Abstract
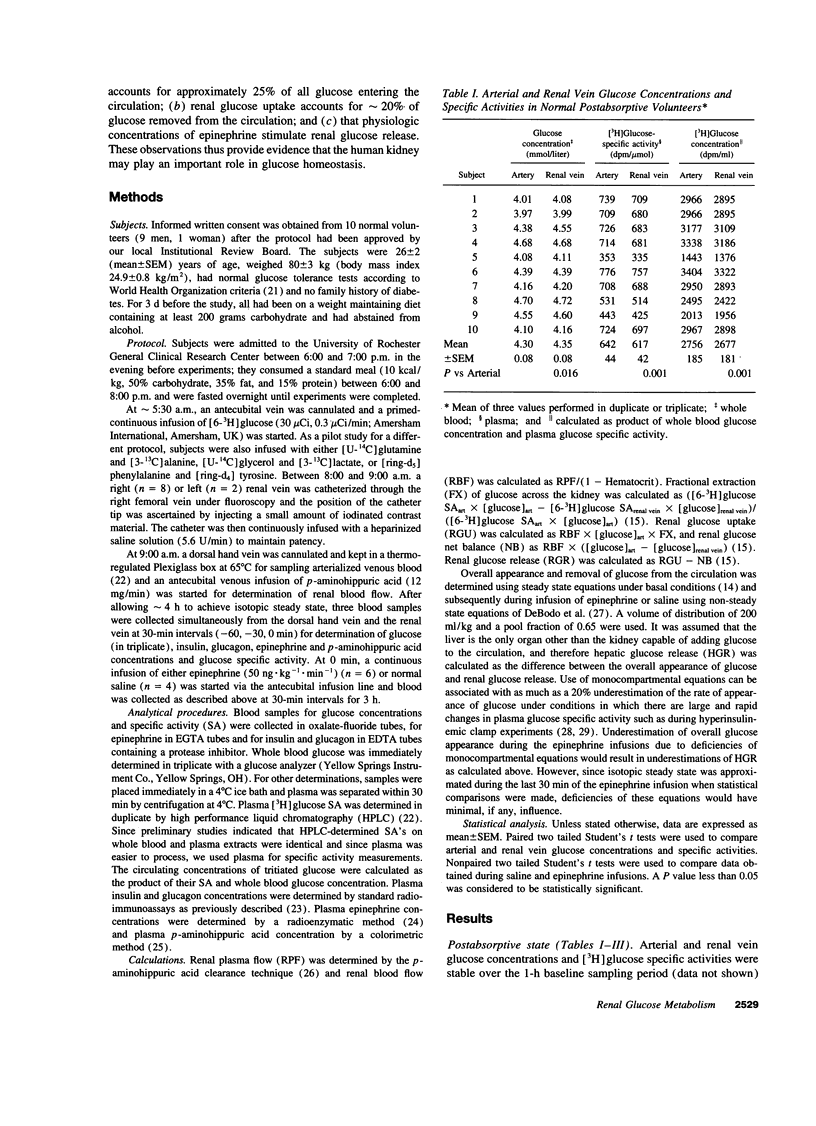
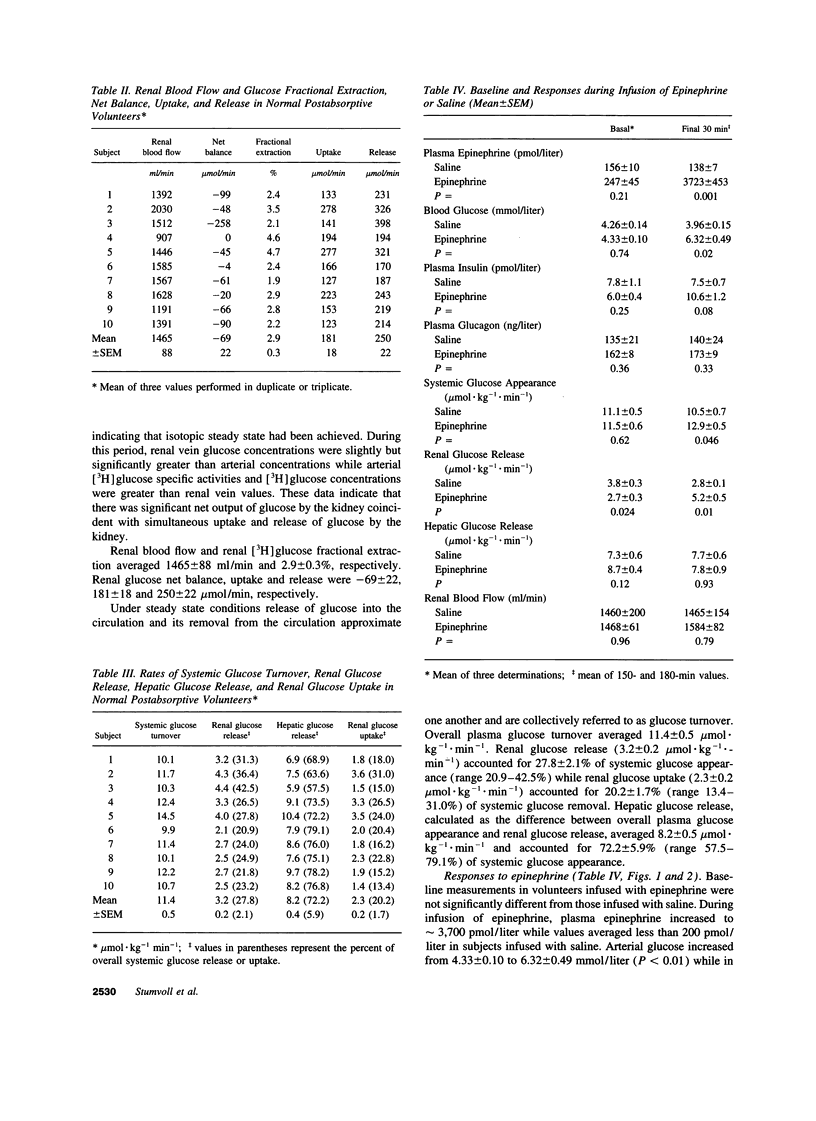
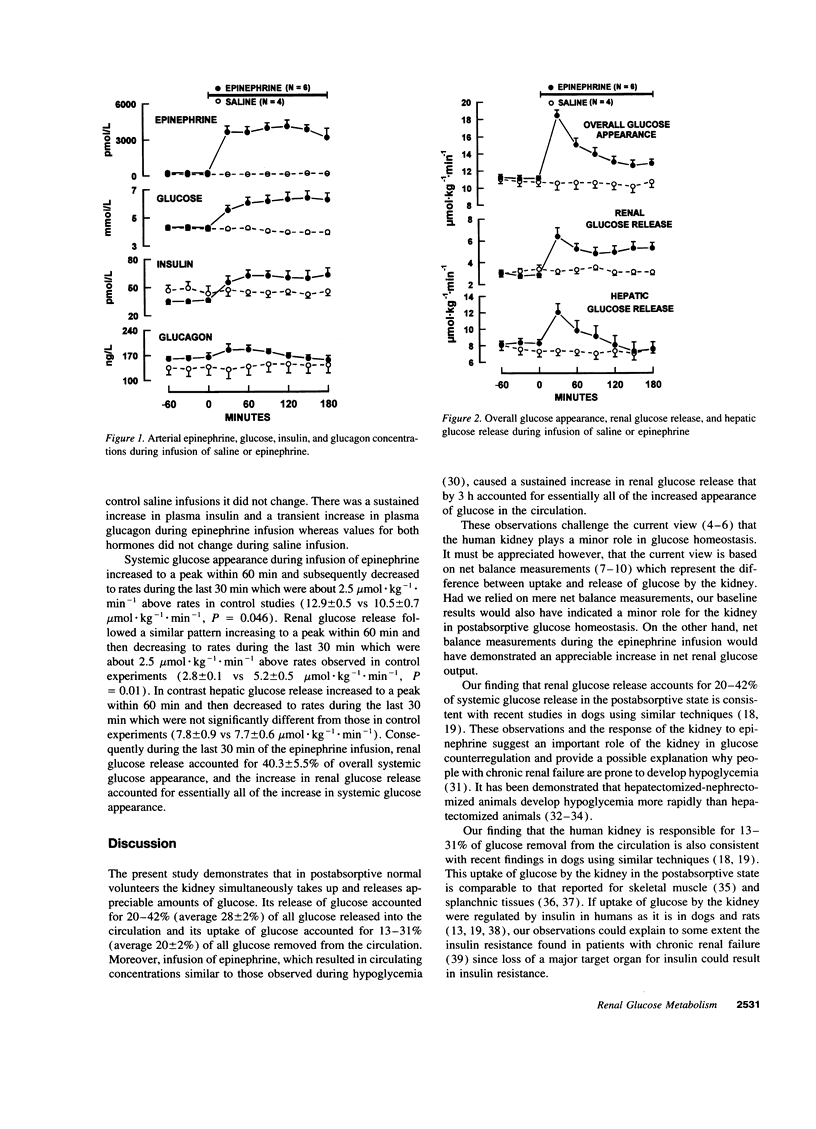
Despite ample evidence that the kidney can both produce and use appreciable amounts of glucose, the human kidney is generally regarded as playing a minor role in glucose homeostasis. This view is based on measurements of arteriorenal vein glucose concentrations indicating little or no net release of glucose. However, inferences from net balance measurements do not take into consideration the simultaneous release and uptake of glucose by the kidney. Therefore, to assess the contribution of release and uptake of glucose by the human kidney to overall entry and removal of plasma glucose, we used a combination of balance and isotope techniques to measure renal glucose net balance, fractional extraction, uptake and release as well as overall plasma glucose appearance and disposal in 10 normal volunteers under basal postabsorptive conditions and during a 3-h epinephrine infusion. In the basal postabsorptive state, there was small but significant net output of glucose by the kidney (66 +/- 22 mumol.min-1, P = 0.016). However, since renal glucose fractional extraction averaged 2.9 +/- 0.3%, there was considerable renal glucose uptake (2.3 +/- 0.2 mumol.kg-1.min-1) which accounted for 20.2 +/- 1.7% of systemic glucose disposal (11.4 +/- 0.5 mumol.kg-1.min-1). Renal glucose release (3.2 +/- 0.2 mumol.kg-1.min-1) accounted for 27.8 +/- 2.1% of systemic glucose appearance (11.4 +/- 0.5 mumol.kg-1.min-1). Epinephrine infusion, which increased plasma epinephrine to levels observed during hypoglycemia (3722 +/- 453 pmol/liter) increased renal glucose release nearly twofold (5.2 +/- 0.5 vs 2.8 +/- 0.1 mol.kg-1.min-1, P = 0.01) so that at the end of the infusion, renal glucose release accounted for 40.3 +/- 5.5% of systemic glucose appearance and essentially all of the increase in systemic glucose appearance. These observations suggest an important role for the human kidney in glucose homeostasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am. 1989 Mar;18(1):103–121. [PubMed] [Google Scholar]
