Abstract
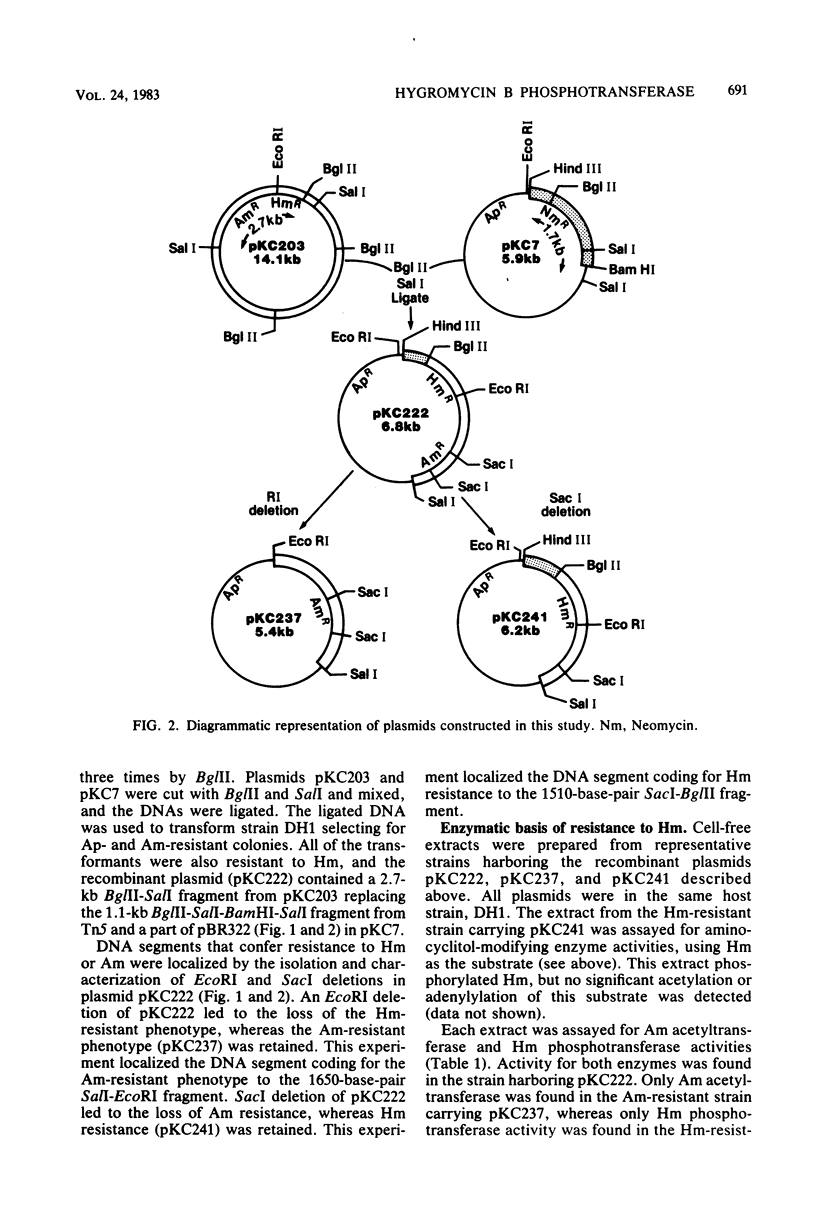
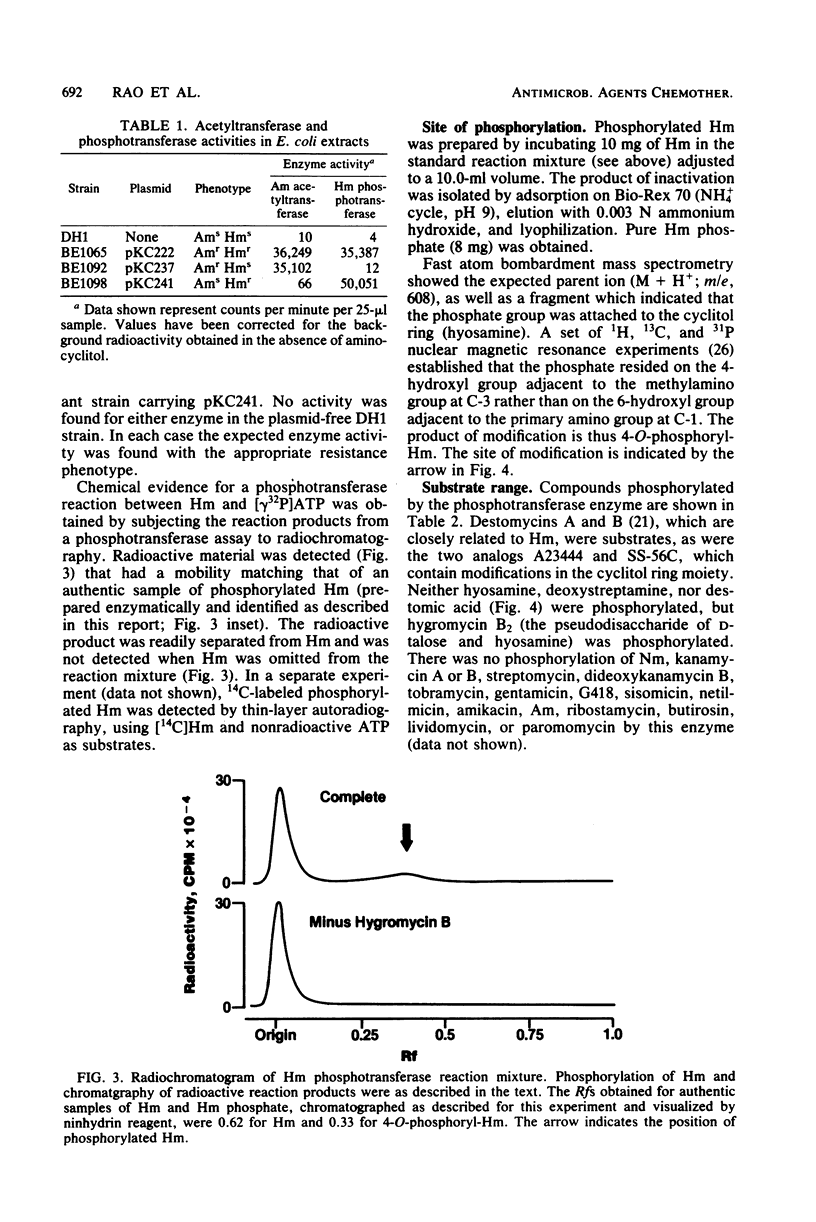
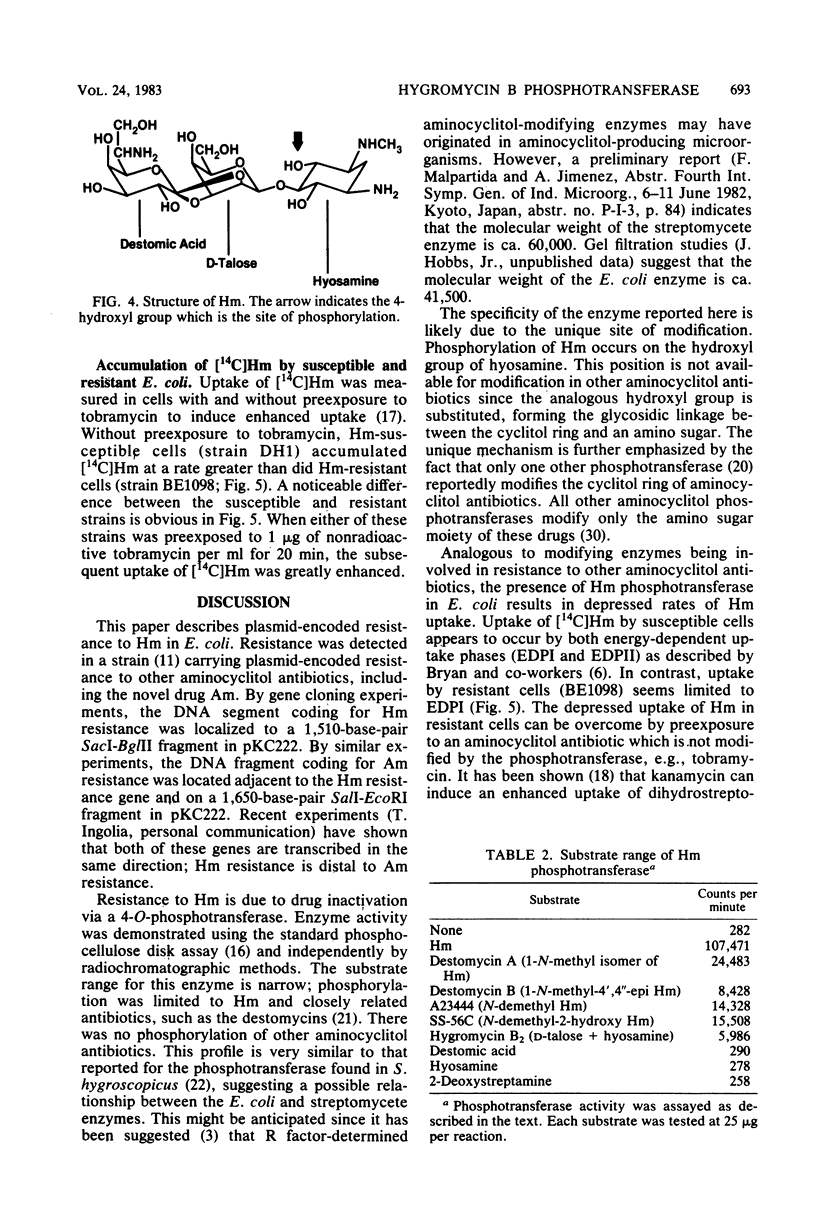
A plasmid conferring resistance to the aminocyclitol antibiotic hygromycin B was isolated from Escherichia coli. The gene conferring resistance to this drug was cloned in pBR322, and the gene was localized to a fragment of ca. 1,510 base pairs. Resistance to hygromycin B is determined by an aminocyclitol phosphotransferase that modifies hygromycin B and structurally related antibiotics. The specific modification of hygromycin B is a phosphorylation of the hydroxyl on the 4 position of the cyclitol ring (hyosamine). The presence of the phosphotransferase in E. coli correlates with reduced accumulation of [14C]hygromycin B.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. H., Rechenmacher A., Böck A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob Agents Chemother. 1980 Nov;18(5):798–806. doi: 10.1128/aac.18.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. E., Alborn W. E., Jr, Hobbs J. N., Jr, Kirst H. A. 7-Hydroxytropolone: an inhibitor of aminoglycoside-2"-O-adenylyltransferase. Antimicrob Agents Chemother. 1982 Nov;22(5):824–831. doi: 10.1128/aac.22.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabañas M. J., Vázquez D., Modolell J. Dual interference of hygromycin B with ribosomal translocation and with aminoacyl-tRNA recognition. Eur J Biochem. 1978 Jun 1;87(1):21–27. doi: 10.1111/j.1432-1033.1978.tb12347.x. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Davies J., Davis B. D. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J Biol Chem. 1968 Jun 25;243(12):3312–3316. [PubMed] [Google Scholar]
- Davies J., Jimenez A. A new selective agent for eukaryotic cloning vectors. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1089–1092. doi: 10.4269/ajtmh.1980.29.1089. [DOI] [PubMed] [Google Scholar]
- Davies J., O'Connor S. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother. 1978 Jul;14(1):69–72. doi: 10.1128/aac.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- González A., Jiménez A., Vázquez D., Davies J. E., Schindler D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim Biophys Acta. 1978 Dec 21;521(2):459–469. doi: 10.1016/0005-2787(78)90287-3. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Davies J. Expression of a transposable antibiotic resistance element in Saccharomyces. Nature. 1980 Oct 30;287(5785):869–871. doi: 10.1038/287869a0. [DOI] [PubMed] [Google Scholar]
- KONDO S. I., SEZAKI M., KOIKE M., SHIMURA M., AKITA E., SATOH K., HARA T. DESTOMYCINS A AND B, TWO NEW ANTIBIOTICS PRODUCED BY A STREPTOMYCES. J Antibiot (Tokyo) 1965 Jan;18:38–42. [PubMed] [Google Scholar]
- Leboul J., Davies J. Enzymatic modification of hygromycin B in Streptomyces hygroscopicus. J Antibiot (Tokyo) 1982 Apr;35(4):527–528. doi: 10.7164/antibiotics.35.527. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Rao R. N., Smith H. O. Cloning of restriction and modification genes in E. coli: the HbaII system from Haemophilus haemolyticus. Gene. 1978 Apr;3(2):97–112. doi: 10.1016/0378-1119(78)90054-9. [DOI] [PubMed] [Google Scholar]
- Neuss N., Koch K. F., Molloy B. B., Day W., Huckstep L. L., Dorman D. E., Roberts J. D. Structure of Hygromycin B, an antibiotic from Streptomyces hygroscopicus: the use of CMR. spectra in structure determination, I. Helv Chim Acta. 1970;53(8):2314–2319. doi: 10.1002/hlca.19700530846. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. A thermoinducible lambda phage-ColE1 plasmid chimera for the overproduction of gene products from cloned DNA segments. Gene. 1978 May;3(3):247–263. doi: 10.1016/0378-1119(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Singh A., Ursic D., Davies J. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature. 1979 Jan 11;277(5692):146–148. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thompson C. J., Skinner R. H., Thompson J., Ward J. M., Hopwood D. A., Cundliffe E. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J Bacteriol. 1982 Aug;151(2):678–685. doi: 10.1128/jb.151.2.678-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic D., Kemp J. D., Helgeson J. P. A new antibiotic with known resistance factors, G418, inhibits plant cells. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1031–1037. doi: 10.1016/0006-291x(81)91852-0. [DOI] [PubMed] [Google Scholar]