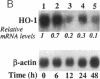
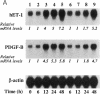
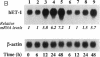
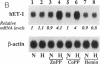
Abstract
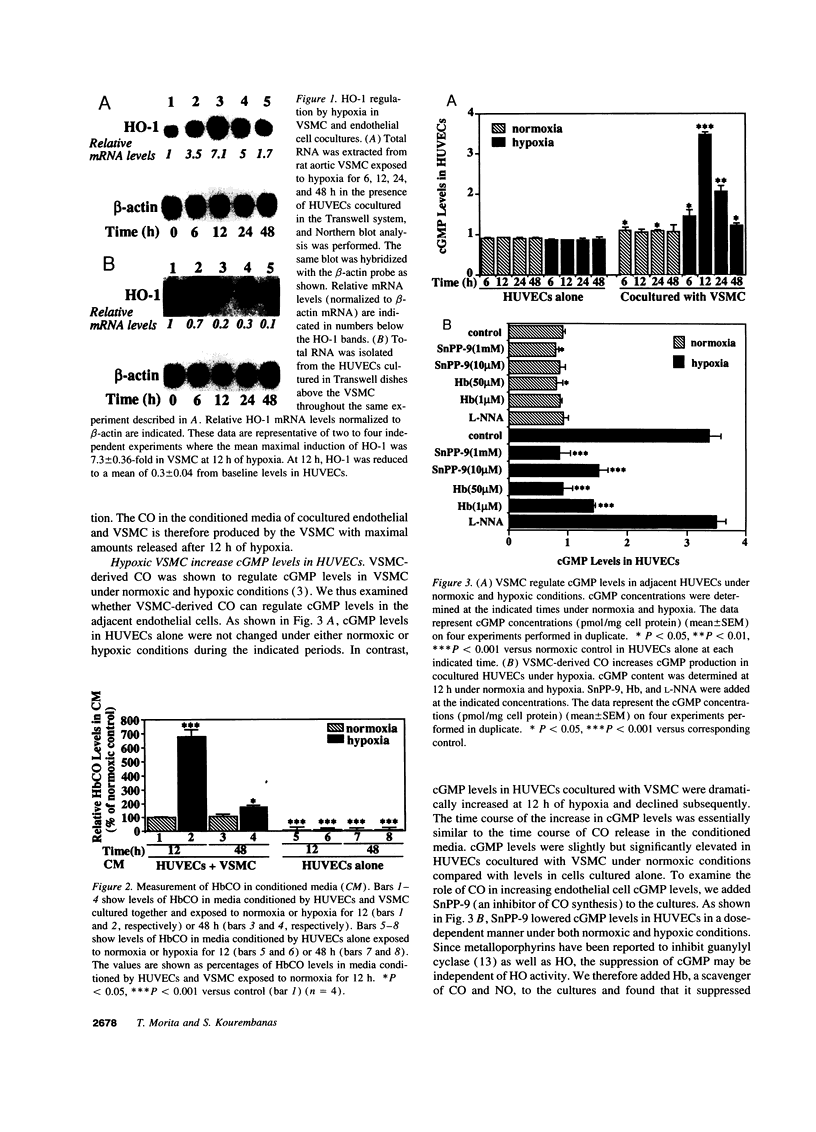
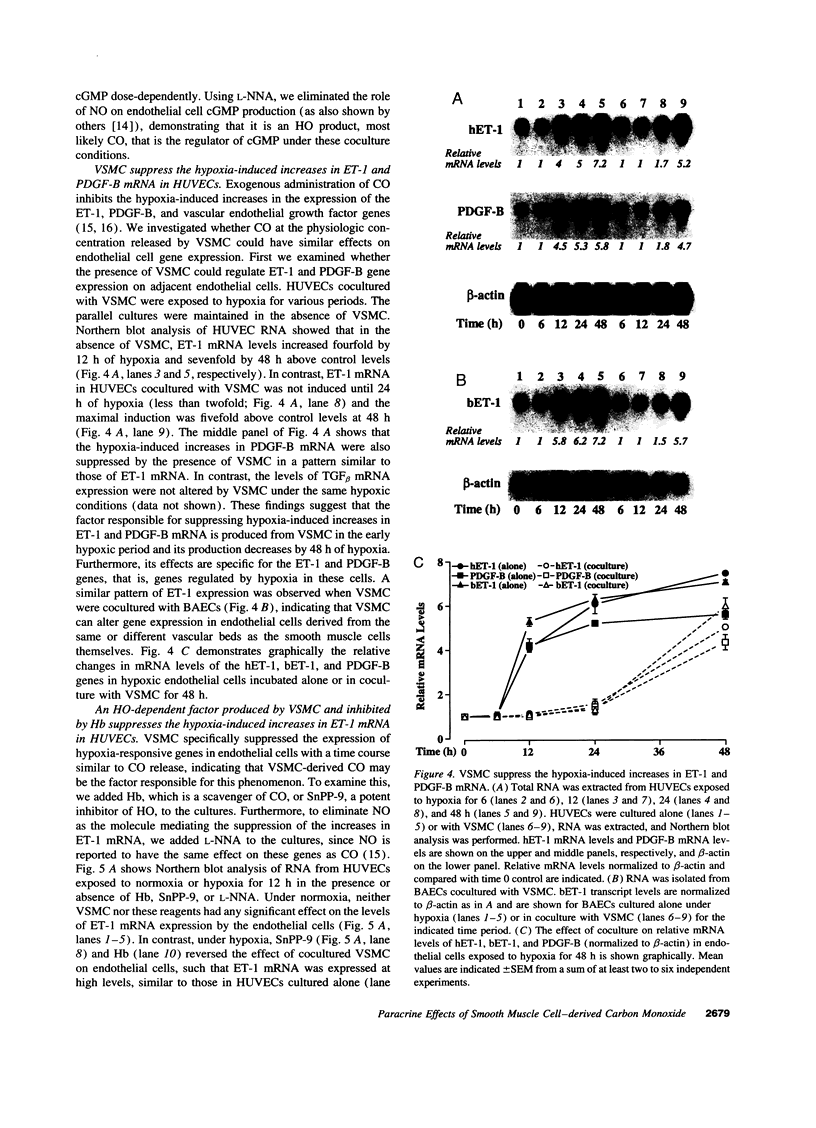
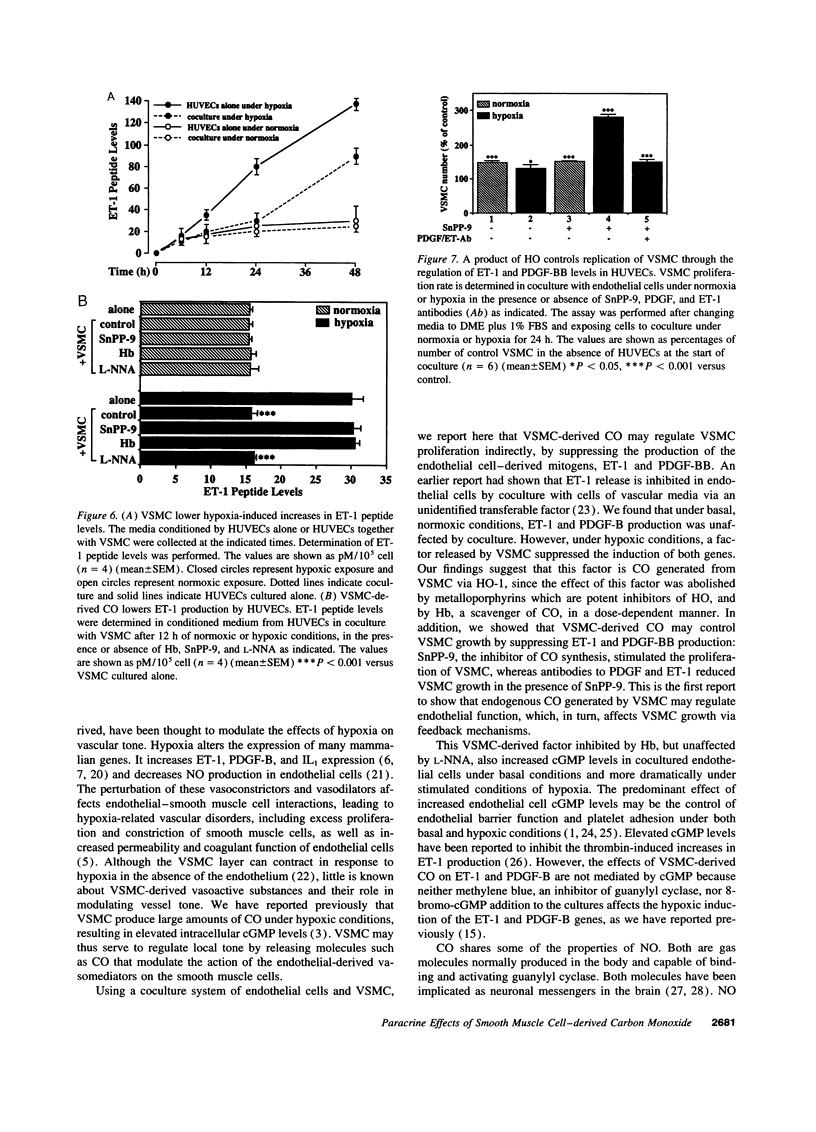
CO is produced in vascular smooth muscle cells (VSMC) by heme oxygenase-1 (HO-1). CO increases cGMP levels in VSMC; however, its possible additional roles in the vasculature have not been examined. We report that a product of HO, released from VSMC and inhibited by hemoglobin, has paracrine effects on endothelial cells: it increases endothelial cGMP content and decreases the expression of the mitogens, endothelin-1 (ET-1) and platelet-derived growth factor-B (PDGF-B). This product has the characteristics of CO, and its production is increased sevenfold under hypoxia. The VSMC-derived CO caused a fourfold rise in endothelial cell cGMP. In addition, it inhibited the hypoxia-induced increases in mRNA levels of the ET-1 and PDGF-B genes. Inhibitors of HO, and hemoglobin, a scavenger of CO, prevented the rise in cGMP and also restored the hypoxic response of these genes. The inhibition of ET-1 and PDGF-B mRNA by CO resulted in decreased production of these endothelial-derived mitogens, and in turn, inhibition of VSMC proliferation. These findings suggest an important physiologic role for VSMC-derived CO in modulating cell-cell interaction and cell proliferation in the vessel wall during hypoxia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger C., Lüscher T. F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990 Feb;85(2):587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogi E., Wu T., Namiki A., Isner J. M. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994 Aug;90(2):649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28(1-3):52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Petri E., Cetta A., Wong-Staal F. Deletions of specific regions of the simian sarcoma-associated virus genome are found in defective viruses and in the simian sarcoma virus. J Virol. 