Abstract
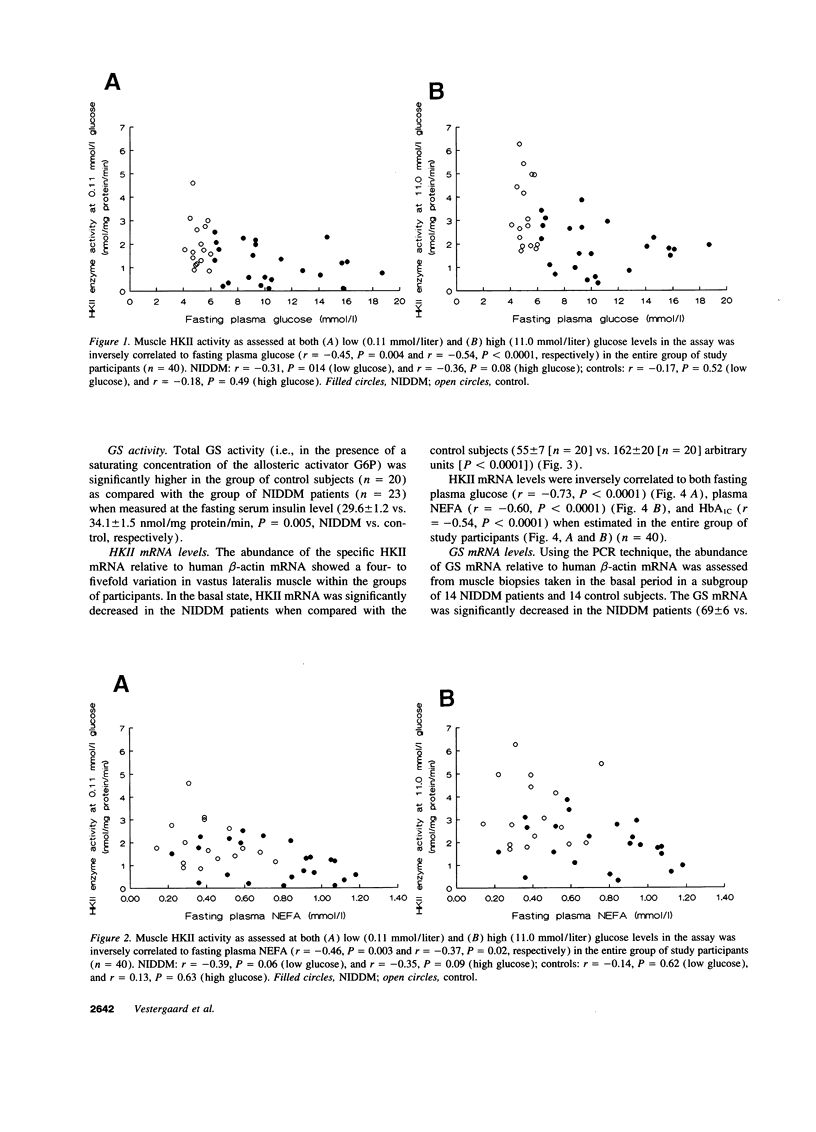
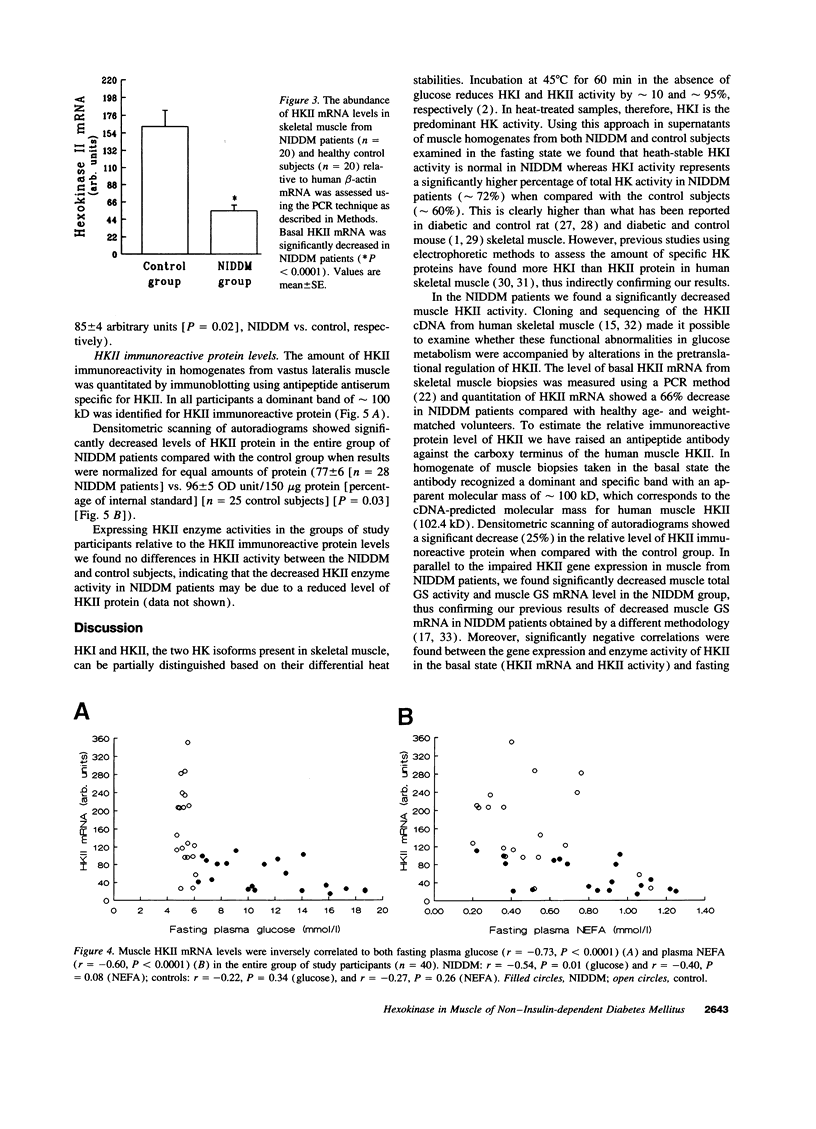
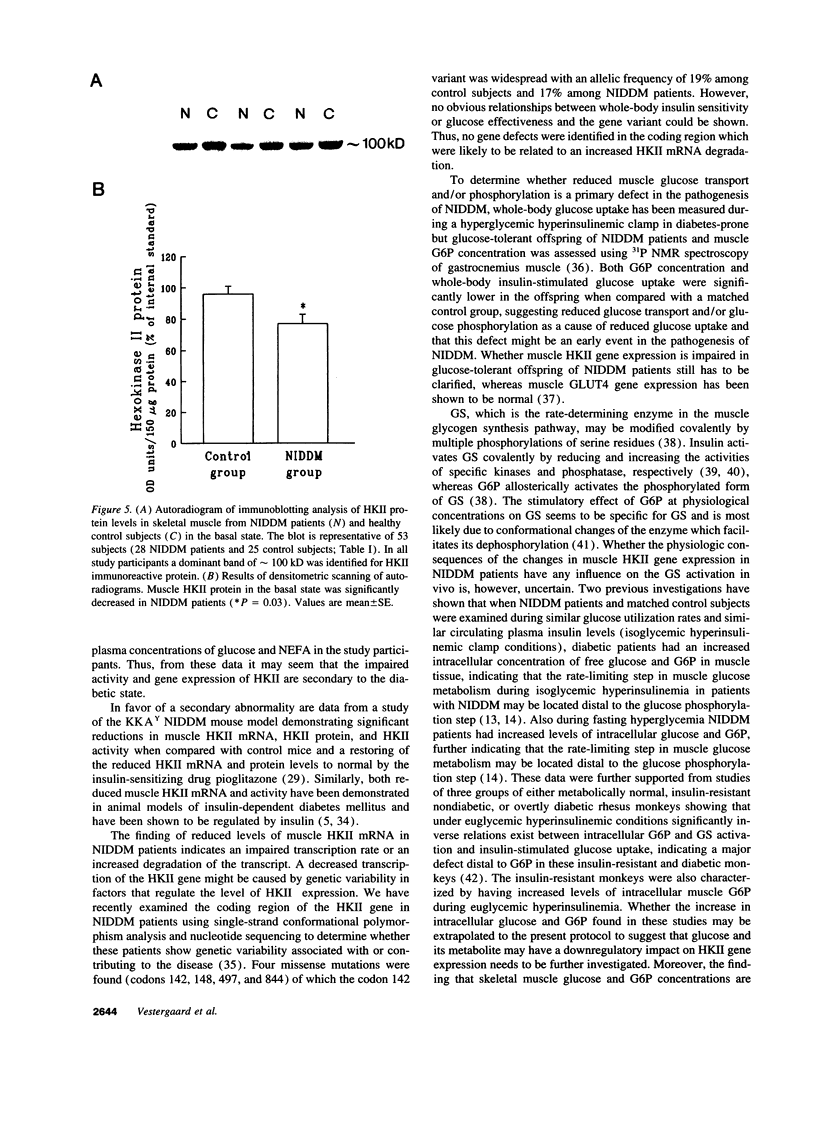
After entering the muscle cell, glucose is immediately and irreversibly phosphorylated to glucose-6-phosphate by hexokinases (HK) I and II. Previous studies in rodents have shown that HKII may be the dominant HK in skeletal muscle. Reduced insulin-stimulated glucose uptake and reduced glucose-6-phosphate concentrations in muscle have been found in non-insulin-dependent diabetes mellitus (NIDDM) patients when examined during a hyperglycemic hyperinsulinemic clamp. These findings [correction of finding] are consistent with a defect in glucose transport and/or phosphorylation. In the present study comprising 29 NIDDM patients and 25 matched controls, we tested the hypothesis that HKII activity and gene expression are impaired in vastus lateralis muscle of NIDDM patients when examined in the fasting state. HKII activity in a supernatant of muscle extract accounted for 28 +/- 5% in NIDDM patients and 40 +/- 5% in controls (P = 0.08) of total muscle HK activity when measured at a glucose media of 0.11 mmol/liter and 31 +/- 4 and 47 +/- 7% (P = 0.02) when measured at 0.11 mmol/liter of glucose. HKII mRNA, HKII immunoreactive protein level, and HKII activity were significantly decreased in NIDDM patients (P < 0.0001, P = 0.03, and P = 0.02, respectively) together with significantly decreased glycogen synthase mRNA level and total glycogen synthase activity (P = 0.02 and P = 0.02, respectively). In the entire study population HKII activity estimated at 0.11 and 11.0 mM glucose was inversely correlated with fasting plasma glucose concentrations (r = -0.45, P = 0.004; r = -0.54, P < 0.0001, respectively) and fasting plasma nonesterified fatty acid concentrations (r = -0.46, P = 0.003; r = -0.37, P = 0.02, respectively). In conclusion, NIDDM patients are characterized by a reduced activity and a reduced gene expression of HKII in muscle which may be secondary to the metabolic peturbations. HKII contributes with about one-third of total HK activity in a supernatant of human vastus lateralis muscle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson K., Galuska D., Thörne A., Sonnenfeld T., Wallberg-Henriksson H. Decreased insulin-stimulated 3-0-methylglucose transport in in vitro incubated muscle strips from type II diabetic subjects. Acta Physiol Scand. 