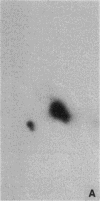
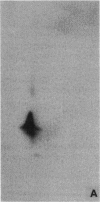
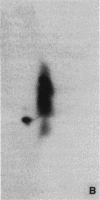
Abstract
The ability of whole serum to promote cell cholesterol efflux and the relationships between apoprotein and lipoprotein components of human serum efflux have been investigated previously (de la Llera Moya, M., V. Atger, J.L. Paul, N. Fournier, N. Moatti, P. Giral, K.E. Friday, and G.H. Rothblat. 1994. Arterioscler. Thromb. 14:1056-1065). We have now used this experimental system to study the selective effects of two human lipoprotein-related proteins, apoprotein AI (apo AI) and cholesteryl ester transfer protein (CETP) on cell cholesterol efflux, when these proteins are expressed in transgenic mice. The percent efflux values for cholesterol released in 4 h from Fu5AH donor cells to 5% sera from the different groups of mice were in the order: background = human apo AI transgenic (HuAITg) > human CETP transgenic (HuCETPTg) > human apo AI and CETP transgenic (HuAICETPTg) >> apo AI knockout mice. In each group of mice a strong, positive correlation (r2 ranging from 0.64 to 0.76) was found between efflux and HDL cholesterol concentrations. The slopes of these regression lines differed between groups of mice, indicating that the cholesterol acceptor efficiencies of the sera differed among groups. These differences in relative efficiencies can explain why cholesterol efflux was not proportional to the different HDL levels in the various groups of mice. We can conclude that: (a) HDL particles from HuAITg mice are less efficient as cholesterol acceptors than HDL from the background mice; (b) despite a lower average efflux due to lower HDL cholesterol concentrations, HDL particles are more efficient in the HuCETPTg mice than in the background mice; and (c) the coexpression of both human apo AI and CETP improves the efficiency of HDL particles in the HuAICETPTg mice when compared with the HuAITg mice. We also demonstrated that the esterification of the free cholesterol released from the cells by lecithin cholesterol acyltransferase in the serum was reduced in the HuAITg and AI knockout mice, whereas it was not different from background values in the two groups of mice expressing human CETP.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agellon L. B., Walsh A., Hayek T., Moulin P., Jiang X. C., Shelanski S. A., Breslow J. L., Tall A. R. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991 Jun 15;266(17):10796–10801. [PubMed] [Google Scholar]
- Arbogast L. Y., Rothblat G. H., Leslie M. H., Cooper R. A. Cellular cholesterol ester accumulation induced by free cholesterol-rich lipid dispersions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3680–3684. doi: 10.1073/pnas.73.10.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos B. F., Sloop C. H., Wong L., Roheim P. S. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993 Sep 8;1169(3):291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- Atger V., Malon D., Bertiere M. C., N'Diaye F., Girard-Globa A. Cholesterol distribution between high-density-lipoprotein subfractions HDL2 and HDL3 determined in serum by discontinuous gradient gel electrophoresis. Clin Chem. 1991 Jul;37(7):1149–1152. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bielicki J. K., Johnson W. J., Weinberg R. B., Glick J. M., Rothblat G. H. Efflux of lipid from fibroblasts to apolipoproteins: dependence on elevated levels of cellular unesterified cholesterol. J Lipid Res. 1992 Nov;33(11):1699–1709. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Burkey B. F., France D., Wang H., Ma X., Brand B., Abuhani C., Diffenderfer M. R., Marsh J. B., Paterniti J. R., Jr, Fisher E. A. Overexpression of human apolipoprotein A-I in transgenic rats and the hyperlipoproteinemia associated with experimental nephrosis. J Lipid Res. 1995 Jul;36(7):1463–1473. [PubMed] [Google Scholar]