1982 Feb;41(2):593–604. doi: 10.1128/jvi.41.2.593-604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. A., Schneider T. J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994 Feb 11;269(6):4355–4359. [PubMed] [Google Scholar]
- Hirata Y., Takagi Y., Fukuda Y., Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989 Aug;78(2-3):225–228. doi: 10.1016/0021-9150(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Ballot B., Wood K. S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem. 1984 May 25;259(10):6201–6207. [PubMed] [Google Scholar]
- Itoh Y., Yanagisawa M., Ohkubo S., Kimura C., Kosaka T., Inoue A., Ishida N., Mitsui Y., Onda H., Fujino M. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988 Apr 25;231(2):440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., Hannan R. L., Faller D. V. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990 Aug;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993 Jul;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P. Nitric oxide-induced microvascular permeability alterations: a regulatory role for cGMP. Am J Physiol. 1993 Dec;265(6 Pt 2):H1909–H1915. doi: 10.1152/ajpheart.1993.265.6.H1909. [DOI] [PubMed] [Google Scholar]
- Lofton C. E., Newman W. H., Currie M. G. Atrial natriuretic peptide regulation of endothelial permeability is mediated by cGMP. Biochem Biophys Res Commun. 1990 Oct 30;172(2):793–799. doi: 10.1016/0006-291x(90)90744-8. [DOI] [PubMed] [Google Scholar]