1991 Jun;142(2):255–260. doi: 10.1111/j.1748-1716.1991.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Bak J. F., Pedersen O. Exercise-enhanced activation of glycogen synthase in human skeletal muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):E957–E963. doi: 10.1152/ajpendo.1990.258.6.E957. [DOI] [PubMed] [Google Scholar]
- Bjørbaek C., Vik T. A., Echwald S. M., Yang P. Y., Vestergaard H., Wang J. P., Webb G. C., Richmond K., Hansen T., Erikson R. L. Cloning of a human insulin-stimulated protein kinase (ISPK-1) gene and analysis of coding regions and mRNA levels of the ISPK-1 and the protein phosphatase-1 genes in muscle from NIDDM patients. Diabetes. 1995 Jan;44(1):90–97. doi: 10.2337/diab.44.1.90. [DOI] [PubMed] [Google Scholar]
- Braithwaite S. S., Palazuk B., Colca J. R., Edwards C. W., 3rd, Hofmann C. Reduced expression of hexokinase II in insulin-resistant diabetes. Diabetes. 1995 Jan;44(1):43–48. doi: 10.2337/diab.44.1.43. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Deeb S. S., Malkki M., Laakso M. Human hexokinase II: sequence and homology to other hexokinases. Biochem Biophys Res Commun. 1993 Nov 30;197(1):68–74. doi: 10.1006/bbrc.1993.2442. [DOI] [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterby J. S., Qadri S. S. Effects of metabolites on the activity of mammalian hexokinases. Biochem Soc Trans. 1981 Feb;9(1):25–27. doi: 10.1042/bst0090025. [DOI] [PubMed] [Google Scholar]
- Easterby J. S., Qadri S. S. Hexokinase type II from rat skeletal muscle. Methods Enzymol. 1982;90(Pt E):11–15. doi: 10.1016/s0076-6879(82)90098-2. [DOI] [PubMed] [Google Scholar]
- Echwald S. M., Bjørbaek C., Hansen T., Clausen J. O., Vestergaard H., Zierath J. R., Printz R. L., Granner D. K., Pedersen O. Identification of four amino acid substitutions in hexokinase II and studies of relationships to NIDDM, glucose effectiveness, and insulin sensitivity. Diabetes. 1995 Mar;44(3):347–353. doi: 10.2337/diab.44.3.347. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Koranyi L., Bourey R., Schalin-Jäntti C., Widén E., Mueckler M., Permutt A. M., Groop L. C. Insulin resistance in type 2 (non-insulin-dependent) diabetic patients and their relatives is not associated with a defect in the expression of the insulin-responsive glucose transporter (GLUT-4) gene in human skeletal muscle. Diabetologia. 