Abstract
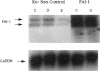
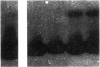
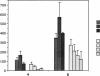
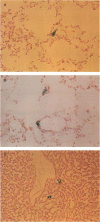
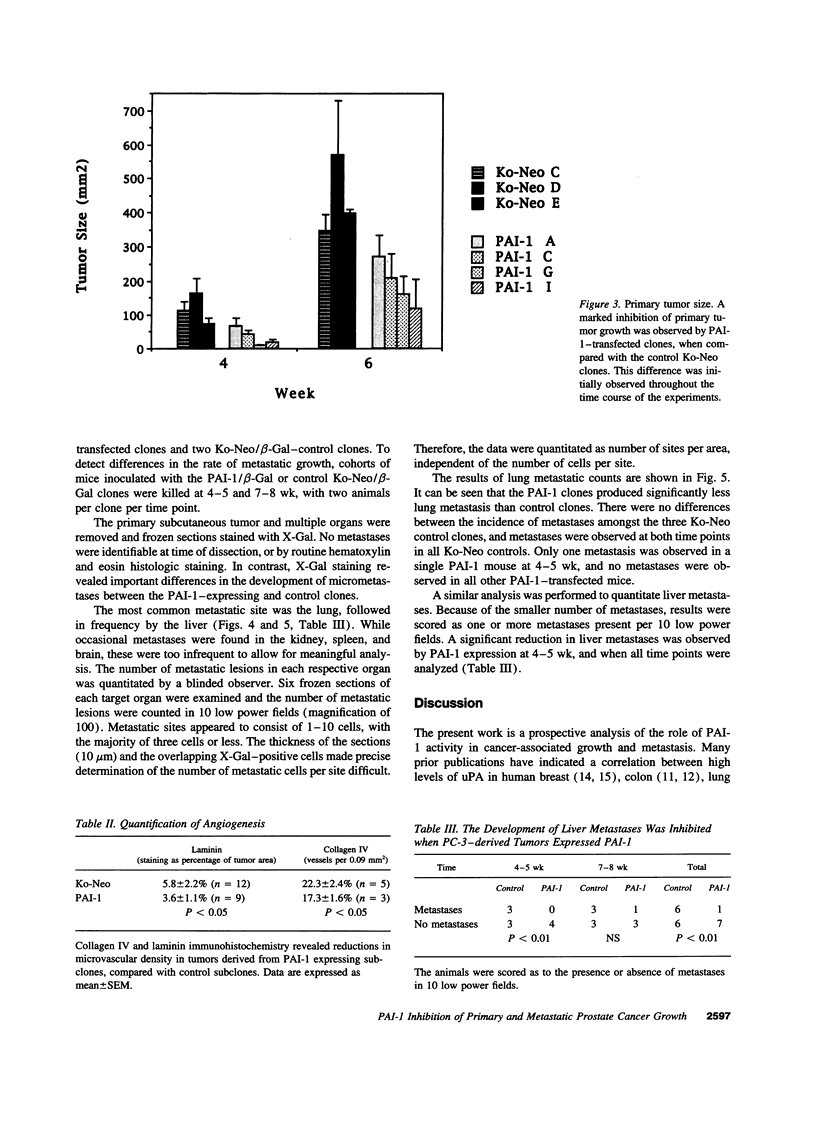
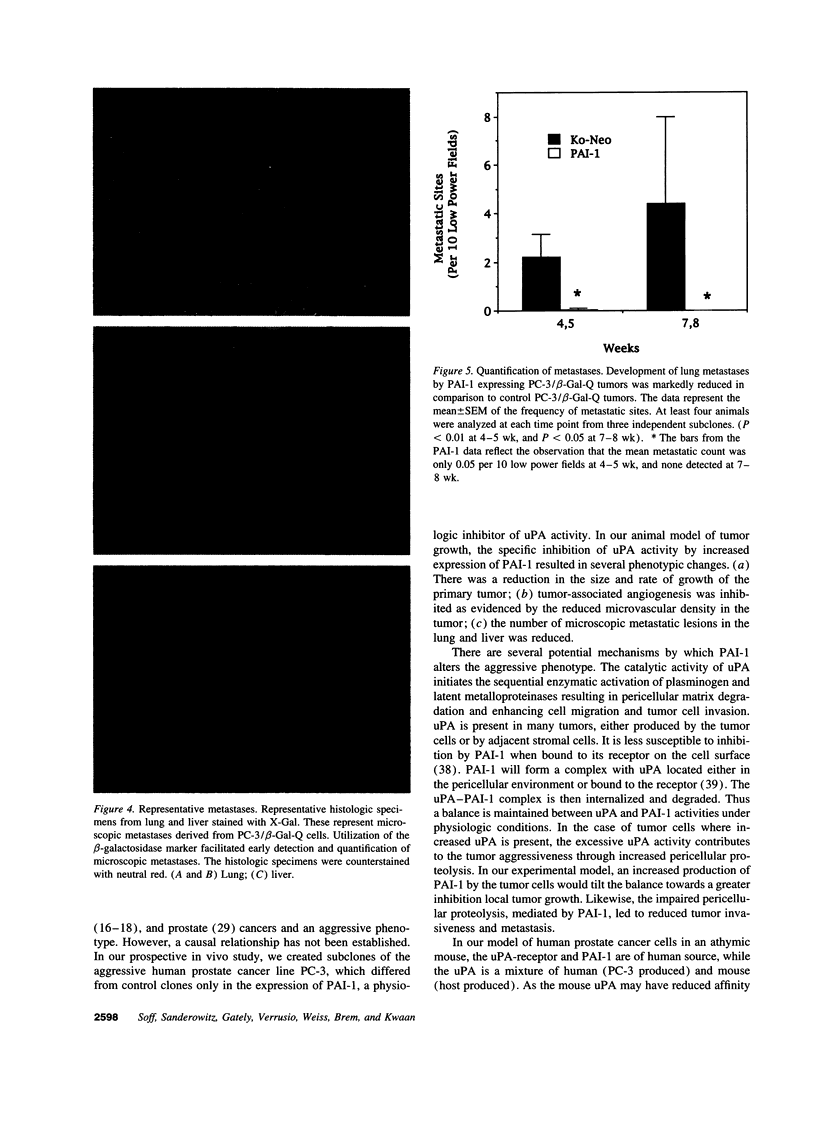
Expression of urokinase-type plasminogen activator (uPA) by malignant cells correlates with an aggressive phenotype, including increased invasiveness, tumor-associated angiogenesis, and metastases. Plasminogen activator inhibitor type 1 (PAI-1) is undetectable in cells of some aggressive malignancies, but present in the stroma of tumor-associated microvasculature. This analysis of an athymic mouse model of prostate carcinoma further defines the role of the uPA/PAI-1/plasmin system in primary growth and metastasis. A marked increase in PAI-1 expression was induced in clones of the aggressive human prostate carcinoma line, PC-3, by stable transfection. Primary PC-3 tumors, in mice, were significantly smaller when derived from PAI-1 expressing versus control cells. PAI-1 expression reduced the density of tumor-associated microvasculature by 22-38%. Microscopic metastases were quantitated using stable expression of the chromogenic label (beta-galactosidase) in control and PAI-1 expressing cells. PAI-1 expression resulted in a significant inhibition of lung metastases, and liver metastases. Expression of PAI-1 by malignant prostate cells resulted in a less aggressive phenotype, presumably by inhibition of uPA activity, suggesting pharmacologic or molecular inhibition of uPA activity as a potential therapeutic target.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cajot J. F., Bamat J., Bergonzelli G. E., Kruithof E. K., Medcalf R. L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camiolo S. M., Siuta M. R., Madeja J. M. Improved medium for extraction of plasminogen activator from tissue. Prep Biochem. 1982;12(4):297–305. doi: 10.1080/00327488208065678. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Wun T. C., Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 1990 Apr;9(4):1079–1085. doi: 10.1002/j.1460-2075.1990.tb08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovich L., Turzó C., Adány R. Factors involved in the plasminogen activation system in human breast tumours. Thromb Haemost. 1994 Jun;71(6):684–691. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., Reilly D., O'Sullivan C., O'Higgins N., Fennelly J. J., Andreasen P. Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res. 1990 Nov 1;50(21):6827–6829. [PubMed] [Google Scholar]
- Ellis V., Wun T. C., Behrendt N., Rønne E., Danø K. Inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990 Jun 15;265(17):9904–9908. [PubMed] [Google Scholar]
