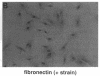
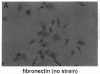
Abstract
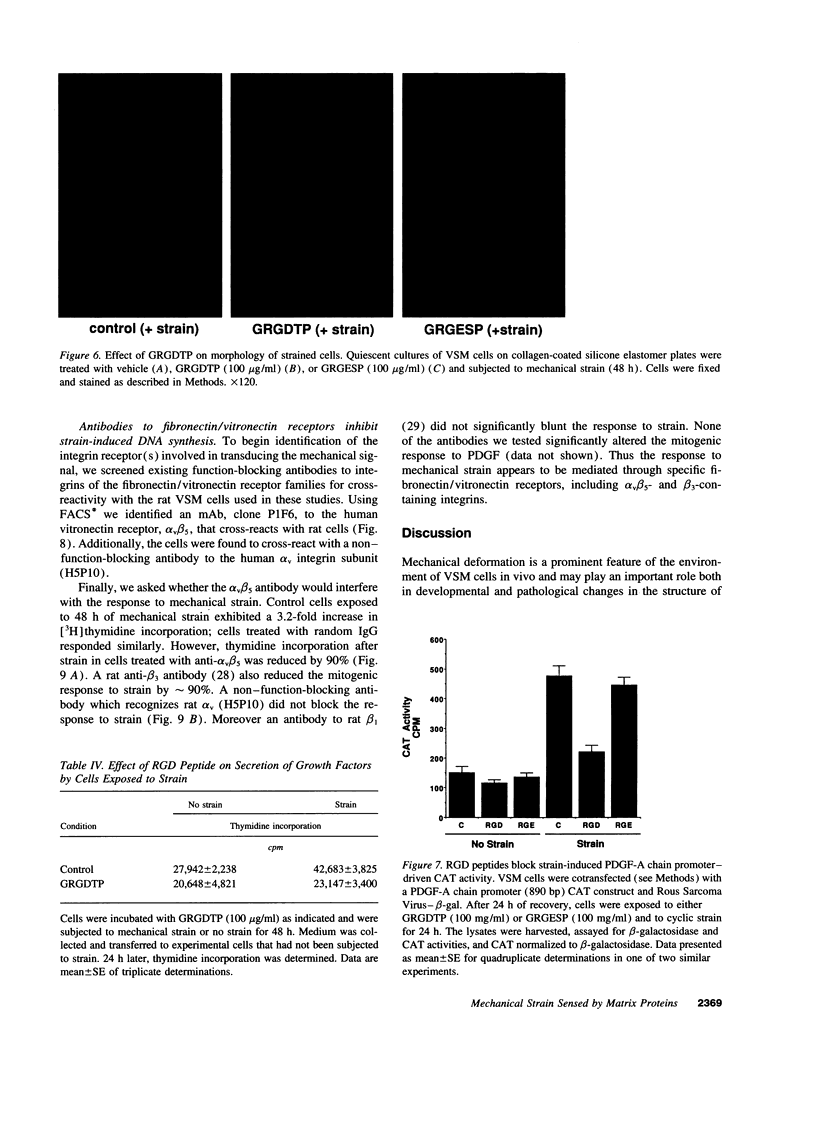
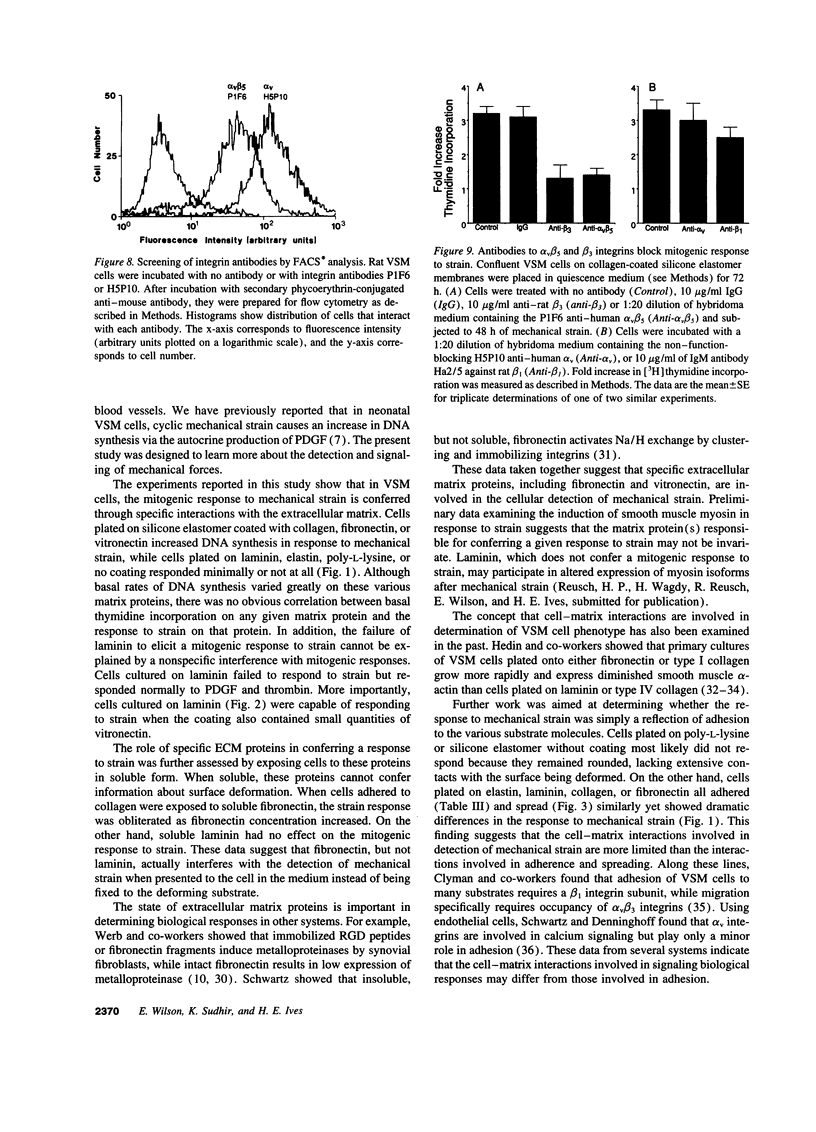
Cyclic mechanical strain (1 Hz) causes a mitogenic response in neonatal rat vascular smooth muscle cells due to production and secretion of PDGF. In this study, the mechanism for sensing mechanical strain was investigated. Silicone elastomer strain plates were coated at varying densities with elastin, laminin, type I collagen, fibronectin, or vitronectin. Strain was applied by cyclic application of a vacuum under the dishes. Cells adhered, spread, and proliferated on each matrix protein, but the mitogenic response to strain was matrix dependent. Strain increased DNA synthesis in cells on collagen, fibronectin, or vitronectin, but not in cells on elastin or laminin. When strain was applied on matrices containing both laminin and vitronectin, the mitogenic response to strain depended upon the vitronectin content of the matrix. Fibronectin, in soluble form (0-50 micrograms/ml), and the integrin binding peptide GRGDTP (100 micrograms/ml) both blocked the mitogenic response to mechanical strain in cells grown on immobilized collagen. Neither soluble laminin nor the inactive peptide GRGESP blocked the response to strain. GRGDTP did not alter the mitogenic response to exogenous PDGF or alpha-thrombin but did prevent the secretion of PDGF in response to strain. Furthermore, GRGDTP, but not GRGESP, prevented strain-induced expression of a PDGF-A chain promoter 890 bp-chloramphenicol acetyltransferase construct that was transiently transfected into vascular smooth muscle cells. Finally, the response to strain was abrogated by antibodies to both beta 3 and alpha v beta 5 integrins but not by an antibody to beta 1 integrins. Thus interaction between integrins and specific matrix proteins is responsible for sensing mechanical strain in vascular smooth muscle cells.
