Abstract
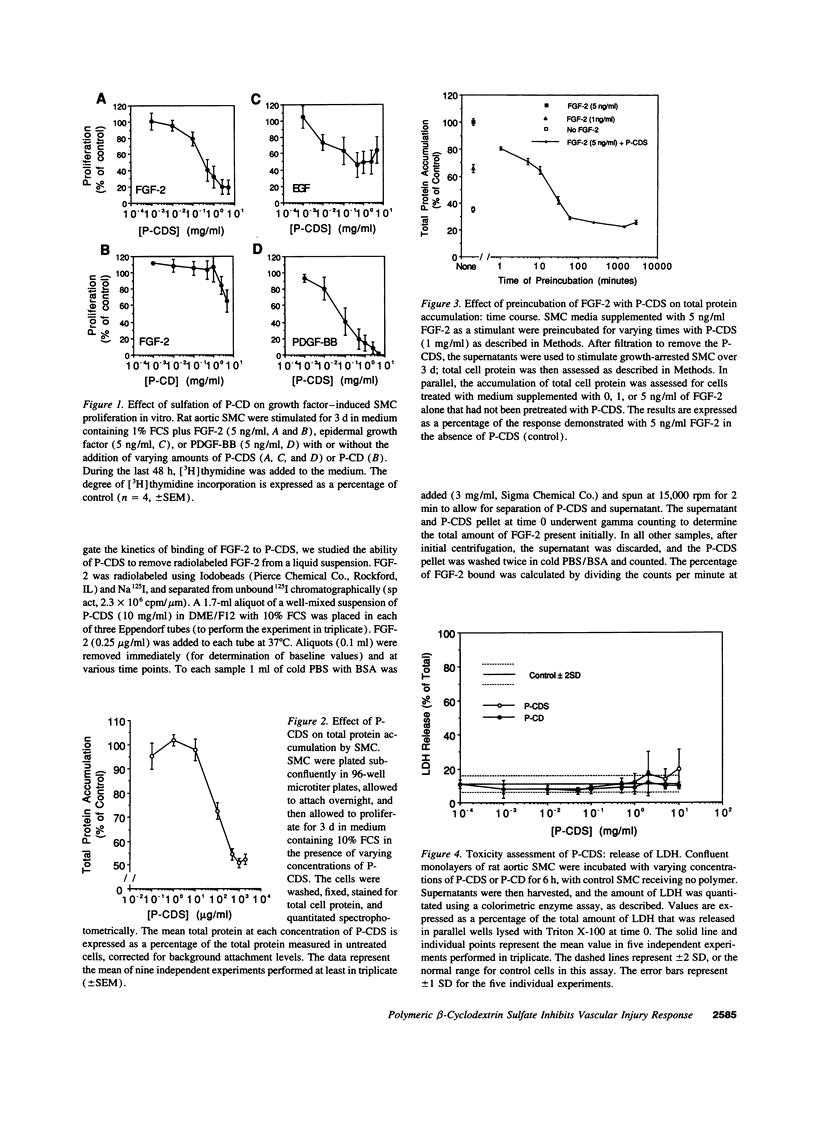
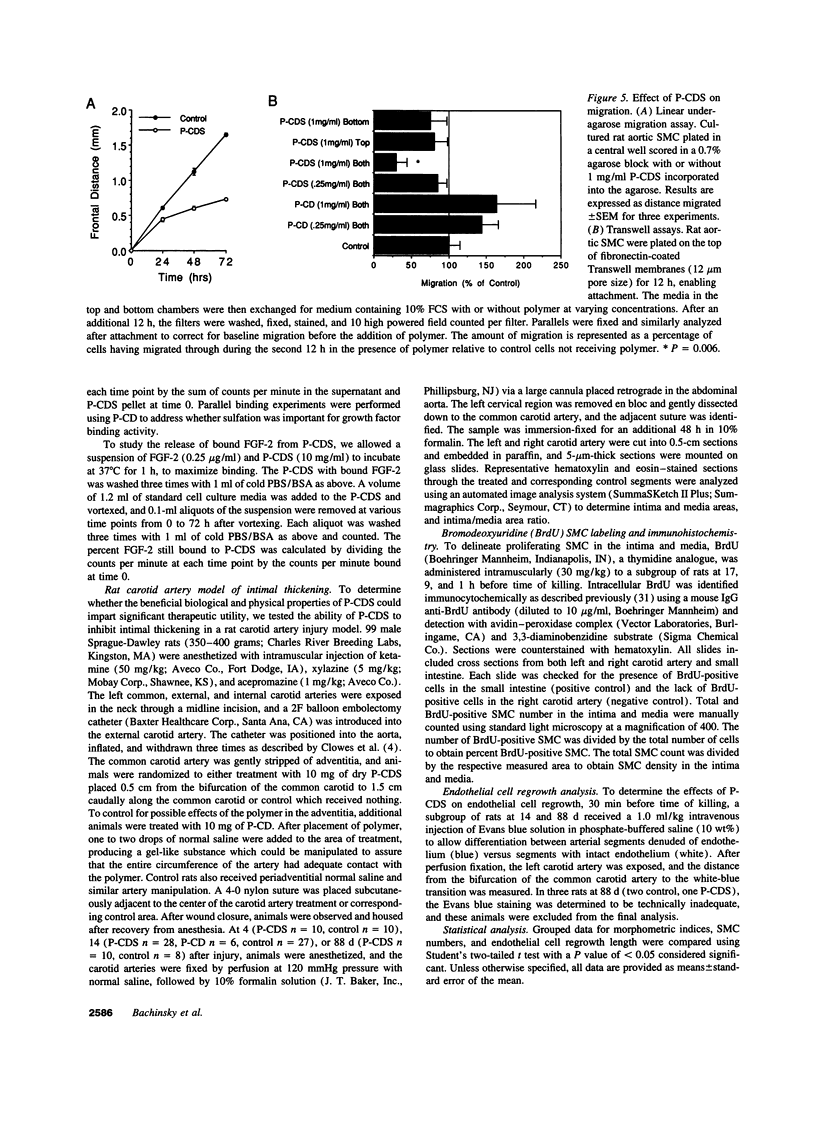
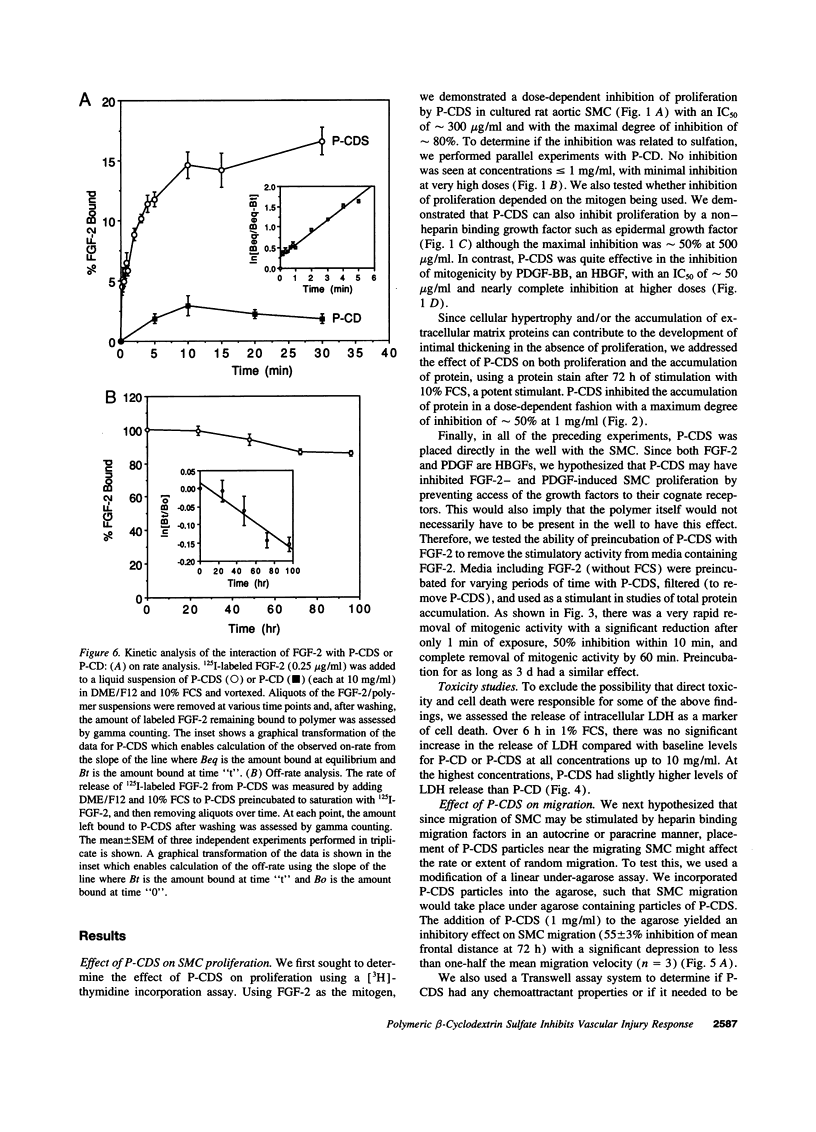
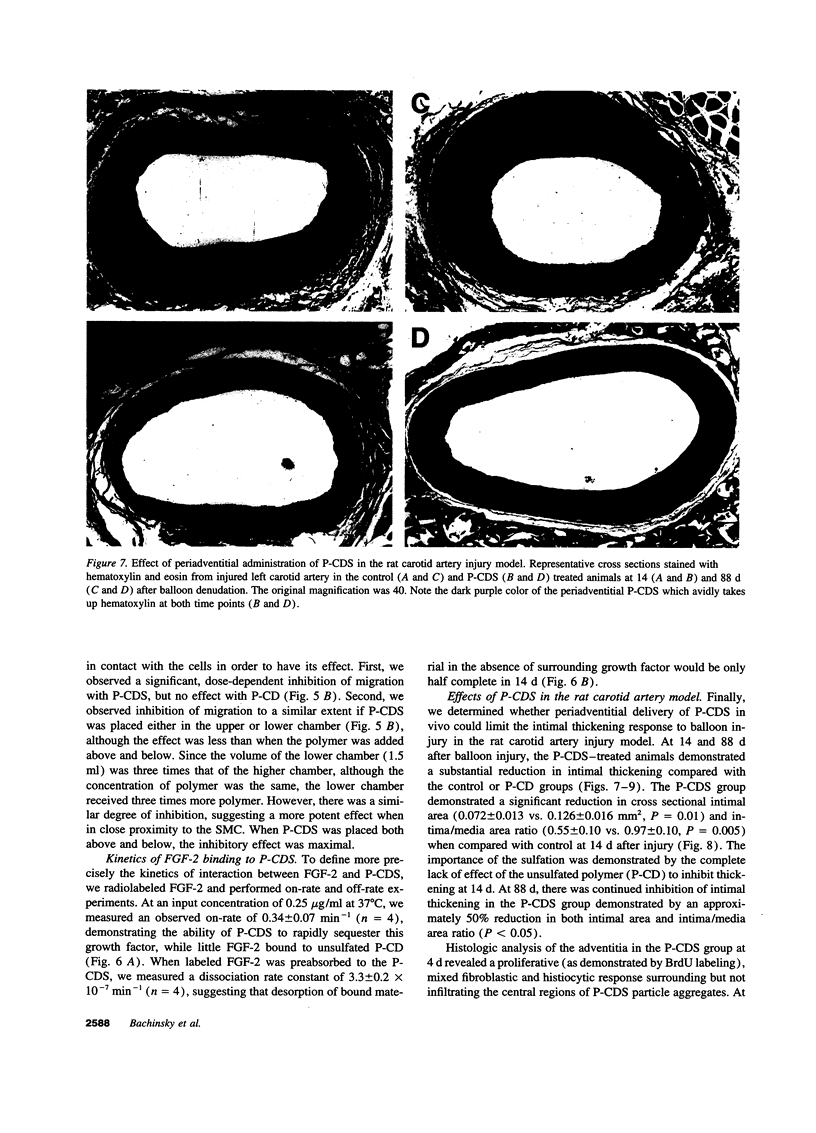
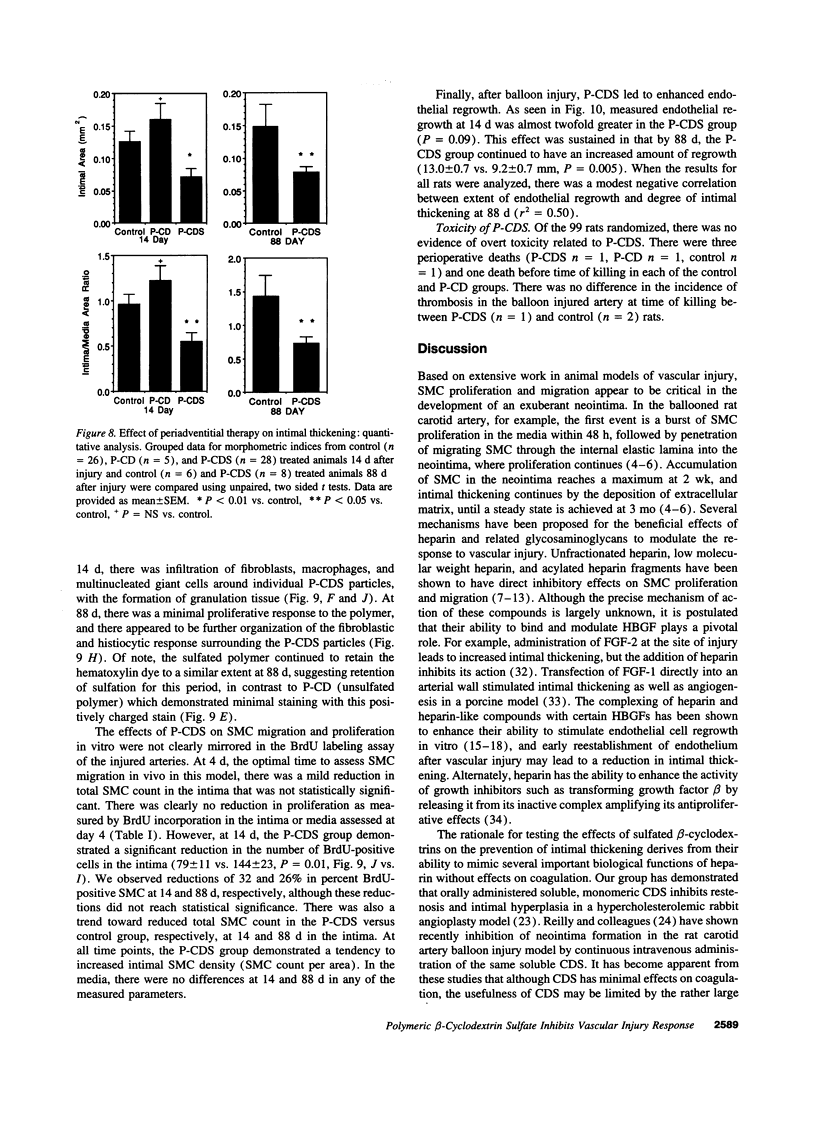
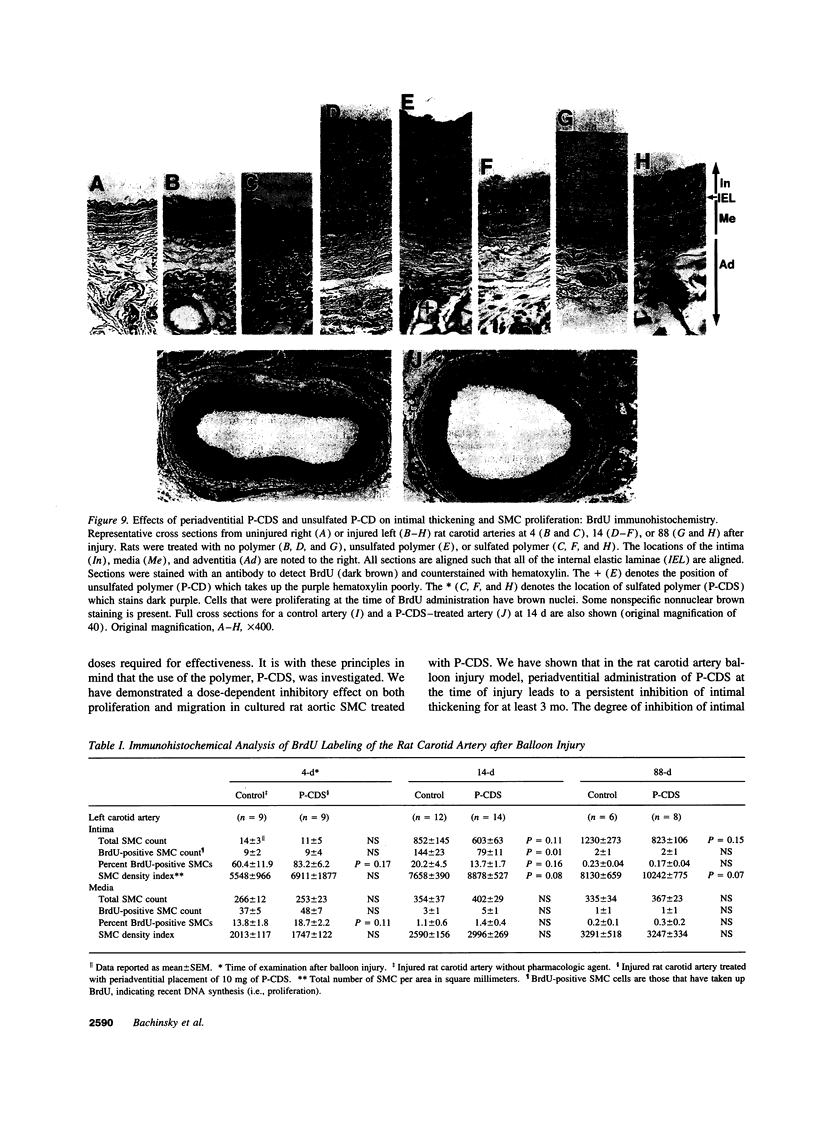
Intimal thickening after vascular injury may be modulated in part by heparin binding growth factors. We hypothesized that placement of a therapeutic polymer in the periadventitial space capable of tightly binding growth factors might alter the