- Madden J. A., Vadula M. S., Kurup V. P. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol. 1992 Sep;263(3 Pt 1):L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- McQuillan L. P., Leung G. K., Marsden P. A., Kostyk S. K., Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994 Nov;267(5 Pt 2):H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- Morita T., Perrella M. A., Lee M. E., Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Koga S., Karakurum M., Pinsky D., Kaiser E., Brett J., Wolitzky B. A., Norton C., Plocinski J., Benjamin W. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992 Dec;90(6):2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Lieberman H. B., Furie M. B., Joseph-Silverstein J. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991 Jan;146(1):8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Langleben D., Cernacek P., Cianflone K. Endothelin release is inhibited by coculture of endothelial cells with cells of vascular media. Am J Physiol. 1990 Dec;259(6 Pt 2):H1928–H1932. doi: 10.1152/ajpheart.1990.259.6.H1928. [DOI] [PubMed] [Google Scholar]
- Takagi K., Isobe Y., Yasukawa K., Okouchi E., Suketa Y. Nitric oxide blocks the cell cycle of mouse macrophage-like cells in the early G2+M phase. FEBS Lett. 1994 Mar 7;340(3):159–162. doi: 10.1016/0014-5793(94)80128-2. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Westendorp R. G., Draijer R., Meinders A. E., van Hinsbergh V. W. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J Vasc Res. 1994 Jan-Feb;31(1):42–51. doi: 10.1159/000159030. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Furumichi T., Furui H., Yokoi T., Ito T., Yamauchi K., Yokota M., Hayashi H., Saito H. Roles of calcium, cyclic nucleotides, and protein kinase C in regulation of endothelial permeability. Arteriosclerosis. 1990 May-Jun;10(3):410–420. doi: 10.1161/01.atv.10.3.410. [DOI] [PubMed] [Google Scholar]
- Yokokawa K., Tahara H., Kohno M., Mandal A. K., Yanagisawa M., Takeda T. Heparin regulates endothelin production through endothelium-derived nitric oxide in human endothelial cells. J Clin Invest. 1993 Oct;92(4):2080–2085. doi: 10.1172/JCI116805. [DOI] [PMC free article] [PubMed] [Google Scholar]