1992 Feb;35(2):143–147. doi: 10.1007/BF00402546. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Wallace P., Brechtel G., Olefsky J. M. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metabolism. 1992 Aug;41(8):897–902. doi: 10.1016/0026-0495(92)90174-9. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Katzen H. M., Soderman D. D., Wiley C. E. Multiple forms of hexokinase. Activities associated with subcellular particulate and soluble fractions of normal and streptozotocin diabetic rat tissues. J Biol Chem. 1970 Aug 25;245(16):4081–4096. [PubMed] [Google Scholar]
- Katzen H. M. The multiple forms of mammalian hexokinase and their significance to the action of insulin. Adv Enzyme Regul. 1967;5:335–356. doi: 10.1016/0065-2571(67)90025-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanng S., Thorsteinsson B., Røder M. E., Orskov C., Holst J. J., Nerup J., Koch C. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (Copenh) 1993 Mar;128(3):207–214. doi: 10.1530/acta.0.1280207. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Consoli A., Kelley D. E., Reilly J. J., Nurjhan N. Fasting hyperglycemia normalizes oxidative and nonoxidative pathways of insulin-stimulated glucose metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990 Dec;71(6):1544–1551. doi: 10.1210/jcem-71-6-1544. [DOI] [PubMed] [Google Scholar]
- O'Doherty R. M., Bracy D. P., Osawa H., Wasserman D. H., Granner D. K. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am J Physiol. 1994 Feb;266(2 Pt 1):E171–E178. doi: 10.1152/ajpendo.1994.266.2.E171. [DOI] [PubMed] [Google Scholar]
- Okubo M., Bogardus C., Lillioja S., Mott D. M. Glucose-6-phosphate stimulation of human muscle glycogen synthase phosphatase. Metabolism. 1988 Dec;37(12):1171–1176. doi: 10.1016/0026-0495(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Ortmeyer H. K., Bodkin N. L., Hansen B. C. Relationship of skeletal muscle glucose 6-phosphate to glucose disposal rate and glycogen synthase activity in insulin-resistant and non-insulin-dependent diabetic rhesus monkeys. Diabetologia. 1994 Feb;37(2):127–133. doi: 10.1007/s001250050082. [DOI] [PubMed] [Google Scholar]
- Printz R. L., Ardehali H., Koch S., Granner D. K. Human hexokinase II mRNA and gene structure. Diabetes. 1995 Mar;44(3):290–294. doi: 10.2337/diab.44.3.290. [DOI] [PubMed] [Google Scholar]
