Abstract
A possible relationship between cognitive abilities and white matter structure as assessed by magnetic resonance diffusion tensor imaging (DTI) was investigated in the pediatric population. DTI was performed on 47 normal children ages 5–18. Using a voxelwise analysis technique, the fractional anisotropy (FA) and mean diffusivity (MD) were tested for significant correlations with Wechsler full‐scale IQ scores, with subject age and gender used as covariates. Regions displaying significant positive correlations of IQ scores with FA were found bilaterally in white matter association areas, including frontal and occipito‐parietal areas. No regions were found exhibiting correlations of IQ with MD except for one frontal area significantly overlapping a region containing a significant correlation with FA. The positive direction of the correlation with FA is the same as that found previously with age, and indicates a positive relationship between fiber organization and/or density with cognitive function. The results are consistent with the hypothesis that regionally specific increased fiber organization is a mechanism responsible for the normal development of white matter tracts. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: diffusion tensor imaging, cognitive function, intelligence, white matter architecture, magnetic resonance imaging
INTRODUCTION
Various studies investigating neurological abnormalities, psychiatric disorders, or developmental issues have demonstrated a connection between white matter properties and cognition. Patients with ischemic leukoariosis [O'Sullivan et al.,2001b] displayed diffusion‐related changes in normal white matter relative to normal controls that correlated with executive function, while in patients with multiple sclerosis (MS), a significant correlation was demonstrated between white matter diffusion properties and measures of verbal fluency and spatial recall [Rovaris et al.,2002]; global white matter volume was shown [Edwards et al.,2001] to correlate with cognitive performance in MS patients as well. Reduced integrity of the association white matter fiber tracts was seen in patients with Alzheimer's disease [Rose et al.,2000] relative to normal controls, and executive function has also been shown to correlate with age‐related declines in diffusion anisotropy and increases in mean diffusivity [O'Sullivan et al.,2001a]. A study performed on geriatric depression patients [Steffens et al.,2002] displayed a correlation between white matter lesions and performance impairment in daily living activities. Reading ability was correlated with a measure of fiber tract organization in a group of healthy and reading‐impaired adults [Klingberg et al.,2000], and inadequate development of normal‐appearing white matter [Mulhern et al.,2001] was demonstrated to account for a significant part of the association of age at the time of craniospinal irradiation and IQ in survivors of childhood medulloblastoma.
In the normal pediatric population, although much is known about normal development of white matter there is limited data available to relate changes in the development and maturation of white matter to measures of cognitive function. Diffusion anisotropy has been observed to increase with age during childhood and adolescence in the pyramidal tract, as well as association areas [Schmithorst et al.,2002], while age‐related decreases in mean diffusivity were observed throughout the white matter. Overall white matter volume increases and region‐specific myelination have been demonstrated [Paus et al.,2001] throughout the developmental period. Using 1H magnetic resonance spectroscopy (MRS) on a cohort of children ages 7–12, improved working memory performance was related to increases in the concentrations of creatine and N‐acetyl‐aspartate (NAA) in white matter in the right frontal lobe [Yeo et al.,2000]. A recent study comparing normal adults who had studied a musical instrument since childhood with those who had not [Schmithorst and Wilke,2002] demonstrated diffusion‐related changes in both white matter association areas and the pyramidal tract, which were hypothesized to correspond to the cognitive and motor effects, respectively, of musical training. These results indicate that significant developmental changes occur in white matter during childhood and adolescence, which may be related to cognitive function. In the current study we wish to test the hypothesis that measures of cognitive function, such as IQ, will correlate with diffusion tensor imaging (DTI) parameters, such as anisotropy and mean diffusivity, in the normal pediatric population. DTI allows for the imaging of white matter microstructure in vivo: as the diffusion of water along axons is less restricted than diffusion perpendicular to the axonal direction, the microstructure of white matter connections can be visualized noninvasively.
SUBJECTS AND METHODS
DTI data was successfully obtained from 47 children (42 Caucasian, two Asian, three Multiethnic, 13 M, 34 F, mean age = 11.0 ± 3.3 years, 41 right‐handed, six left‐handed). Institutional review board approval and informed consent were obtained for all subjects. Exclusion criteria were: previous neurological illness; learning disability; head trauma with loss of consciousness; current or past use of psychostimulant medication; pregnancy; IQ less than 80, measured via the Wechsler Intelligence Scale for Children, Full‐Scale IQ, Third Edition (WISC‐IQ); birth at 37 weeks gestational age or earlier; or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist. The WISC‐IQ test was administered to all subjects by a psychologist under the supervision of a board‐certified pediatric neuropsychologist, who scored all tests. All Verbal and Performance subtests were administered and the Full Scale IQ was computed according to the standard formula. The anatomical scans were read as normal by a pediatric neuroradiologist. The age and gender breakdown of the study participants is shown in Table I.
