Abstract
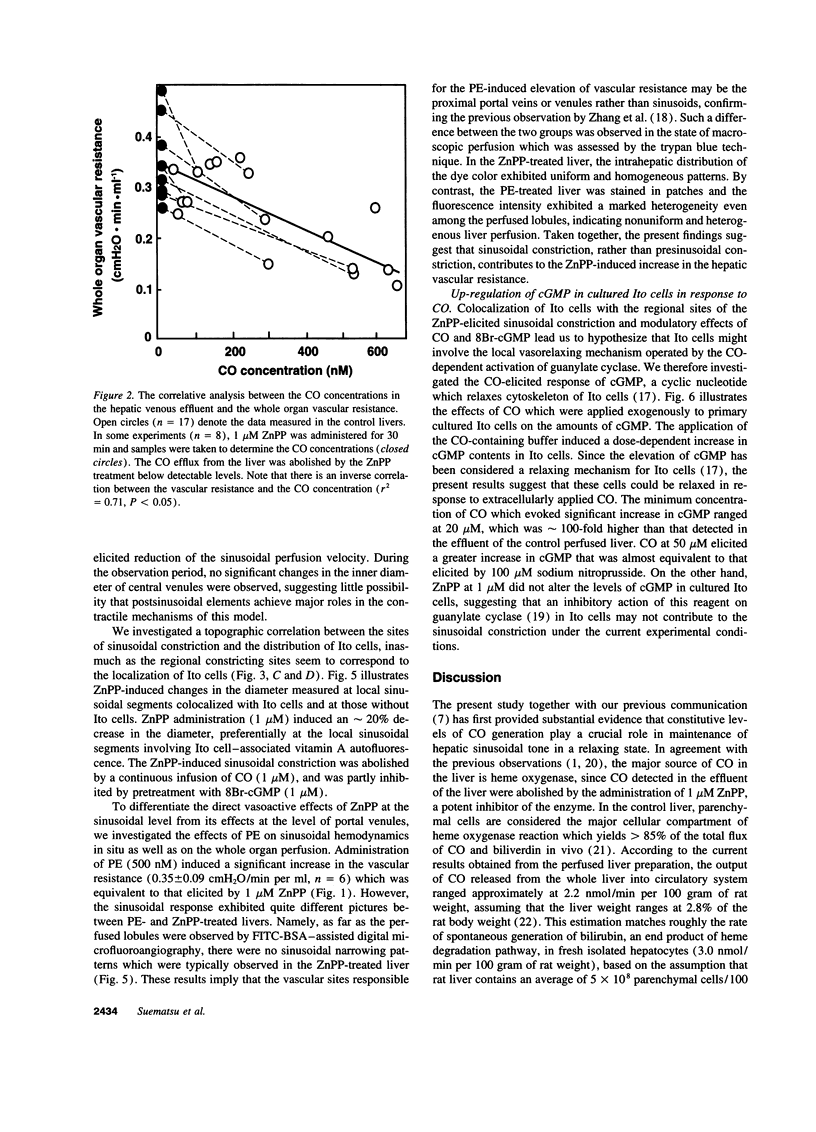
Heme oxygenase is a heme-oxidizing enzyme which generates biliverdin and carbon monoxide (CO). The present study was designed to elucidate whether CO endogenously produced by this enzyme serves as an active vasorelaxant in the hepatic microcirculation. Microvasculature of the isolated perfused rat liver was visualized by dual-color digital microfluorography to alternately monitor sinusoidal lining and fat-storing Ito cells. In the control liver, the CO flux in the venous effluent ranged at 0.7 nmol/min per gram of liver. Administration of a heme oxygenase inhibitor zinc protoporphyrin IX (1 microM) eliminated the baseline CO generation, and the vascular resistance exhibited a 30% elevation concurrent with discrete patterns of constriction in sinusoids and reduction of the sinusoidal perfusion velocity. The major sites of the constriction corresponded to local sinusoidal segments colocalized with Ito cell which were identified by imaging their vitamin A autofluorescence. The increase in the vascular resistance and sinusoidal constriction were attenuated significantly by adding CO (1 microM) or a cGMP analogue 8-bromo-cGMP (1 microM) in the perfusate. From these findings, we propose that CO can function as an endogenous modulator of hepatic sinusoidal perfusion through a relaxing mechanism involving Ito cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautista A. P., Spitzer J. J. Inhibition of nitric oxide formation in vivo enhances superoxide release by the perfused liver. Am J Physiol. 1994 May;266(5 Pt 1):G783–G788. doi: 10.1152/ajpgi.1994.266.5.G783. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Hemoglobin and erythrocyte catabolism in rat liver: the separate roles of parenchymal and sinusoidal cells. Blood. 1972 Dec;40(6):812–822. [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987 Oct;32(4):497–504. [PubMed] [Google Scholar]
- Grossman H. J., Grossman V. L., Bhathal P. S. Enhanced vasoconstrictor response of the isolated perfused cirrhotic rat liver to humoral vasoconstrictor substances found in portal venous blood. J Gastroenterol Hepatol. 1992 May-Jun;7(3):283–287. doi: 10.1111/j.1440-1746.1992.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Ballot B., Wood K. S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem. 1984 May 25;259(10):6201–6207. [PubMed] [Google Scholar]
- Jungermann K. Regulation von Stoffwechsel und Hämodynamik der Leber durch die hepatischen Nerven. Z Gastroenterol. 1987 Apr;25 (Suppl 1):44–54. [PubMed] [Google Scholar]
- Kawada N., Tran-Thi T. A., Klein H., Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993 Apr 15;213(2):815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Watanabe M., Yoshihara H., Harada N., Shiga T. Detection of nitric oxide production in lipopolysaccharide-treated rats by ESR using carbon monoxide hemoglobin. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1119–1124. doi: 10.1016/0006-291x(92)90708-s. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993 Jul;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Pinzani M., Failli P., Ruocco C., Casini A., Milani S., Baldi E., Giotti A., Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992 Aug;90(2):642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993 Oct;265(4 Pt 1):G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- Reilly F. D., McCuskey R. S., Cilento E. V. Hepatic microvascular regulatory mechanisms. I. Adrenergic mechanisms. Microvasc Res. 1981 Jan;21(1):103–116. doi: 10.1016/0026-2862(81)90008-x. [DOI] [PubMed] [Google Scholar]