- Eriksdotter-Nilsson M., Björklund H., Olson L. Laminin immunohistochemistry: a simple method to visualize and quantitate vascular structures in the mammalian brain. J Neurosci Methods. 1986 Sep;17(4):275–286. doi: 10.1016/0165-0270(86)90128-7. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R., Moscatelli D., Saksela O., Rifkin D. B. Role of extracellular matrix in the action of basic fibroblast growth factor: matrix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J Cell Physiol. 1989 Jul;140(1):75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- Ganesh S., Sier C. F., Griffioen G., Vloedgraven H. J., de Boer A., Welvaart K., van de Velde C. J., van Krieken J. H., Verheijen J. H., Lamers C. B. Prognostic relevance of plasminogen activators and their inhibitors in colorectal cancer. Cancer Res. 1994 Aug 1;54(15):4065–4071. [PubMed] [Google Scholar]
- Gaylis F. D., Keer H. N., Wilson M. J., Kwaan H. C., Sinha A. A., Kozlowski J. M. Plasminogen activators in human prostate cancer cell lines and tumors: correlation with the aggressive phenotype. J Urol. 1989 Jul;142(1):193–198. doi: 10.1016/s0022-5347(17)38709-8. [DOI] [PubMed] [Google Scholar]
- Gossler A., Joyner A. L., Rossant J., Skarnes W. C. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989 Apr 28;244(4903):463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienert G., Kirchheimer J. C., Pflüger H., Binder B. R. Urokinase-type plasminogen activator as a marker for the formation of distant metastases in prostatic carcinomas. J Urol. 1988 Dec;140(6):1466–1469. doi: 10.1016/s0022-5347(17)42074-x. [DOI] [PubMed] [Google Scholar]
- Hollas W., Hoosein N., Chung L. W., Mazar A., Henkin J., Kariko K., Barnathan E. S., Boyd D. Expression of urokinase and its receptor in invasive and non-invasive prostate cancer cell lines. Thromb Haemost. 1992 Dec 7;68(6):662–666. [PubMed] [Google Scholar]
- Keer H. N., Gaylis F. D., Kozlowski J. M., Kwaan H. C., Bauer K. D., Sinha A. A., Wilson M. J. Heterogeneity in plasminogen activator (PA) levels in human prostate cancer cell lines: increased PA activity correlates with biologically aggressive behavior. Prostate. 1991;18(3):201–214. doi: 10.1002/pros.2990180303. [DOI] [PubMed] [Google Scholar]
- Kellen J. A., Mirakian A., Kolin A. Antimetastatic effect of amiloride in an animal tumour model. Anticancer Res. 1988 Nov-Dec;8(6):1373–1376. [PubMed] [Google Scholar]
- Kent R. B., Emanuel J. R., Ben Neriah Y., Levenson R., Housman D. E. Ouabain resistance conferred by expression of the cDNA for a murine Na+, K+-ATPase alpha subunit. Science. 1987 Aug 21;237(4817):901–903. doi: 10.1126/science.3039660. [DOI] [PubMed] [Google Scholar]
- Kwaan H. C., Keer H. N., Radosevich J. A., Cajot J. F., Ernst R. Components of the plasminogen-plasmin system in human tumor cell lines. Semin Thromb Hemost. 1991 Jul;17(3):175–182. doi: 10.1055/s-2007-1002607. [DOI] [PubMed] [Google Scholar]