Table I.
Age and gender breakdown of the subjects for which DTI data was successfully acquired (n = 47)
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 0 | 0 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 0 | 13 |
| F | 1 | 3 | 3 | 7 | 4 | 4 | 0 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 34 |
M, male; F, female.
All DTI scans were visually inspected for gross motion artifacts. In addition to the 47 successful scans, datasets from 15 subjects (all Caucasian) were excluded due to motion artifacts, and datasets from two subjects (Caucasian) were excluded due to technical problems. The included subjects, as a group, had IQ scores within the high end of the normal range (Full‐Scale IQ range: 83–146, mean ± σ: 114.4 ± 13.4; Performance IQ range: 79–151, mean ± σ: 111.2 ± 13.8; Verbal IQ range: 90–150, mean ± σ: 115.2 ± 13.6). The excluded subjects had an IQ distribution similar to the included ones (Full‐Scale IQ range: 91–137, mean ± σ: 116.5 ± 12.1; Performance IQ range: 96–141, mean ± σ: 114.2 ± 12.5; Verbal IQ range: 87–139, mean ± σ: 115.5 ± 11.9), suggesting that there was no relationship between IQ and quality of the data. However, due to the younger age range (8.0 ± 3.2 years) and gender composition (10 males, 5 females) of the excluded subjects, together with the imbalanced gender composition of the included subjects, age and gender were included as covariates in the analysis. The mismatch of females compared to males was due to the fact that the DTI sequence was added to the protocol of an ongoing fMRI study investigating language development in normal children during the summer of 2001; unfortunately, during that portion of the study (late summer 2001) there were more females than males enrolled. The mismatch was also exacerbated due to the higher failure rate in boys due to subject motion.
All images were acquired on a Bruker 3T Medspec system (Bruker Medizintechnik, Ettlingen, Germany). A 24‐slice, diffusion‐weighted, spin‐echo, echo‐planar imaging (EPI) scan was acquired in the transverse plane with the following parameters: TR = 6070 ms, TE = 87 ms, FOV = 19.2 × 25.6 cm, slice thickness = 5 mm, matrix = 64 × 128, Δ = 40 ms, δ = 18 ms, diffusion gradient strength = 30 mT/m (resulting in a b‐value of 710 s/mm2). Three scans were acquired with no diffusion weighting, and 25 diffusion‐weighted scans were acquired, each with different diffusion directions determined using an electrostatic repulsive model [Jones et al.,1999]. Total scan time for the DTI sequence was 2 min, 50 s. In addition, a fluid‐attenuated inversion recovery (FLAIR)‐EPI scan with the same scan parameters and no diffusion weighting, and a T1‐weighted Modified Driven Equilibrium Fourier Transform (MDEFT) [Duewll et al.,1996] whole‐brain anatomical scan were also acquired. While no external apparatus was used to reduce head motion, previously developed techniques [Byars et al.,2002] were used to acclimatize the subjects to the MRI procedure and make them more comfortable inside the scanner.
Geometric distortion due to gradient eddy currents was minimized using an automated gradient preemphasis adjustment routine [Schmithorst and Dardzinski2002]. Any residual distortion due to gradient eddy currents was corrected for by coregistering the diffusion‐weighted images to the FLAIR‐EPI scan with no diffusion weighting using a Levenberg‐Marquardt iterative least‐squares algorithm. Geometric distortion due to B0 inhomogeneity was corrected for using a multiecho reference scan [Schmithorst et al.,2001].
In each subject, only the voxels determined to be in white matter, using the segmentation procedure available in SPM99 (Wellcome Department of Cognitive Neurology, London, UK) on the high‐resolution anatomical images, were retained for further analysis in order to minimize any possible partial volume effects. Specifically, the higher‐resolution white matter images generated from SPM were transformed into the space of the lower‐resolution DTI images (using neighborhood averaging) and only voxels with a probability greater than 80% of being in white matter were analyzed. Using routines written in IDL (Research Systems, Boulder, CO), the components of the diffusion tensor were computed and transformed into stereotaxic coordinates [Talairach and Tournoux,1988] using a linear affine transformation, with normalization parameters calculated from the high‐resolution T1‐whole brain image.
