Abstract
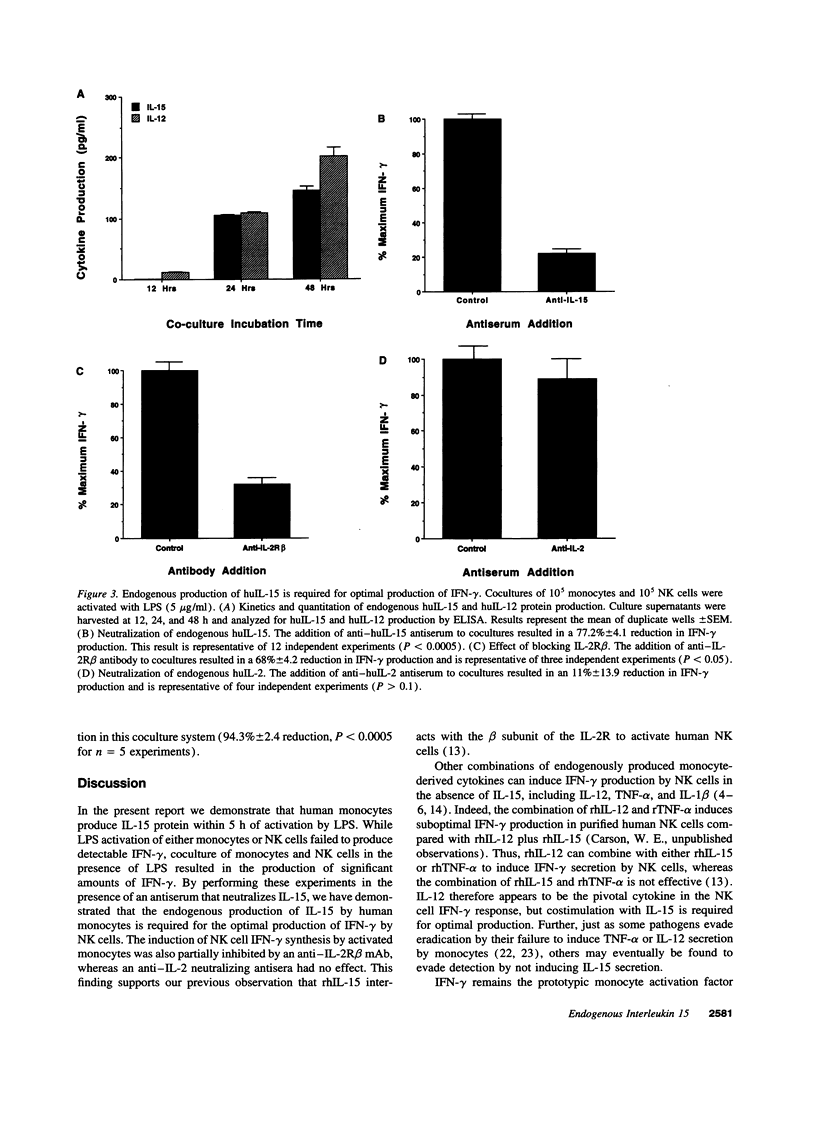
Natural killer (NK) cells are large granular lymphocytes that constitutively express functional IL-2 receptors. We have shown that recombinant human IL-15 uses the IL-2 receptor to activate human NK cells and can synergize with recombinant human IL-12 to stimulate NK cell production of IFN-gamma in vitro. IFN-gamma production by NK cells is critical in the prevention of overwhelming infection by obligate intracellular microbial pathogens in several experimental animal models. Herein, we demonstrate that human monocytes produce IL-15 protein within 5 h of activation with LPS. Using an IL-15-neutralizing antiserum in a coculture of LPS-activated monocytes and NK cells, we demonstrate that monocyte-derived IL-15 is critical for optimal NK cell production of IFN-gamma. Endogenous IL-15 activates NK cells through the IL-2 receptor, and with endogenous IL-12, regulates NK cell IFN-gamma after monocyte activation by LPS. These in vitro studies are the first to characterize a function for endogenous IL-15, and as such, suggest an important role for IL-15 during the innate immune response. IL-15 may be an important ligand for the NK cell IL-2 receptor in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baiocchi R. A., Ross M. E., Tan J. C., Chou C. C., Sullivan L., Haldar S., Monne M., Seiden M. V., Narula S. K., Sklar J. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood. 1995 Feb 15;85(4):1063–1074. [PubMed] [Google Scholar]
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson W. E., Giri J. G., Lindemann M. J., Linett M. L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M. A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994 Oct 1;180(4):1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Hunter C. A., Subauste C. S., Van Cleave V. H., Remington J. S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994 Jul;62(7):2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993 Aug;5(4):518–523. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M. E., Schnier G. S., Beecher M. S., Ashman L. K., William D. E., Caligiuri M. A. Expression of a functional c-kit receptor on a subset of natural killer cells. J Exp Med. 1993 Sep 1;178(3):1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Wang Z. E., Stowring L., Locksley R. M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994 Feb 1;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Sondel P. M., Robb R. J. Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R gamma chain with the IL-2R beta chain in functional intermediate-affinity IL-2R. J Exp Med. 1992 Aug 1;176(2):531–541. doi: 10.1084/jem.176.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]