- Printz R. L., Koch S., Potter L. R., O'Doherty R. M., Tiesinga J. J., Moritz S., Granner D. K. Hexokinase II mRNA and gene structure, regulation by insulin, and evolution. J Biol Chem. 1993 Mar 5;268(7):5209–5219. [PubMed] [Google Scholar]
- Printz R. L., Magnuson M. A., Granner D. K. Mammalian glucokinase. Annu Rev Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- Reid S., Masters C. On the developmental properties and tissue interactions of hexokinase. Mech Ageing Dev. 1985 Jul-Aug;31(2):197–212. doi: 10.1016/s0047-6374(85)80030-0. [DOI] [PubMed] [Google Scholar]
- Rogers P. A., Fisher R. A., Harris H. An electrophoretic study of the distribution and properties of human hexokinases. Biochem Genet. 1975 Dec;13(11-12):857–866. doi: 10.1007/BF00484416. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaag A., Damsbo P., Hother-Nielsen O., Beck-Nielsen H. Hyperglycaemia compensates for the defects in insulin-mediated glucose metabolism and in the activation of glycogen synthase in the skeletal muscle of patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992 Jan;35(1):80–88. doi: 10.1007/BF00400856. [DOI] [PubMed] [Google Scholar]
- Vestergaard H., Bjørbaek C., Andersen P. H., Bak J. F., Pedersen O. Impaired expression of glycogen synthase mRNA in skeletal muscle of NIDDM patients. Diabetes. 1991 Dec;40(12):1740–1745. doi: 10.2337/diab.40.12.1740. [DOI] [PubMed] [Google Scholar]
- Vestergaard H., Lund S., Larsen F. S., Bjerrum O. J., Pedersen O. Glycogen synthase and phosphofructokinase protein and mRNA levels in skeletal muscle from insulin-resistant patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1993 Jun;91(6):2342–2350. doi: 10.1172/JCI116466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig A., Printz R. L., Stratton I. M., Granner D. K., Moller D. E. Analysis of the hexokinase II gene in subjects with insulin resistance and NIDDM and detection of a Gln142-->His substitution. Diabetes. 1995 Mar;44(3):340–346. doi: 10.2337/diab.44.3.340. [DOI] [PubMed] [Google Scholar]
- Villar-Palasi C. Substrate specific activation by glucose 6-phosphate of the dephosphorylation of muscle glycogen synthase. Biochim Biophys Acta. 1991 Nov 12;1095(3):261–267. doi: 10.1016/0167-4889(91)90109-b. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]




