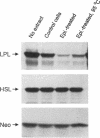
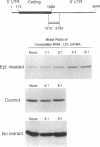
Abstract
Lipoprotein lipase (LPL) is a central enzyme in lipoprotein metabolism and is in part responsible for adipocyte lipid accumulation. Catecholamines are known to decrease the activity of LPL in adipocytes, and we have previously demonstrated that this inhibition occurs posttranscriptionally, with a prominent inhibition of LPL translation. To better characterize the inhibition of LPL translation, 3T3-L1 cells were differentiated into adipocytes, and exposed to epinephrine. Epinephrine induced a dose-dependent decrease in LPL synthesis using [35S]methionine incorporation, with no change in LPL mRNA levels, demonstrating translational regulation of LPL in this cell line. The poly A-enriched RNA from epinephrine-treated cells was translated well in vitro, and there was no difference in the polysome profiles from control and epinephrine-treated cells, suggesting that epinephrine did not affect mRNA editing, and did not induce an inhibition of translation initiation. To obtain evidence for the presence of an inhibitory factor, a cytoplasmic extract from control, and epinephrine-treated adipocytes was human. When compared to the control cell extract, the epinephrine-treated cell extract sharply inhibited LPL translation in vitro, yet had no effect on the translation of other mRNAs. Epinephrine-treated cells had fourfold more of this inhibitor activity than control cells, and this translation inhibition was partially reversed by heat treatment. To determine what region of the LPL mRNA was involved in the translation inhibition, different LPL constructs were synthesized. The inhibitory effect of the epinephrine-treated cell extract was dependent on the presence of the first 40 nucleotides of the 3' (untranslated region UTR) (nucleotides 1599-1638), whereas deletion of the 5' UTR and other areas of the 3' UTR had no effect on translation inhibition. When a sense RNA strand corresponding to this region was added to the in vitro translation reaction, it restored translation towards normal, suggesting that the sense strand was competing for a transacting binding protein. Thus, epinephrine-treated adipocytes produced a transacting factor, probably a protein, that interacted with a region on the LPL mRNA between nucleotides 1599 and 1638, resulting in an inhibition of translation. These studies add new insight into the hormonal regulation of LPL.
Full text
PDF

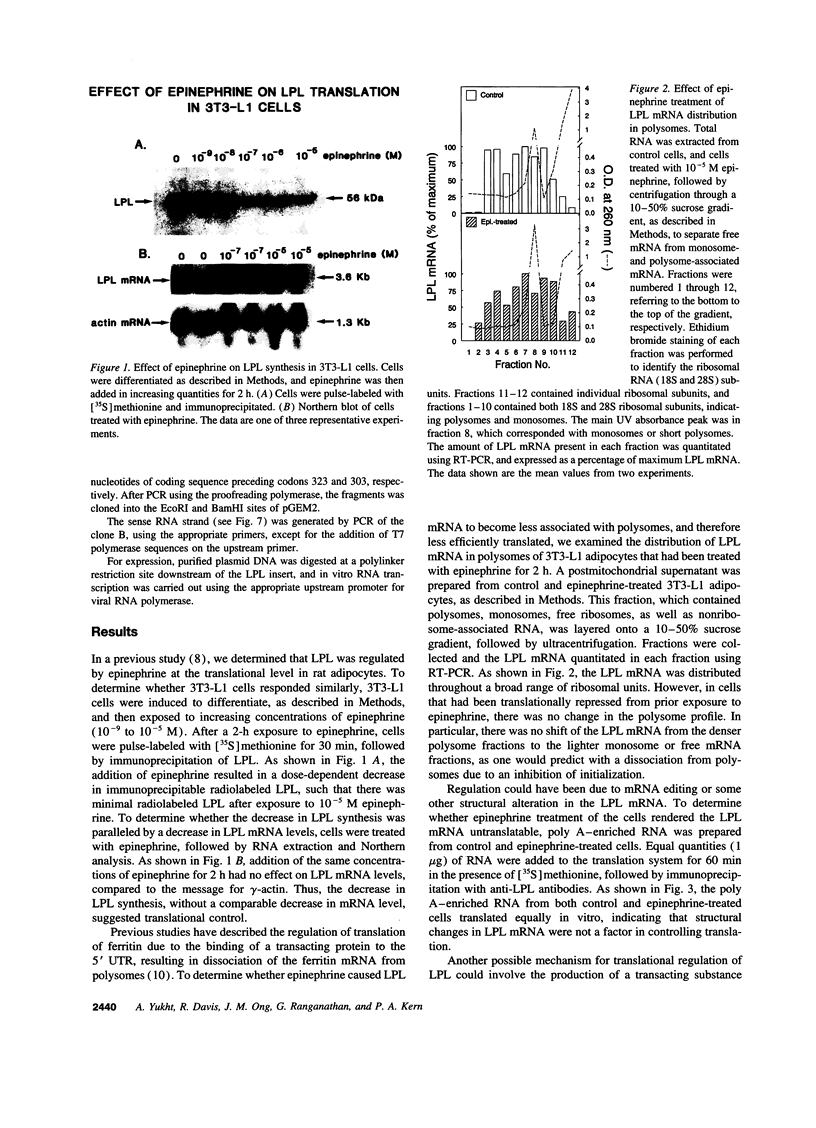
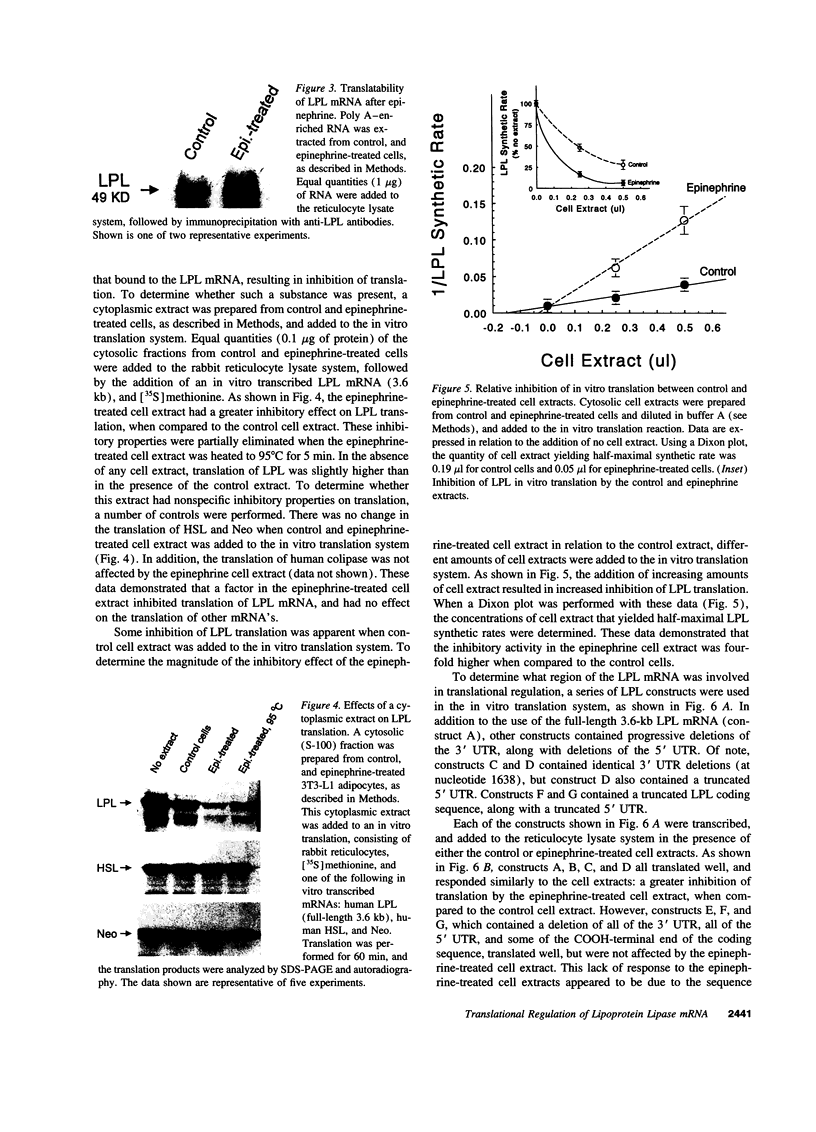