Full text
PDF

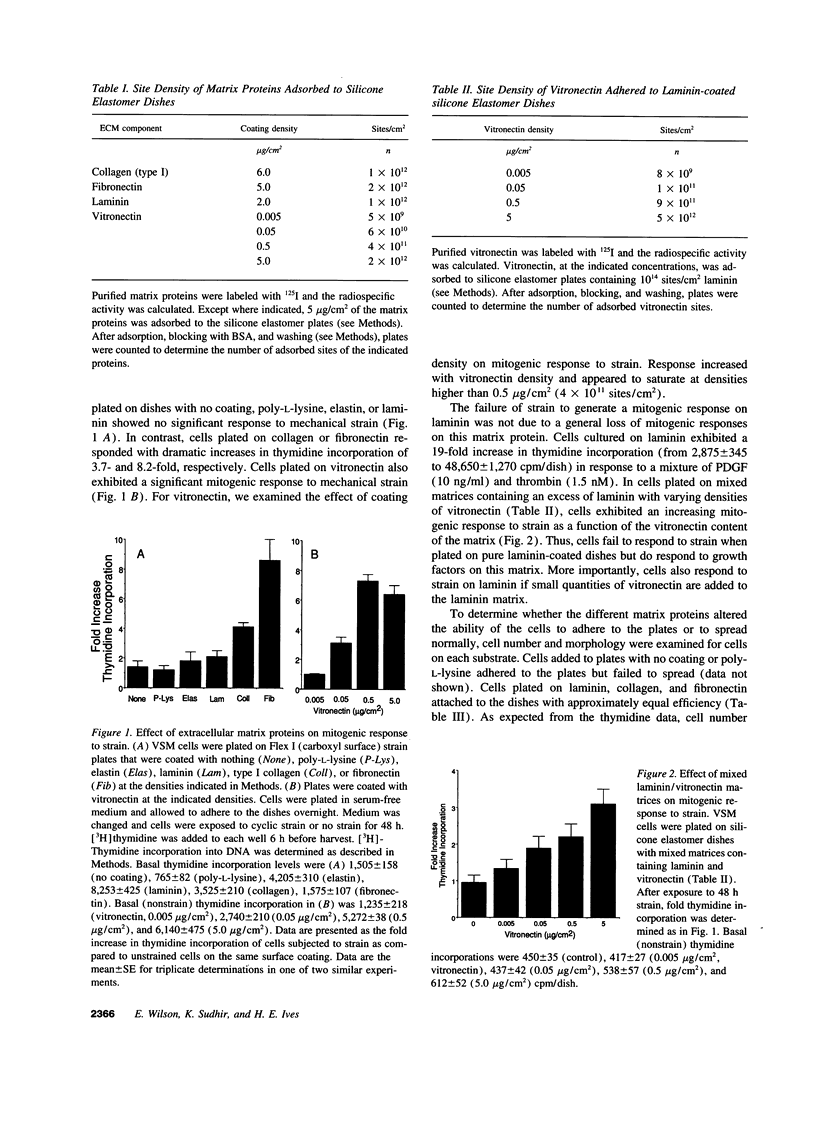

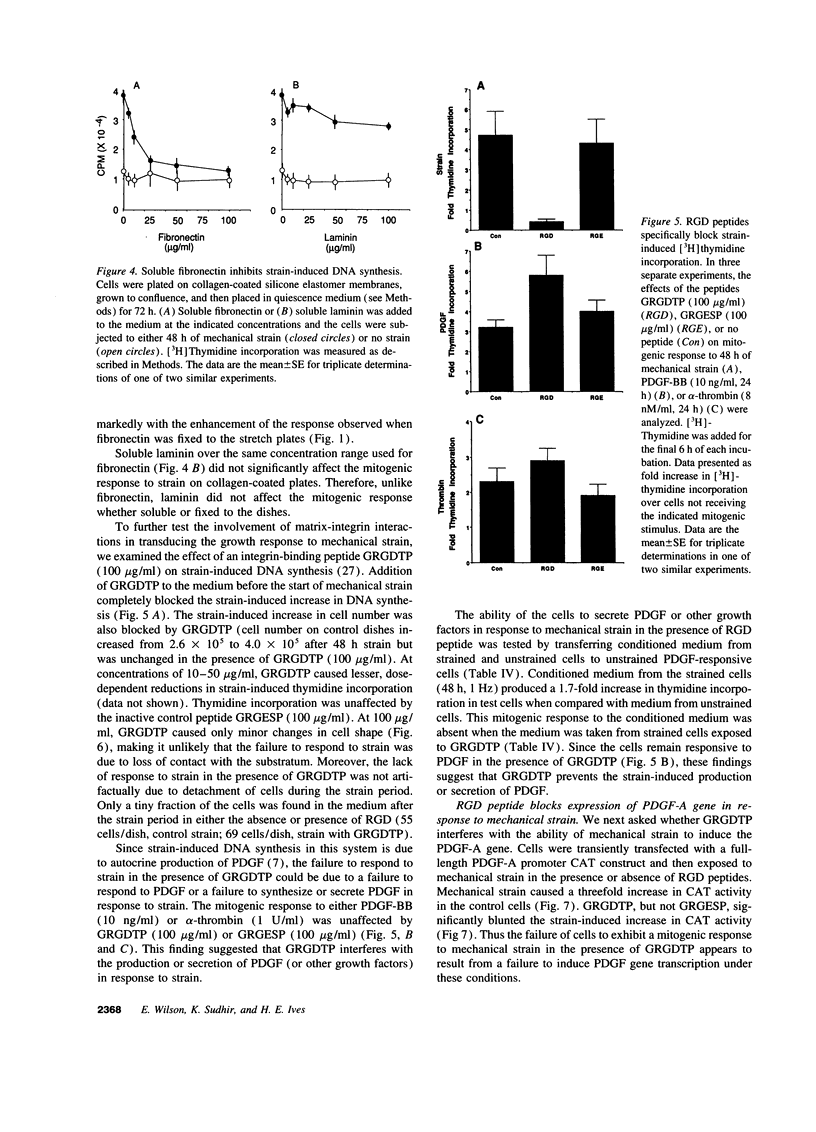


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banes A. J., Gilbert J., Taylor D., Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985 Apr;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- Banes A. J., Link G. W., Jr, Gilbert J. W., Tran Son Tay R., Monbureau O. Culturing cells in a mechanically active environment. Am Biotechnol Lab. 1990 May;8(7):12–22. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton C. T., Sennett B. J., Farmer J. C., Iannotti J. P., Hansen C. A., Williams J. L., Williamson J. The inositol phosphate pathway as a mediator in the proliferative response of rat calvarial bone cells to cyclical biaxial mechanical strain. J Orthop Res. 1992 May;10(3):385–393. doi: 10.1002/jor.1100100311. [DOI] [PubMed] [Google Scholar]
- Brophy C. M., Mills I., Rosales O., Isales C., Sumpio B. E. Phospholipase C: a putative mechanotransducer for endothelial cell response to acute hemodynamic changes. Biochem Biophys Res Commun. 1993 Jan 29;190(2):576–581. doi: 10.1006/bbrc.1993.1087. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Kramer R. H. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992 Jun;200(2):272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Robotewskyj A., Griem M. L. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest. 1994 May;93(5):2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKOW B., GRIMBY G., THULESIUS O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand. 1958 Dec 15;44(3-4):255–272. doi: 10.1111/j.1748-1716.1958.