Abstract
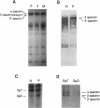
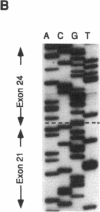
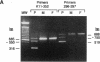
We describe a spectrin variant characterized by a truncated beta chain and associated with hereditary spherocytosis. The clinical phenotype consists of a moderate hemolytic anemia with striking spherocytosis and mild spiculation of the red cells. We describe the biochemical characteristics of this truncated protein which constitutes only 10% of the total beta spectrin present on the membrane, resulting in spectrin deficiency. Analysis of reticulocyte cDNA revealed the deletion of exons 22 and 23. We show, using Southern blot analysis, that this truncation results from a 4.6-kb genomic deletion. To elucidate the basis for the decreased amount of the truncated protein on the membrane and the overall spectrin deficiency, we show that (a) the mutated gene is efficiently transcribed and its mRNA abundant in reticulocytes, (b) the mutant protein is normally synthesized in erythroid progenitor cells, (c) the stability of the mutant protein in the cytoplasm of erythroblasts parallels that of the normal beta spectrin, and (d) the abnormal protein is inefficiently incorporated into the membrane of erythroblasts. We conclude that the truncation within the beta spectrin leads to inefficient incorporation of the mutant protein into the skeleton despite its normal synthesis and stability. We postulate that this misincorporation results from conformational changes of the beta spectrin subunit affecting the binding of the abnormal heterodimer to ankyrin, and we provide evidence based on binding assays of recombinant synthetic peptides to inside-out-vesicles to support this model.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Tse W. T., Lux S. E., Forget B. G. Beta spectrin kissimmee: a spectrin variant associated with autosomal dominant hereditary spherocytosis and defective binding to protein 4.1. J Clin Invest. 1993 Aug;92(2):612–616. doi: 10.1172/JCI116628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Gilligan D. M. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Coetzer T. L., Lawler J., Liu S. C., Prchal J. T., Gualtieri R. J., Brain M. C., Dacie J. V., Palek J. Partial ankyrin and spectrin deficiency in severe, atypical hereditary spherocytosis. N Engl J Med. 1988 Jan 28;318(4):230–234. doi: 10.1056/NEJM198801283180407. [DOI] [PubMed] [Google Scholar]
- Coetzer T. L., Palek J. Partial spectrin deficiency in hereditary pyropoikilocytosis. Blood. 1986 Apr;67(4):919–924. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Delaunay J., Dhermy D. Mutations involving the spectrin heterodimer contact site: clinical expression and alterations in specific function. Semin Hematol. 1993 Jan;30(1):21–33. [PubMed] [Google Scholar]
- Fibach E., Manor D., Oppenheim A., Rachmilewitz E. A. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989 Jan;73(1):100–103. [PubMed] [Google Scholar]
- Frangioni J. V., Neel B. G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993 Apr;210(1):179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Ghanem A., Pothier B., Marechal J., Ducluzeau M. T., Morle L., Alloisio N., Feo C., Ben Abdeladhim A., Fattoum S., Delaunay J. A haemolytic syndrome associated with the complete absence of red cell membrane protein 4.2 in two Tunisian siblings. Br J Haematol. 1990 Jul;75(3):414–420. doi: 10.1111/j.1365-2141.1990.tb04357.x. [DOI] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Hanspal M., Hanspal J. S., Kalraiya R., Liu S. C., Sahr K. E., Howard D., Palek J. Asynchronous synthesis of membrane skeletal proteins during terminal maturation of murine erythroblasts. Blood. 1992 Jul 15;80(2):530–539. [PubMed] [Google Scholar]
- Hanspal M., Hanspal J. S., Sahr K. E., Fibach E., Nachman J., Palek J. Molecular basis of spectrin deficiency in hereditary pyropoikilocytosis. Blood. 1993 Sep 1;82(5):1652–1660. [PubMed] [Google Scholar]
- Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987 Sep;105(3):1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi H., Nishimura J., Nawata H., Hamasaki N. A genetic defect of erythrocyte band 4.2 protein associated with hereditary spherocytosis. Br J Haematol. 1990 Mar;74(3):347–353. doi: 10.1111/j.1365-2141.1990.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Iolascon A., Miraglia del Giudice E., Camaschella C., Pinto L., Nobili B., Perrotta S., Cutillo S. Ankyrin deficiency in dominant hereditary spherocytosis: report of three cases. Br J Haematol. 1991 Aug;78(4):551–554. doi: 10.1111/j.1365-2141.1991.tb04487.x. [DOI] [PubMed] [Google Scholar]
- Jarolim P., Rubin H. L., Liu S. C., Cho M. R., Brabec V., Derick L. H., Yi S. J., Saad S. T., Alper S., Brugnara C. Duplication of 10 nucleotides in the erythroid band 3 (AE1) gene in a kindred with hereditary spherocytosis and band 3 protein deficiency (band 3PRAGUE). J Clin Invest. 1994 Jan;93(1):121–130. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. P., Warren S. L., Forget B. G., Morrow J. S. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991 Oct;115(1):267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia del Giudice E., Perrotta S., Pinto L., Cappellini M. D., Fiorelli G., Cutillo S., Iolascon A. Hereditary spherocytosis characterized by increased spectrin/band 3 ratio. Br J Haematol. 1992 Jan;80(1):133–134. doi: 10.1111/j.1365-2141.1992.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis, spherocytosis and related disorders: consequences of a deficiency or a mutation of membrane skeletal proteins. Blood Rev. 1987 Sep;1(3):147–168. doi: 10.1016/0268-960x(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Palek J., Sahr K. E. Mutations of the red blood cell membrane proteins: from clinical evaluation to detection of the underlying genetic defect. Blood. 1992 Jul 15;80(2):308–330. [PubMed] [Google Scholar]
- Rybicki A. C., Heath R., Wolf J. L., Lubin B., Schwartz R. S. Deficiency of protein 4.2 in erythrocytes from a patient with a Coombs negative hemolytic anemia. Evidence for a role of protein 4.2 in stabilizing ankyrin on the membrane. J Clin Invest. 1988 Mar;81(3):893–901. doi: 10.1172/JCI113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki A. C., Qiu J. J., Musto S., Rosen N. L., Nagel R. L., Schwartz R. S. Human erythrocyte protein 4.2 deficiency associated with hemolytic anemia and a homozygous 40glutamic acid-->lysine substitution in the cytoplasmic domain of band 3 (band 3Montefiore). Blood. 1993 Apr 15;81(8):2155–2165. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides P., Shalev O., John K. M., Lux S. E. Combined spectrin and ankyrin deficiency is common in autosomal dominant hereditary spherocytosis. Blood. 1993 Nov 15;82(10):2953–2960. [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Speicher D. W., Weglarz L., DeSilva T. M. Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. J Biol Chem. 1992 Jul 25;267(21):14775–14782. [PubMed] [Google Scholar]
- Sykes B. C. DNA in heritable disease. Lancet. 1983 Oct 1;2(8353):787–788. doi: 10.1016/s0140-6736(83)92314-0. [DOI] [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Yan Y., Winograd E., Viel A., Cronin T., Harrison S. C., Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993 Dec 24;262(5142):2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]
- Yoshino H., Marchesi V. T. Isolation of spectrin subunits and reassociation in vitro. Analysis by fluorescence polarization. J Biol Chem. 1984 Apr 10;259(7):4496–4500. [PubMed] [Google Scholar]