- BRUN C. A rapid method for the determination of para-aminohippuric acid in kidney function tests. J Lab Clin Med. 1951 Jun;37(6):955–958. [PubMed] [Google Scholar]
- Biava C., Grossman A., West M. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Lab Invest. 1966 Jan;15(1 Pt 2):330–356. [PubMed] [Google Scholar]
- Björkman O., Felig P. Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes. 1982 Jun;31(6 Pt 1):516–520. doi: 10.2337/diab.31.6.516. [DOI] [PubMed] [Google Scholar]
- Björkman O., Felig P., Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980 Aug;29(8):610–616. doi: 10.2337/diab.29.8.610. [DOI] [PubMed] [Google Scholar]
- Brooks D. C., Black P. R., Arcangeli M. A., Aoki T. T., Wilmore D. W. The heated dorsal hand vein: an alternative arterial sampling site. JPEN J Parenter Enteral Nutr. 1989 Jan-Feb;13(1):102–105. doi: 10.1177/0148607189013001102. [DOI] [PubMed] [Google Scholar]
- Brundin T., Wahren J. Renal oxygen consumption, thermogenesis, and amino acid utilization during i.v. infusion of amino acids in man. Am J Physiol. 1994 Nov;267(5 Pt 1):E648–E655. doi: 10.1152/ajpendo.1994.267.5.E648. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Narins R. G., Chu C., Fagioli S., Choi S., McCarthy W., Lowry O. H. Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol. 1978 Sep;235(3):F246–F253. doi: 10.1152/ajprenal.1978.235.3.F246. [DOI] [PubMed] [Google Scholar]
- Castellino P., DeFronzo R. A. Glucose metabolism and the kidney. Semin Nephrol. 1990 Sep;10(5):458–463. [PubMed] [Google Scholar]
- Cersosimo E., Judd R. L., Miles J. M. Insulin regulation of renal glucose metabolism in conscious dogs. J Clin Invest. 1994 Jun;93(6):2584–2589. doi: 10.1172/JCI117270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Wasserman D. H., McGinness O. P. Renal contribution to glucose production after a brief fast: fact or fancy? J Clin Invest. 1994 Jun;93(6):2303–2303. doi: 10.1172/JCI117232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Consoli A., Kennedy F., Miles J., Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987 Nov;80(5):1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am J Physiol. 