Abstract
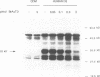
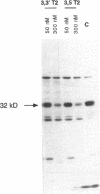
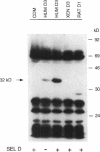
Type 3 iodothyronine deiodinase (D3) catalyzes the conversion of T4 and T3 to inactive metabolites. It is highly expressed in placenta and thus can regulate circulating fetal thyroid hormone concentrations throughout gestation. We have cloned and expressed a 2.1-kb human placental D3 cDNA which encodes a 32-kD protein with a Km of 1.2 nM for 5 deiodination of T3 and 340 nM for 5' deiodination of reverse T3. The reaction requires DTT and is not inhibited by 6n-propylthiouracil. We quantitated transiently expressed D3 by specifically labeling the protein with bromoacetyl [125I]T3. The Kcat/Km ratio for 5 deiodination of T3 was over 1,000-fold that for 5' deiodination of reverse T3. Human D3 is a selenoenzyme as evidenced by (a) the presence of an in frame UGA codon at position 144, (b) the synthesis of a 32-kD 75Se-labeled protein in D3 cDNA transfected cells, and (c) the presence of a selenocysteine insertion sequence element in the 3' untranslated region of the mRNA which is required for its expression. The D3 selenocysteine insertion sequence element is more potent than that in the type 1 deiodinase or glutathione peroxidase gene, suggesting a high priority for selenocysteine incorporation into this enzyme. The conservation of this enzyme from Xenopus laevis tadpoles to humans implies an essential role for regulation of thyroid hormone inactivation during embryological development.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuid J., Stinson D. A., Larsen P. R. Serum triiodothyronine and thyroxine in the neonate and the acute increases in these hormones following delivery. J Clin Invest. 1973 May;52(5):1195–1199. doi: 10.1172/JCI107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. R., Nicol F., Beckett G. J. Hepatic iodothyronine 5'-deiodinase. The role of selenium. Biochem J. 1990 Dec 1;272(2):537–540. doi: 10.1042/bj2720537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axley M. J., Böck A., Stadtman T. C. Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8450–8454. doi: 10.1073/pnas.88.19.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett G. J., MacDougall D. A., Nicol F., Arthur R. Inhibition of type I and type II iodothyronine deiodinase activity in rat liver, kidney and brain produced by selenium deficiency. Biochem J. 1989 May 1;259(3):887–892. doi: 10.1042/bj2590887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Chen Y. Y., Mandel S. J., Kieffer J. D., Harney J. W., Larsen P. R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991 Sep 19;353(6341):273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Harney J. W., Larsen P. R. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993 Aug;12(8):3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Harney J. W., Ohama T., Hatfield D. L. Selenocysteine insertion or termination: factors affecting UGA codon fate and complementary anticodon:codon mutations. Nucleic Acids Res. 1994 Sep 11;22(18):3753–3759. doi: 10.1093/nar/22.18.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Kieffer J. D., Harney J. W., Larsen P. R. Selenocysteine confers the biochemical properties characteristic of the type I iodothyronine deiodinase. J Biol Chem. 1991 Aug 5;266(22):14155–14158. [PubMed] [Google Scholar]
- Berry M. J., Larsen P. R. The role of selenium in thyroid hormone action. Endocr Rev. 1992 May;13(2):207–219. doi: 10.1210/edrv-13-2-207. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Maia A. L., Kieffer J. D., Harney J. W., Larsen P. R. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992 Oct;131(4):1848–1852. doi: 10.1210/endo.131.4.1396330. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol. 1989 Dec;3(12):1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Larsen P. R., Harney J. W., Koenig R. J., Moore D. D. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J Biol Chem. 1989 Jan 5;264(1):178–182. [PubMed] [Google Scholar]
- Burrow G. N., Fisher D. A., Larsen P. R. Maternal and fetal thyroid function. N Engl J Med. 1994 Oct 20;331(16):1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Chanoine J. P., Alex S., Stone S., Fang S. L., Veronikis I., Leonard J. L., Braverman L. E. Placental 5-deiodinase activity and fetal thyroid hormone economy are unaffected by selenium deficiency in the rat. Pediatr Res. 1993 Sep;34(3):288–292. doi: 10.1203/00006450-199309000-00009. [DOI] [PubMed] [Google Scholar]
- Chanoine J. P., Braverman L. E., Farwell A. P., Safran M., Alex S., Dubord S., Leonard J. L. The thyroid gland is a major source of circulating T3 in the rat. J Clin Invest. 1993 Jun;91(6):2709–2713. doi: 10.1172/JCI116510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contempré B., Jauniaux E., Calvo R., Jurkovic D., Campbell S., de Escobar G. M. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993 Dec;77(6):1719–1722. doi: 10.1210/jcem.77.6.8263162. [DOI] [PubMed] [Google Scholar]