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Mulligan R. C., Schimmel P., Holmes E. W. Antisense RNA complementary to 3' coding and noncoding sequences of creatine kinase is a potent inhibitor of translation in vivo. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10006–10010. doi: 10.1073/pnas.86.24.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng J. L., Shoemaker D. L., Schimmel P., Holmes E. W. Reversal of creatine kinase translational repression by 3' untranslated sequences. Science. 1990 May 25;248(4958):1003–1006. doi: 10.1126/science.2343304. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davidson N. O. Apolipoprotein B mRNA editing: a key controlling element targeting fats to proper tissue. Ann Med. 1993 Dec;25(6):539–543. [PubMed] [Google Scholar]
- Davis R. C., Xia Y. R., Mohandas T., Schotz M. C., Lusis A. J. Assignment of the human pancreatic colipase gene to chromosome 6p21.1 to pter. Genomics. 1991 May;10(1):262–265. doi: 10.1016/0888-7543(91)90509-d. [DOI] [PubMed] [Google Scholar]
- Deshaies Y., Géloën A., Paulin A., Marette A., Bukowiecki L. J. Tissue-specific alterations in lipoprotein lipase activity in the rat after chronic infusion of isoproterenol. Horm Metab Res. 1993 Jan;25(1):13–16. doi: 10.1055/s-2007-1002036. [DOI] [PubMed] [Google Scholar]
- Doolittle M. H., Ben-Zeev O., Elovson J., Martin D., Kirchgessner T. G. The response of lipoprotein lipase to feeding and fasting. Evidence for posttranslational regulation. J Biol Chem. 1990 Mar 15;265(8):4570–4577. [PubMed] [Google Scholar]
- Enerbäck S., Gimble J. M. Lipoprotein lipase gene expression: physiological regulators at the transcriptional and post-transcriptional level. Biochim Biophys Acta. 1993 Aug 11;1169(2):107–125. doi: 10.1016/0005-2760(93)90196-g. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fried S. K., Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J Lipid Res. 1989 Dec;30(12):1917–1923. [PubMed] [Google Scholar]
- Friedman G., Ben-Naim M., Halimi O., Etienne J., Stein O., Stein Y. The expression of lipoprotein lipase activity and mRNA in mesenchymal rat heart cell cultures is modulated by bFGF. Biochim Biophys Acta. 1991 Feb 26;1082(1):27–32. doi: 10.1016/0005-2760(91)90295-s. [DOI] [PubMed] [Google Scholar]
- Giralt M., Martin I., Vilaró S., Villarroya F., Mampel T., Iglesias R., Viñas O. Lipoprotein lipase mRNA expression in brown adipose tissue: translational and/or posttranslational events are involved in the modulation of enzyme activity. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):270–273. doi: 10.1016/0167-4781(90)90066-b. [DOI] [PubMed] [Google Scholar]