1990 Nov;259(5 Pt 1):E677–E684. doi: 10.1152/ajpendo.1990.259.5.E677. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo R. A., Alvestrand A., Smith D., Hendler R., Hendler E., Wahren J. Insulin resistance in uremia. J Clin Invest. 1981 Feb;67(2):563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- Dinneen S., Gerich J., Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992 Sep 3;327(10):707–713. doi: 10.1056/NEJM199209033271007. [DOI] [PubMed] [Google Scholar]
- Gregg C. M., Cohen J. J., Black A. J., Espeland M. A., Feldstein M. L. Effects of glucose and insulin on metabolism and function of perfused rat kidney. Am J Physiol. 1978 Jul;235(1):F52–F61. doi: 10.1152/ajprenal.1978.235.1.F52. [DOI] [PubMed] [Google Scholar]
- Guder W. G., Ross B. D. Enzyme distribution along the nephron. Kidney Int. 1984 Aug;26(2):101–111. doi: 10.1038/ki.1984.143. [DOI] [PubMed] [Google Scholar]
- Haüssinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990 Apr 15;267(2):281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Kelley D., Mitrakou A., Marsh H., Schwenk F., Benn J., Sonnenberg G., Arcangeli M., Aoki T., Sorensen J., Berger M. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest. 1988 May;81(5):1563–1571. doi: 10.1172/JCI113489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida K., Nakajo S., Kamiya F., Toyama Y., Nishio T., Nakagawa H. Renal net glucose release in vivo and its contribution to blood glucose in rats. J Clin Invest. 1978 Oct;62(4):721–726. doi: 10.1172/JCI109182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B. R., Wahren J., Chandramouli V., Schumann W. C., Ekberg K., Kalhan S. C. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest. 1995 Jan;95(1):172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux G., Achkar M., Vinay P., Gougoux A. Characteristics of ammoniagenesis and gluconeogenesis by the diabetic kidney. In vitro studies in the rat. Contrib Nephrol. 1982;31:23–28. doi: 10.1159/000406612. [DOI] [PubMed] [Google Scholar]
