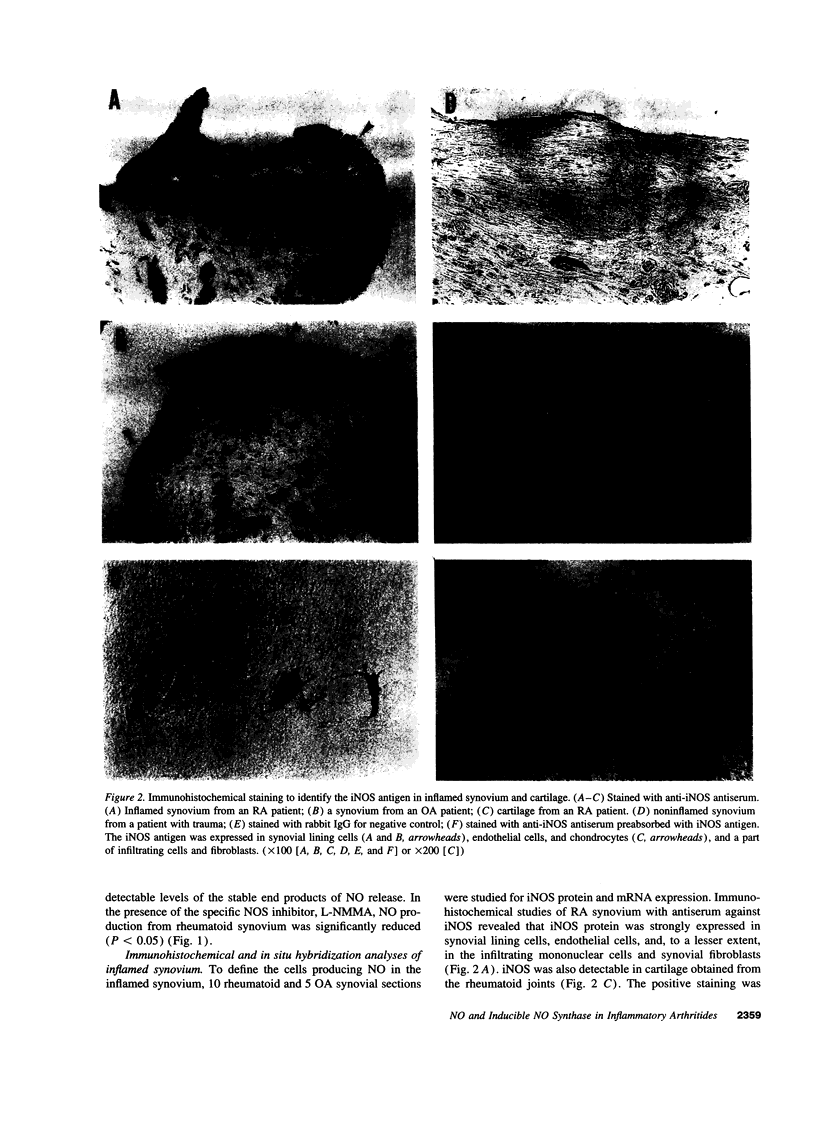



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergroth V., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. III. Rheumatoid arthritis monocytes are not unusually sensitive to gamma-interferon, but have defective gamma-interferon-mediated HLA-DQ and HLA-DR induction. Arthritis Rheum. 1989 Sep;32(9):1074–1079. doi: 10.1002/anr.1780320904. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Paik J., Xie Q. W., Nathan C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol. 1994 Feb;55(2):227–233. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- Charles I. G., Palmer R. M., Hickery M. S., Bayliss M. T., Chubb A. P., Hall V. S., Moss D. W., Moncada S. Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11419–11423. doi: 10.1073/pnas.90.23.11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Moncada S., Liew F. Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Farahat M. N., Yanni G., Poston R., Panayi G. S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993 Dec;52(12):870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A. J., Blake D. R., Palmer R. M., Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992 Nov;51(11):1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G. V., Villanueva T., Schumacher H. R., Gohel V. Autopsy study correlating degree of osteoarthritis, synovitis and evidence of articular calcification. J Rheumatol. 1984 Oct;11(5):681–686. [PubMed] [Google Scholar]
- Hamerman D. The biology of osteoarthritis. N Engl J Med. 1989 May 18;320(20):1322–1330. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Marumo F. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension. 1993 Jul;22(1):34–39. doi: 10.1161/01.hyp.22.1.34. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Langrehr J. M., Murase N., Markus P. M., Cai X., Neuhaus P., Schraut W., Simmons R. L., Hoffman R. A. Nitric oxide production in host-versus-graft and graft-versus-host reactions in the rat. J Clin Invest. 1992 Aug;90(2):679–683. doi: 10.1172/JCI115911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Fong T. W., Harlow L. A., Koch A. E. Production of angiogenic activity by human monocytes requires an L-arginine/nitric oxide-synthase-dependent effector mechanism. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4190–4194. doi: 10.1073/pnas.91.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Kekow J., Carson D. A. Transforming growth factor-beta and cellular immune responses in synovial fluids. J Immunol. 1990 Jun 1;144(11):4189–4194. [PubMed] [Google Scholar]
- Lotz M., Moats T., Villiger P. M. Leukemia inhibitory factor is expressed in cartilage and synovium and can contribute to the pathogenesis of arthritis. J Clin Invest. 1992 Sep;90(3):888–896. doi: 10.1172/JCI115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J. D., Willenborg D. O., Weidemann M. J., Rockett K. A., Cowden W. B. Elevated secretion of reactive nitrogen and oxygen intermediates by inflammatory leukocytes in hyperacute experimental autoimmune encephalomyelitis: enhancement by the soluble products of encephalitogenic T cells. J Exp Med. 1992 Jul 1;176(1):303–307. doi: 10.1084/jem.176.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrinat G., Mason S. N., Shami P. J., Weinberg J. B. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992 Oct 15;80(8):1880–1884. [PubMed] [Google Scholar]