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Cheresh D. A. Vitronectin and its receptors. Curr Opin Cell Biol. 1993 Oct;5(5):864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Forsberg E., Johansson S., Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988 Jul;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin U., Sjölund M., Hultgårdh-Nilsson A., Thyberg J. Changes in expression and organization of smooth-muscle-specific alpha-actin during fibronectin-mediated modulation of arterial smooth muscle cell phenotype. Differentiation. 1990 Sep;44(3):222–231. doi: 10.1111/j.1432-0436.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Hedin U., Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33(3):239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Horton M. A. Integrins on rat osteoclasts: characterization of two monoclonal antibodies (F4 and F11) to rat beta 3. J Bone Miner Res. 1992 Mar;7(3):345–351. doi: 10.1002/jbmr.5650070315. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991 Oct;3(5):841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Scott-Burden T., Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille B. L. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21 (Suppl 1):S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- Letsou G. V., Rosales O., Maitz S., Vogt A., Sumpio B. E. Stimulation of adenylate cyclase activity in cultured endothelial cells subjected to cyclic stretch. J Cardiovasc Surg (Torino) 1990 Sep-Oct;31(5):634–639. [PubMed] [Google Scholar]
- Liaw L., Almeida M., Hart C. E., Schwartz S. M., Giachelli C. M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994 Feb;74(2):214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Grinnell F. Decreased level of PDGF-stimulated receptor autophosphorylation by fibroblasts in mechanically relaxed collagen matrices. J Cell Biol. 1993 Aug;122(3):663–672. doi: 10.1083/jcb.122.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrick D. L., Kelly D. M. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab Invest. 1993 Dec;69(6):690–702. [PubMed] [Google Scholar]