- Lemieux G., Aranda M. R., Fournel P., Lemieux C. Renal enzymes during experimental diabetes mellitus in the rat. Role of insulin, carbohydrate metabolism, and ketoacidosis. Can J Physiol Pharmacol. 1984 Jan;62(1):70–75. doi: 10.1139/y84-010. [DOI] [PubMed] [Google Scholar]
- MACKLER B., AMMENTORP P., GRAUBARTH H., GUEST G. M. Glucose formation by kidneys in eviscerated dogs. Proc Soc Exp Biol Med. 1951 Nov;78(2):479–480. doi: 10.3181/00379727-78-19110. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A., Wahren J., DeFronzo R. A., Ferrannini E. Glucose absorption and production following oral glucose: comparison of compartmental and arteriovenous-difference methods. Metabolism. 1994 Nov;43(11):1419–1425. doi: 10.1016/0026-0495(94)90038-8. [DOI] [PubMed] [Google Scholar]
- McGuinness O. P., Fugiwara T., Murrell S., Bracy D., Neal D., O'Connor D., Cherrington A. D. Impact of chronic stress hormone infusion on hepatic carbohydrate metabolism in the conscious dog. Am J Physiol. 1993 Aug;265(2 Pt 1):E314–E322. doi: 10.1152/ajpendo.1993.265.2.E314. [DOI] [PubMed] [Google Scholar]
- Mitrakou A., Ryan C., Veneman T., Mokan M., Jenssen T., Kiss I., Durrant J., Cryer P., Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991 Jan;260(1 Pt 1):E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Kennedy F., Consoli A., Martin C., Miles J., Gerich J. Quantification of the glycolytic origin of plasma glycerol: implications for the use of the rate of appearance of plasma glycerol as an index of lipolysis in vivo. Metabolism. 1988 Apr;37(4):386–389. doi: 10.1016/0026-0495(88)90140-0. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolwerth A. C., Smith B. C., Culpepper R. M. Renal gluconeogenesis. Miner Electrolyte Metab. 1988;14(6):347–361. [PubMed] [Google Scholar]
- Stanley W. C., Gertz E. W., Wisneski J. A., Neese R. A., Morris D. L., Brooks G. A. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol (1985) 1986 Apr;60(4):1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- Wirthensohn G., Guder W. G. Renal substrate metabolism. Physiol Rev. 1986 Apr;66(2):469–497. doi: 10.1152/physrev.1986.66.2.469. [DOI] [PubMed] [Google Scholar]