- Mayhan W. G. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5'-diphosphate and bradykinin. Inflammation. 1992 Aug;16(4):295–305. doi: 10.1007/BF00917622. [DOI] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N., Hirata Y., Ando K., Sato K., Morita H., Shichiri M., Kanno K., Tomita K., Marumo F. Increased production of endothelin-1 in patients with inflammatory arthritides. Arthritis Rheum. 1992 Apr;35(4):397–400. doi: 10.1002/art.1780350406. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Yamamoto K., Goto M., Nishioka K. Immunological and immunohistochemical analysis of rheumatoid nodules. Ann Rheum Dis. 1989 Mar;48(3):220–226. doi: 10.1136/ard.48.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama D. K., Geller D. A., Lowenstein C. J., Chern H. D., Davies P., Pitt B. R., Simmons R. L., Billiar T. R. Cytokines and lipopolysaccharide induce nitric oxide synthase in cultured rat pulmonary artery smooth muscle. Am J Respir Cell Mol Biol. 1992 Nov;7(5):471–476. doi: 10.1165/ajrcmb/7.5.471. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nozaki T., Dvorak J. A. Trypanosoma cruzi: flow cytometric analysis of developmental stage differences in DNA. J Protozool. 1991 May-Jun;38(3):234–243. doi: 10.1111/j.1550-7408.1991.tb04435.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Hickery M. S., Charles I. G., Moncada S., Bayliss M. T. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993 May 28;193(1):398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- Rediske J. J., Koehne C. F., Zhang B., Lotz M. The inducible production of nitric oxide by articular cell types. Osteoarthritis Cartilage. 1994 Sep;2(3):199–206. doi: 10.1016/s1063-4584(05)80069-x. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stefanovic-Racic M., Meyers K., Meschter C., Coffey J. W., Hoffman R. A., Evans C. H. N-monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthritis Rheum. 1994 Jul;37(7):1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Tsujino M., Hirata Y., Imai T., Kanno K., Eguchi S., Ito H., Marumo F. Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiocytes. Circulation. 1994 Jul;90(1):375–383. doi: 10.1161/01.cir.90.1.375. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Weinberg J. B., Granger D. L., Pisetsky D. S., Seldin M. F., Misukonis M. A., Mason S. N., Pippen A. M., Ruiz P., Wood E. R., Gilkeson G. S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994 Feb 1;179(2):651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Gunn C., Beckman J. S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- Ziff M. Pathways of mononuclear cell infiltration in rheumatoid synovitis. Rheumatol Int. 1989;9(3-5):97–103. doi: 10.1007/BF00271865. [DOI] [PubMed] [Google Scholar]