- Galton V. A., Hiebert A. Hepatic iodothyronine 5-deiodinase activity in Rana catesbeiana tadpoles at different stages of the life cycle. Endocrinology. 1987 Jul;121(1):42–47. doi: 10.1210/endo-121-1-42. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981 Apr;67(4):1208–1214. doi: 10.1172/JCI110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar M. G., Prabarkaran D., Godbole M. M. 5'-Monodeiodinase activity in developing human cerebral cortex. Am J Clin Nutr. 1993 Feb;57(2 Suppl):291S–294S. doi: 10.1093/ajcn/57.2.291S. [DOI] [PubMed] [Google Scholar]
- Klein A. H., Hobel C. J., Sack J., Fisher D. A. Effect of intraamniotic fluid thyroxine injection on fetal serum and amniotic fluid iodothyronine concentrations. J Clin Endocrinol Metab. 1978 Nov;47(5):1034–1037. doi: 10.1210/jcem-47-5-1034. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Berry M. J. Type I iodothyronine deiodinase: unexpected complexities in a simple deiodination reaction. Thyroid. 1994 Fall;4(3):357–362. doi: 10.1089/thy.1994.4.357. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Veprek B., Zehelein E., Böck A. In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc Natl Acad Sci U S A. 1990 Jan;87(2):543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. L., Ekenbarger D. M., Frank S. J., Farwell A. P., Koehrle J. Localization of type I iodothyronine 5'-deiodinase to the basolateral plasma membrane in renal cortical epithelial cells. J Biol Chem. 1991 Jun 15;266(17):11262–11269. [PubMed] [Google Scholar]
- Low S. C., Harney J. W., Berry M. J. Cloning and functional characterization of human selenophosphate synthetase, an essential component of selenoprotein synthesis. J Biol Chem. 1995 Sep 15;270(37):21659–21664. doi: 10.1074/jbc.270.37.21659. [DOI] [PubMed] [Google Scholar]
- Mandel S. J., Berry M. J., Kieffer J. D., Harney J. W., Warne R. L., Larsen P. R. Cloning and in vitro expression of the human selenoprotein, type I iodothyronine deiodinase. J Clin Endocrinol Metab. 1992 Oct;75(4):1133–1139. doi: 10.1210/jcem.75.4.1400883. [DOI] [PubMed] [Google Scholar]
- Meinhold H., Campos-Barros A., Walzog B., Köhler R., Müller F., Behne D. Effects of selenium and iodine deficiency on type I, type II and type III iodothyronine deiodinases and circulating thyroid hormones in the rat. Exp Clin Endocrinol. 1993;101(2):87–93. doi: 10.1055/s-0029-1211212. [DOI] [PubMed] [Google Scholar]
- Mol J. A., Docter R., Hennemann G., Visser T. J. Modification of rat liver iodothyronine 5'-deiodinase activity with diethylpyrocarbonate and rose bengal; evidence for an active site histidine residue. Biochem Biophys Res Commun. 1984 Apr 16;120(1):28–36. doi: 10.1016/0006-291x(84)91409-8. [DOI] [PubMed] [Google Scholar]
- Mori K., Yoshida K., Kayama T., Kaise N., Fukazawa H., Kiso Y., Kikuchi K., Aizawa Y., Abe K. Thyroxine 5-deiodinase in human brain tumors. J Clin Endocrinol Metab. 1993 Nov;77(5):1198–1202. doi: 10.1210/jcem.77.5.8077312. [DOI] [PubMed] [Google Scholar]
- Santini F., Chopra I. J., Hurd R. E., Solomon D. H., Teco G. N. A study of the characteristics of the rat placental iodothyronine 5-monodeiodinase: evidence that it is distinct from the rat hepatic iodothyronine 5'-monodeiodinase. Endocrinology. 1992 Apr;130(4):2325–2332. doi: 10.1210/endo.130.4.1547744. [DOI] [PubMed] [Google Scholar]
- Santini F., Hurd R. E., Chopra I. J. A study of metabolism of deaminated and sulfoconjugated iodothyronines by rat placental iodothyronine 5-monodeiodinase. Endocrinology. 1992 Oct;131(4):1689–1694. doi: 10.1210/endo.131.4.1396315. [DOI] [PubMed] [Google Scholar]
- Schoenmakers C. H., Pigmans I. G., Visser T. J. Investigation of type I and type III iodothyronine deiodinases in rat tissues using N-bromoacetyl-iodothyronine affinity labels. Mol Cell Endocrinol. 1995 Feb;107(2):173–180. doi: 10.1016/0303-7207(94)03440-5. [DOI] [PubMed] [Google Scholar]
- St Germain D. L., Schwartzman R. A., Croteau W., Kanamori A., Wang Z., Brown D. D., Galton V. A. A thyroid hormone-regulated gene in Xenopus laevis encodes a type III iodothyronine 5-deiodinase. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7767–7771. doi: 10.1073/pnas.91.16.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe-Beeston J. G., Nicolaides K. H., McGregor A. M. Fetal thyroid function. Thyroid. 1992 Fall;2(3):207–217. doi: 10.1089/thy.1992.2.207. [DOI] [PubMed] [Google Scholar]
- Toyoda N., Berry M. J., Harney J. W., Larsen P. R. Topological analysis of the integral membrane protein, type 1 iodothyronine deiodinase (D1). J Biol Chem. 1995 May 19;270(20):12310–12318. doi: 10.1074/jbc.270.20.12310. [DOI] [PubMed] [Google Scholar]
- Toyoda N., Harney J. W., Berry M. J., Larsen P. R. Identification of critical amino acids for 3,5,3'-triiodothyronine deiodination by human type 1 deiodinase based on comparative functional-structural analyses of the human, dog, and rat enzymes. J Biol Chem. 1994 Aug 12;269(32):20329–20334. [PubMed] [Google Scholar]