- Goers J. W., Pedersen M. E., Kern P. A., Ong J., Schotz M. C. An enzyme-linked immunoassay for lipoprotein lipase. Anal Biochem. 1987 Oct;166(1):27–35. doi: 10.1016/0003-2697(87)90541-0. [DOI] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J. B., Klausner R. D. Coordinate post-transcriptional regulation of ferritin and transferrin receptor expression: the role of regulated RNA-protein interaction. Enzyme. 1990;44(1-4):28–41. doi: 10.1159/000468745. [DOI] [PubMed] [Google Scholar]
- Holm C., Kirchgessner T. G., Svenson K. L., Fredrikson G., Nilsson S., Miller C. G., Shively J. E., Heinzmann C., Sparkes R. S., Mohandas T. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science. 1988 Sep 16;241(4872):1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kirchgessner T. G., Svenson K. L., Lusis A. J., Schotz M. C. The sequence of cDNA encoding lipoprotein lipase. A member of a lipase gene family. J Biol Chem. 1987 Jun 25;262(18):8463–8466. [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Kruys V., Wathelet M., Poupart P., Contreras R., Fiers W., Content J., Huez G. The 3' untranslated region of the human interferon-beta mRNA has an inhibitory effect on translation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6030–6034. doi: 10.1073/pnas.84.17.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. Iron regulation of ferritin gene expression. J Cell Biochem. 1990 Oct;44(2):107–115. doi: 10.1002/jcb.240440205. [DOI] [PubMed] [Google Scholar]
- Ong J. M., Kern P. A. Effect of feeding and obesity on lipoprotein lipase activity, immunoreactive protein, and messenger RNA levels in human adipose tissue. J Clin Invest. 1989 Jul;84(1):305–311. doi: 10.1172/JCI114155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J. M., Kern P. A. The role of glucose and glycosylation in the regulation of lipoprotein lipase synthesis and secretion in rat adipocytes. J Biol Chem. 1989 Feb 25;264(6):3177–3182. [PubMed] [Google Scholar]
- Ong J. M., Kirchgessner T. G., Schotz M. C., Kern P. A. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J Biol Chem. 1988 Sep 15;263(26):12933–12938. [PubMed] [Google Scholar]
- Ong J. M., Saffari B., Simsolo R. B., Kern P. A. Epinephrine inhibits lipoprotein lipase gene expression in rat adipocytes through multiple steps in posttranscriptional processing. Mol Endocrinol. 1992 Jan;6(1):61–69. doi: 10.1210/mend.6.1.1738372. [DOI] [PubMed] [Google Scholar]
- Ong J. M., Simsolo R. B., Saffari B., Kern P. A. The regulation of lipoprotein lipase gene expression by dexamethasone in isolated rat adipocytes. Endocrinology. 1992 Apr;130(4):2310–2316. doi: 10.1210/endo.130.4.1547742. [DOI] [PubMed] [Google Scholar]