vascular response to injury. We first demonstrated that incubation of rat aortic smooth muscle cells with an insoluble, sulfated polymer of beta-cyclodextrin (P-CDS) was associated with a dose-dependent inhibition of proliferation induced by fetal calf serum, fibroblast growth factor-2 (FGF-2), platelet-derived growth factor BB, or epidermal growth factor. Preincubation studies of P-CDS with FGF-2 revealed a very rapid removal of mitogenic activity. Using radiolabeled FGF-2 (0.25 microg/ml), we observed a very rapid association rate (0.34 +/- 0.07 min-1, n=4) and a very slow dissociation rate (3.3 +/- 0.2 X 10(-7) min-1) at 37 degrees C, suggesting a high affinity interaction. Using both Transwell and linear under-agarose assays, we demonstrated a significant inhibition of random migration (chemokinesis) by P-CDS. Unsulfated polymeric beta-cyclodextrin (P-CD) had little if any of these effects, suggesting that the high negative charge density of P-CDS was important for the effects. Finally, rats undergoing carotid artery balloon injury were randomized to treatment with periadventitial P-CDS or no treatment, and were killed at 4 (n=20), 14 (n=59), and 88 d (n=14). Morphometric analysis demonstrated significant and sustained inhibition of intimal thickening in P-CDS-treated rats at 14 (P < 0.01) and 88 d (P < 0.05) using absolute intimal area or intima/media area ratios. No inhibition was seen in a group of rats treated with P-CD. In P-CDS-treated rats, bromodeoxyuridine labeling studies revealed fewer labeled smooth muscle cells in the intima at 14 d (P=0.01), while staining with Evans blue revealed enhanced late endothelial cell regrowth. Thus, periadventitially applied sulfated beta-cyclodextrin polymer, which can tightly bind heparin binding growth factors, inhibits intimal thickening in vivo in a sustained fashion without using an additional delivery system. These studies suggest that cellular processes mediated by heparin binding growth factors may be modulated by P-CDS.