- Rich A., Farrugia G., Rae J. L. Carbon monoxide stimulates a potassium-selective current in rabbit corneal epithelial cells. Am J Physiol. 1994 Aug;267(2 Pt 1):C435–C442. doi: 10.1152/ajpcell.1994.267.2.C435. [DOI] [PubMed] [Google Scholar]
- Stock R. J., Cilento E. V., McCuskey R. S. A quantitative study of fluorescein isothiocyanate-dextran transport in the microcirculation of the isolated perfused rat liver. Hepatology. 1989 Jan;9(1):75–82. doi: 10.1002/hep.1840090112. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Kashiwagi S., Sano T., Goda N., Shinoda Y., Ishimura Y. Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1333–1337. doi: 10.1006/bbrc.1994.2811. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Oda M., Suzuki H., Kaneko H., Watanabe N., Furusho T., Masushige S., Tsuchiya M. Intravital and electron microscopic observation of Ito cells in rat hepatic microcirculation. Microvasc Res. 1993 Jul;46(1):28–42. doi: 10.1006/mvre.1993.1033. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Suzuki H., Ishii H., Kato S., Hamamatsu H., Miura S., Tsuchiya M. Topographic dissociation between mitochondrial dysfunction and cell death during low-flow hypoxia in perfused rat liver. Lab Invest. 1992 Oct;67(4):434–442. [PubMed] [Google Scholar]
- Suematsu M., Suzuki H., Ishii H., Kato S., Yanagisawa T., Asako H., Suzuki M., Tsuchiya M. Early midzonal oxidative stress preceding cell death in hypoperfused rat liver. Gastroenterology. 1992 Sep;103(3):994–1001. doi: 10.1016/0016-5085(92)90034-v. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Suematsu M., Ishii H., Kato S., Miki H., Mori M., Ishimura Y., Nishino T., Tsuchiya M. Prostaglandin E1 abrogates early reductive stress and zone-specific paradoxical oxidative injury in hypoperfused rat liver. J Clin Invest. 1994 Jan;93(1):155–164. doi: 10.1172/JCI116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Katagiri K., Hoshino M., Hayakawa T., Tsukada K., Takeuchi T. Endothelin-1 stimulates bile acid secretion and vesicular transport in the isolated perfused rat liver. Am J Physiol. 1994 Feb;266(2 Pt 1):G324–G329. doi: 10.1152/ajpgi.1994.266.2.G324. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Gräser T., Vanin A. F. Similar endothelium-independent arterial relaxation by carbon monoxide and nitric oxide. Biomed Biochim Acta. 1989;48(8):601–603. [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E., De Zanger R. B., Charels K., Van Der Smissen P., McCuskey R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985 Jul-Aug;5(4):683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Features of the reaction of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1978 Jun 25;253(12):4230–4236. [PubMed] [Google Scholar]
- Zhang J. X., Pegoli W., Jr, Clemens M. G. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994 Apr;266(4 Pt 1):G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Small S. A., Kandel E. R., Hawkins R. D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993 Jun 25;260(5116):1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]