- Ong J. M., Simsolo R. B., Saghizadeh M., Pauer A., Kern P. A. Expression of lipoprotein lipase in rat muscle: regulation by feeding and hypothyroidism. J Lipid Res. 1994 Sep;35(9):1542–1551. [PubMed] [Google Scholar]
- Ostareck-Lederer A., Ostareck D. H., Standart N., Thiele B. J. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3' untranslated region. EMBO J. 1994 Mar 15;13(6):1476–1481. doi: 10.1002/j.1460-2075.1994.tb06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato L., Parrott C. L., Santamarina-Fojo S., Brewer H. B., Jr Transcriptional regulation of the human lipoprotein lipase gene in 3T3-L1 adipocytes. J Biol Chem. 1991 Oct 5;266(28):18958–18963. [PubMed] [Google Scholar]
- Ranganathan G., Ong J. M., Yukht A., Saghizadeh M., Simsolo R. B., Pauer A., Kern P. A. Tissue-specific expression of human lipoprotein lipase. Effect of the 3'-untranslated region on translation. J Biol Chem. 1995 Mar 31;270(13):7149–7155. doi: 10.1074/jbc.270.13.7149. [DOI] [PubMed] [Google Scholar]
- Raynolds M. V., Awald P. D., Gordon D. F., Gutierrez-Hartmann A., Rule D. C., Wood W. M., Eckel R. H. Lipoprotein lipase gene expression in rat adipocytes is regulated by isoproterenol and insulin through different mechanisms. Mol Endocrinol. 1990 Sep;4(9):1416–1422. doi: 10.1210/mend-4-9-1416. [DOI] [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari B., Ong J. M., Kern P. A. Regulation of adipose tissue lipoprotein lipase gene expression by thyroid hormone in rats. J Lipid Res. 1992 Feb;33(2):241–249. [PubMed] [Google Scholar]
- Simsolo R. B., Ong J. M., Kern P. A. Characterization of lipoprotein lipase activity, secretion, and degradation at different sites of post-translational processing in primary cultures of rat adipocytes. J Lipid Res. 1992 Dec;33(12):1777–1784. [PubMed] [Google Scholar]
- Simsolo R. B., Ong J. M., Kern P. A. The regulation of adipose tissue and muscle lipoprotein lipase in runners by detraining. J Clin Invest. 1993 Nov;92(5):2124–2130. doi: 10.1172/JCI116813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsolo R. B., Ong J. M., Saffari B., Kern P. A. Effect of improved diabetes control on the expression of lipoprotein lipase in human adipose tissue. J Lipid Res. 1992 Jan;33(1):89–95. [PubMed] [Google Scholar]
- Sonenberg N. mRNA translation: influence of the 5' and 3' untranslated regions. Curr Opin Genet Dev. 1994 Apr;4(2):310–315. doi: 10.1016/s0959-437x(05)80059-0. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Wion K. L., Kirchgessner T. G., Lusis A. J., Schotz M. C., Lawn R. M. Human lipoprotein lipase complementary DNA sequence. Science. 1987 Mar 27;235(4796):1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]