- Burns C. H., Rothblat G. H. Cholesterol excretion by tissue culture cells: effect of serum lipids. Biochim Biophys Acta. 1969 Apr 29;176(3):616–625. doi: 10.1016/0005-2760(69)90228-8. [DOI] [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 1988 Jan 12;27(1):25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- Chajek-Shaul T., Hayek T., Walsh A., Breslow J. L. Expression of the human apolipoprotein A-I gene in transgenic mice alters high density lipoprotein (HDL) particle size distribution and diminishes selective uptake of HDL cholesteryl esters. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6731–6735. doi: 10.1073/pnas.88.15.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau I. Y., Geyer R. P. The effects of serum albumin and phospholipid on sterol excretion in tissue culture cells. Biochim Biophys Acta. 1978 Aug 17;542(2):214–221. doi: 10.1016/0304-4165(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Davidson W. S., Gillotte K. L., Lund-Katz S., Johnson W. J., Rothblat G. H., Phillips M. C. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem. 1995 Mar 17;270(11):5882–5890. doi: 10.1074/jbc.270.11.5882. [DOI] [PubMed] [Google Scholar]
- Davidson W. S., Lund-Katz S., Johnson W. J., Anantharamaiah G. M., Palgunachari M. N., Segrest J. P., Rothblat G. H., Phillips M. C. The influence of apolipoprotein structure on the efflux of cellular free cholesterol to high density lipoprotein. J Biol Chem. 1994 Sep 16;269(37):22975–22982. [PubMed] [Google Scholar]
- Davidson W. S., Rodrigueza W. V., Lund-Katz S., Johnson W. J., Rothblat G. H., Phillips M. C. Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem. 1995 Jul 21;270(29):17106–17113. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- DeLamatre J., Wolfbauer G., Phillips M. C., Rothblat G. H. Role of apolipoproteins in cellular cholesterol efflux. Biochim Biophys Acta. 1986 Feb 28;875(3):419–428. doi: 10.1016/0005-2760(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995 Feb;36(2):211–228. [PubMed] [Google Scholar]
- Fielding C. J., Moser K. Evidence for the separation of albumin- and apo A-I-dependent mechanisms of cholesterol efflux from cultured fibroblasts into human plasma. J Biol Chem. 1982 Sep 25;257(18):10955–10960. [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J., Havel R. J., Kane J. P., Tun P. Cholesterol net transport, esterification, and transfer in human hyperlipidemic plasma. J Clin Invest. 1983 Mar;71(3):449–460. doi: 10.1172/JCI110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francone O. L., Fielding C. J. Initial steps in reverse cholesterol transport: the role of short-lived cholesterol acceptors. Eur Heart J. 1990 Aug;11 (Suppl E):218–224. doi: 10.1093/eurheartj/11.suppl_e.218. [DOI] [PubMed] [Google Scholar]
- Francone O. L., Gong E. L., Ng D. S., Fielding C. J., Rubin E. M. Expression of human lecithin-cholesterol acyltransferase in transgenic mice. Effect of human apolipoprotein AI and human apolipoprotein all on plasma lipoprotein cholesterol metabolism. J Clin Invest. 1995 Sep;96(3):1440–1448. doi: 10.1172/JCI118180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francone O. L., Gurakar A., Fielding C. Distribution and functions of lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in plasma lipoproteins. Evidence for a functional unit containing these activities together with apolipoproteins A-I and D that catalyzes the esterification and transfer of cell-derived cholesterol. J Biol Chem. 1989 Apr 25;264(12):7066–7072. [PubMed] [Google Scholar]
- Gallo L. L., Atasoy R., Vahouny G. V., Treadwell C. R. Enzymatic assay for cholesterol ester hydrolase activity. J Lipid Res. 1978 Sep;19(7):913–916. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Hara H., Hara H., Komaba A., Yokoyama S. Alpha-helical requirements for free apolipoproteins to generate HDL and to induce cellular lipid efflux. Lipids. 1992 Apr;27(4):302–304. doi: 10.1007/BF02536480. [DOI] [PubMed] [Google Scholar]
- Hara H., Yokoyama S. Role of apolipoproteins in cholesterol efflux from macrophages to lipid microemulsion: proposal of a putative model for the pre-beta high-density lipoprotein pathway. Biochemistry. 1992 Feb 25;31(7):2040–2046. doi: 10.1021/bi00122a021. [DOI] [PubMed] [Google Scholar]
- Hayek T., Chajek-Shaul T., Walsh A., Agellon L. B., Moulin P., Tall A. R., Breslow J. L. An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-I genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J Clin Invest. 1992 Aug;90(2):505–510. doi: 10.1172/JCI115887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., von Eckardstein A., Wu S., Maeda N., Assmann G. A plasma lipoprotein containing only apolipoprotein E and with gamma mobility on electrophoresis releases cholesterol from cells. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1834–1838. doi: 10.1073/pnas.91.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. M., Bucher N. L., Faris B., Franzblau C., Zannis V. I. Tissue-specific posttranslational modification of rat apoE. Synthesis of sialated apoE forms by neonatal rat aortic smooth muscle cells. J Lipid Res. 1988 Jul;29(7):915–923. [PubMed] [Google Scholar]
- Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995 Jul 21;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Komaba A., Li Q., Hara H., Yokoyama S. Resistance of smooth muscle cells to assembly of high density lipoproteins with extracellular free apolipoproteins and to reduction of intracellularly accumulated cholesterol. J Biol Chem. 1992 Sep 5;267(25):17560–17566. [PubMed] [Google Scholar]
- Kotite L., Bergeron N., Havel R. J. Quantification of apolipoproteins B-100, B-48, and E in human triglyceride-rich lipoproteins. J Lipid Res. 1995 Apr;36(4):890–900. [PubMed] [Google Scholar]
- Melchior G. W., Castle C. K., Murray R. W., Blake W. L., Dinh D. M., Marotti K. R. Apolipoprotein A-I metabolism in cholesteryl ester transfer protein transgenic mice. Insights into the mechanisms responsible for low plasma high density lipoprotein levels. J Biol Chem. 1994 Mar 18;269(11):8044–8051. [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Johnson W. J., Rothblat G. H. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987 Jun 24;906(2):223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Scott C. J., Breslow J. L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pászty C., Maeda N., Verstuyft J., Rubin E. M. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994 Aug;94(2):899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl D. Extravascular circulation of lipoproteins: their role in reverse transport of cholesterol. Atherosclerosis. 1994 Feb;105(2):117–129. doi: 10.1016/0021-9150(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Reichl D. Lipoproteins of human peripheral lymph. Eur Heart J. 1990 Aug;11 (Suppl E):230–236. doi: 10.1093/eurheartj/11.suppl_e.230. [DOI] [PubMed] [Google Scholar]
- Reichl D., Miller N. E. Pathophysiology of reverse cholesterol transport. Insights from inherited disorders of lipoprotein metabolism. Arteriosclerosis. 1989 Nov-Dec;9(6):785–797. doi: 10.1161/01.atv.9.6.785. [DOI] [PubMed] [Google Scholar]
- Roheim P. S. Atherosclerosis and lipoprotein metabolism: role of reverse cholesterol transport. Am J Cardiol. 1986 Feb 12;57(5):3C–10C. doi: 10.1016/0002-9149(86)91020-9. [DOI] [PubMed] [Google Scholar]
- Roheim P. S., Dory L., Lefevre M., Sloop C. H. Lipoproteins in interstitial fluid of dogs: implications for a role in reverse cholesterol transport. Eur Heart J. 1990 Aug;11 (Suppl E):225–229. doi: 10.1093/eurheartj/11.suppl_e.225. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991 Sep 19;353(6341):265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- Schultz J. R., Verstuyft J. G., Gong E. L., Nichols A. V., Rubin E. M. Protein composition determines the anti-atherogenic properties of HDL in transgenic mice. Nature. 1993 Oct 21;365(6448):762–764. doi: 10.1038/365762a0. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Lefevre M., Roheim P. S. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim Biophys Acta. 1986 Aug 14;878(1):7–13. doi: 10.1016/0005-2760(86)90337-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz A., Barbaras R., Ghalim N., Clavey V., Fruchart J. C., Ailhaud G. Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J Biol Chem. 1990 May 15;265(14):7859–7863. [PubMed] [Google Scholar]
- Tall A. R. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993 Aug;34(8):1255–1274. [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]
- Yancey P. G., Bielicki J. K., Johnson W. J., Lund-Katz S., Palgunachari M. N., Anantharamaiah G. M., Segrest J. P., Phillips M. C., Rothblat G. H. Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry. 1995 Jun 20;34(24):7955–7965. doi: 10.1021/bi00024a021. [DOI] [PubMed] [Google Scholar]
- de la Llera Moya M., Atger V., Paul J. L., Fournier N., Moatti N., Giral P., Friday K. E., Rothblat G. A cell culture system for screening human serum for ability to promote cellular cholesterol efflux. Relations between serum components and efflux, esterification, and transfer. Arterioscler Thromb. 1994 Jul;14(7):1056–1065. doi: 10.1161/01.atv.14.7.1056. [DOI] [PubMed] [Google Scholar]