- Kwaan H. C. The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev. 1992 Nov;11(3-4):291–311. doi: 10.1007/BF01307184. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landau B. J., Kwaan H. C., Verrusio E. N., Brem S. S. Elevated levels of urokinase-type plasminogen activator and plasminogen activator inhibitor type-1 in malignant human brain tumors. Cancer Res. 1994 Feb 15;54(4):1105–1108. [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus G., Takita H., Camiolo S. M., Corasanti J. G., Evers J. L., Hobika G. H. Content and characterization of plasminogen activators in human lung tumors and normal lung tissue. Cancer Res. 1980 Mar;40(3):841–848. [PubMed] [Google Scholar]
- Markus G. The relevance of plasminogen activators to neoplastic growth. A review of recent literature. Enzyme. 1988;40(2-3):158–172. doi: 10.1159/000469158. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Ishida T., Nishino T., Sugimachi K. Immunohistochemical evidence of urokinase-type plasminogen activator in primary and metastatic tumors of pulmonary adenocarcinoma. Cancer Res. 1991 Jul 1;51(13):3522–3525. [PubMed] [Google Scholar]
- Ossowski L., Clunie G., Masucci M. T., Blasi F. In vivo paracrine interaction between urokinase and its receptor: effect on tumor cell invasion. J Cell Biol. 1991 Nov;115(4):1107–1112. doi: 10.1083/jcb.115.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen H., Grøndahl-Hansen J., Francis D., Osterlind K., Hansen H. H., Danø K., Brünner N. Urokinase and plasminogen activator inhibitor type 1 in pulmonary adenocarcinoma. Cancer Res. 1994 Jan 1;54(1):120–123. [PubMed] [Google Scholar]
- Pepper M. S., Belin D., Montesano R., Orci L., Vassalli J. D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990 Aug;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu K., Kamo H., Akiguchi I., Kimura J., Kameyama M., Kimura H. Neovascularization in kainic acid-induced lesions of rat striatum. An immunohistochemical study with laminin. Brain Res. 1989 Nov 6;501(2):215–222. doi: 10.1016/0006-8993(89)90639-2. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Testa J. E., Quigley J. P. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990 Dec;9(4):353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987 Apr 6;214(1):187–191. doi: 10.1016/0014-5793(87)80039-x. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga C., Nakashima Y., Sueishi K. A role of fibrinolytic activity in angiogenesis. Quantitative assay using in vitro method. Lab Invest. 1989 Dec;61(6):698–704. [PubMed] [Google Scholar]
- de Bruin P. A., Griffioen G., Verspaget H. W., Verheijen J. H., Lamers C. B. Plasminogen activators and tumor development in the human colon: activity levels in normal mucosa, adenomatous polyps, and adenocarcinomas. Cancer Res. 1987 Sep 1;47(17):4654–4657. [PubMed] [Google Scholar]