Full text
PDF



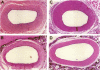
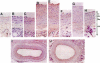
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Ratliff N. B., Hollman J., Tabei S., Phillips D. F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985 Aug;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Inhibition of smooth muscle cell proliferation by heparin molecules. Transplant Proc. 1989 Aug;21(4):3700–3701. [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. IV. Heparin inhibits rat smooth muscle mitogenesis and migration. Circ Res. 1986 Jun;58(6):839–845. doi: 10.1161/01.res.58.6.839. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W. Regulation of smooth muscle cell function by heparin. J Vasc Surg. 1992 May;15(5):911–913. doi: 10.1016/0741-5214(92)90746-u. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Karnovsky M. J. Contrasting effects of the intermittent and continuous administration of heparin in experimental restenosis. Circulation. 1994 Feb;89(2):770–776. doi: 10.1161/01.cir.89.2.770. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager G., Hansson G. K., Ottosson P., Dahllöf B., Bondjers G. Human arterial smooth muscle cells in culture. Effects of platelet-derived growth factor and heparin on growth in vitro. Exp Cell Res. 1988 Jun;176(2):319–335. doi: 10.1016/0014-4827(88)90334-5. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Folkman J., Weisz P. B., Joullié M. M., Li W. W., Ewing W. R. Control of angiogenesis with synthetic heparin substitutes. Science. 1989 Mar 17;243(4897):1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hanke H., Oberhoff M., Hanke S., Hassenstein S., Kamenz J., Schmid K. M., Betz E., Karsch K. R. Inhibition of cellular proliferation after experimental balloon angioplasty by low-molecular-weight heparin. Circulation. 1992 Apr;85(4):1548–1556. doi: 10.1161/01.cir.85.4.1548. [DOI] [PubMed] [Google Scholar]
- Herrmann H. C., Okada S. S., Hozakowska E., LeVeen R. F., Golden M. A., Tomaszewski J. E., Weisz P. B., Barnathan E. S. Inhibition of smooth muscle cell proliferation and experimental angioplasty restenosis by beta-cyclodextrin tetradecasulfate. Arterioscler Thromb. 1993 Jun;13(6):924–931. doi: 10.1161/01.atv.13.6.924. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. Mediators of angiogenesis: the biological significance of basic fibroblast growth factor (bFGF)-heparin and heparan sulfate interactions. Semin Cancer Biol. 1992 Apr;3(2):81–87. [PubMed] [Google Scholar]
- Kockx M. M., Cambier B. A., Bortier H. E., De Meyer G. R., Van Cauwelaert P. A. The modulation of smooth muscle cell phenotype is an early event in human aorto-coronary saphenous vein grafts. Virchows Arch A Pathol Anat Histopathol. 1992;420(2):155–162. doi: 10.1007/BF02358807. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Yang Z. Y., Plautz G., Forough R., Zhan X., Haudenschild C. C., Maciag T., Nabel G. J. Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature. 1993 Apr 29;362(6423):844–846. doi: 10.1038/362844a0. [DOI] [PubMed] [Google Scholar]
- Okada S. S., Kuo A., Muttreja M. R., Hozakowska E., Weisz P. B., Barnathan E. S. Inhibition of human vascular smooth muscle cell migration and proliferation by beta-cyclodextrin tetradecasulfate. J Pharmacol Exp Ther. 1995 May;273(2):948–954. [PubMed] [Google Scholar]
- Okada T., Bark D. H., Mayberg M. R. Localized release of perivascular heparin inhibits intimal proliferation after endothelial injury without systemic anticoagulation. Neurosurgery. 1989 Dec;25(6):892–898. doi: 10.1097/00006123-198912000-00007. [DOI] [PubMed] [Google Scholar]
- Peppas N. A., Langer R. New challenges in biomaterials. Science. 1994 Mar 25;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- Pukac L. A., Hirsch G. M., Lormeau J. C., Petitou M., Choay J., Karnovsky M. J. Antiproliferative effects of novel, nonanticoagulant heparin derivatives on vascular smooth muscle cells in vitro and in vivo. Am J Pathol. 1991 Dec;139(6):1501–1509. [PMC free article] [PubMed] [Google Scholar]
- Schutte B., Reynders M. M., Bosman F. T., Blijham G. H. Effect of tissue fixation on anti-bromodeoxyuridine immunohistochemistry. J Histochem Cytochem. 1987 Nov;35(11):1343–1345. doi: 10.1177/35.11.3116075. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Rifkin D. B. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J Cell Physiol. 1989 Jan;138(1):215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- Villa A. E., Guzman L. A., Chen W., Golomb G., Levy R. J., Topol E. J. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest. 1994 Mar;93(3):1243–1249. doi: 10.1172/JCI117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. B., Hermann H. C., Joullié M. M., Kumor K., Levine E. M., Macarak E. J., Weiner D. B. Angiogenesis and heparin mimics. EXS. 1992;61:107–117. doi: 10.1007/978-3-0348-7001-6_18. [DOI] [PubMed] [Google Scholar]
- Yuen S. N., Folkman J., Weisz P. B., Joullie M. M., Ewing W. R. Affinity of fibroblast growth factors for beta-cyclodextrin tetradecasulfate. Anal Biochem. 1990 Feb 15;185(1):108–111. doi: 10.1016/0003-2697(90)90263-9. [DOI] [PubMed] [Google Scholar]