- Mills I., Letsou G., Rabban J., Sumpio B., Gewirtz H. Mechanosensitive adenylate cyclase activity in coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 Aug 31;171(1):143–147. doi: 10.1016/0006-291x(90)91368-3. [DOI] [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Denninghoff K. Alpha v integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994 Apr 15;269(15):11133–11137. [PubMed] [Google Scholar]
- Schwartz M. A. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993 Apr 1;53(7):1503–1506. [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Sudhir K., Wilson E., Chatterjee K., Ives H. E. Mechanical strain and collagen potentiate mitogenic activity of angiotensin II in rat vascular smooth muscle cells. J Clin Invest. 1993 Dec;92(6):3003–3007. doi: 10.1172/JCI116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpio B. E., Banes A. J., Buckley M., Johnson G., Jr Alterations in aortic endothelial cell morphology and cytoskeletal protein synthesis during cyclic tensional deformation. J Vasc Surg. 1988 Jan;7(1):130–138. [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P., Damsky C. H. Regulation of extracellular matrix degradation by cell-extracellular matrix interactions. Cell Differ Dev. 1990 Dec 2;32(3):299–306. doi: 10.1016/0922-3371(90)90043-v. [DOI] [PubMed] [Google Scholar]
- Wiersbitzky M., Mills I., Sumpio B. E., Gewirtz H. Chronic cyclic strain reduces adenylate cyclase activity and stimulatory G protein subunit levels in coronary smooth muscle cells. Exp Cell Res. 1994 Jan;210(1):52–55. doi: 10.1006/excr.1994.1008. [DOI] [PubMed] [Google Scholar]
- Wilson E., Mai Q., Sudhir K., Weiss R. H., Ives H. E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993 Nov;123(3):741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz H. R., Dobbs L. G. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990 Nov 30;250(4985):1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]