The fractional anisotropy (FA) [Papadakis et al.,1999] and mean diffusivity (MD) values were computed for each subject. FA is a marker for diffusion anisotropy and has a range of zero, in the case of completely isotropic diffusion, to one, in the case of completely anisotropic diffusion, while MD is a directionally averaged measure of water diffusion, reflecting tissue density. As a further safeguard against partial‐volume effects, only voxels with an MD less than 1e‐5 cm2/s were retained for the subsequent correlational analyses, which were only performed on the subset of voxels judged to be in white matter in 25 or more subjects. This resulted in the inclusion of 5,126 voxels, from all regions of the brain (temporal, frontal, occipital, and parietal lobes) in the analysis. Across all subjects where the given voxel was retained for analysis, the FA and MD values were tested for significant correlation with the WISC Full‐Scale IQ scores, using subject age and gender as covariates in order to account for any effects due to gross morphological differences. The (T‐score) results were transformed into Z‐score maps, which were then filtered with a Gaussian filter of width 4 mm in order to increase contrast‐to‐noise. After filtering, the results were masked to the original set of voxels used in the analysis to prevent “bleeding” of correlated regions into areas containing gray matter or CSF. The clustering method [Xiong et al.,1995] was used to increase sensitivity, since it is expected that regions of white matter containing significant correlations with IQ will be presented in extended areas of white matter tracts. Regions with statistically significant correlations were determined using the criterion of at least 30 contiguous voxels (corresponding to a volume of ∼0.8 cc) with Z > 4.5. Using a procedure similar to that described in Ledberg et al. [1998], the chosen filter width, Z‐threshold, and cluster size was shown to correspond to a corrected double‐tailed P < 0.05, using the fit residuals to estimate the intrinsic smoothness present in the data. Since the intrinsic smoothness was ∼2 mm in each direction, the significantly greater exogenous filtering applied reduces the chance of the results being biased toward regions of greater smoothness. The regions determined to be significantly correlated with the WISC Full‐Scale IQ scores were then overlaid on the averaged anatomical whole‐brain dataset for visualization purposes (Figs. 1, 2, 3). For the regions correlated with FA, scatterplots were generated (Fig. 4) and additional analyses were performed. Using subject age and gender as covariates, the partial correlations of the mean FA values (averaged over the region) with Full‐Scale, Performance, and Verbal IQ, and the Talairach coordinates of the voxel containing the greatest correlation (using the unfiltered Z‐score maps) are displayed in Table II.
Figure 1.

Regions of statistically significant positive correlations of FA with Full‐Scale IQ, controlling for age and gender, overlaid on the averaged whole‐brain anatomical dataset (slice range Z = +5 mm to +45 mm). Colored voxels have double‐tailed P < 0.05 (corrected). All images in radiological orientation.
Figure 2.

Sagittal view containing regions of statistically significant positive correlations of FA with Full‐Scale IQ, controlling for age and gender, overlaid on the averaged whole‐brain anatomical dataset (slice position, x = −32 mm (left); +32 mm (right)), displaying positive correlations of FA with IQ in the arcuate fasciculus bilaterally. Colored voxels have double‐tailed P < 0.05 (corrected).
Figure 3.
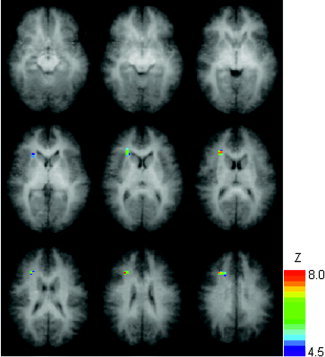
Regions of statistically significant negative correlations of MD with Full‐Scale IQ, controlling for age and gender, overlaid on the averaged whole‐brain anatomical dataset (slice range Z = +5 mm to +45 mm). Colored voxels have double‐tailed P < 0.05 (corrected). All images in radiological orientation.
Figure 4.

Mean FA (y‐axis) as a function of Full‐Scale IQ (x axis) for all regions shown in Table II.
Table II.
Voxel with largest correlation (Talairach coordinates) of regions with positive correlations of FA with Wechsler Full‐Scale IQ shown in Figure 1
| Anatomical location | x, y, z | R Full | R Perf. | R Verbal |
|---|---|---|---|---|
| Left frontal | −29, 21, 25 | 0.42 | 0.36 | 0.37 |
| Left occipito‐temporo‐parietal | −29, −65, 15 | 0.39 | 0.36 | 0.36 |
| Left Parietal | −32, −13, 25 | 0.51 | 0.33 | 0.57 |
| Right Frontal | 23, 23, 25 | 0.46 | 0.31 | 0.48 |
| Right Occipito‐Temporo‐Parietal | 35, −45, 25 | 0.41 | 0.41 | 0.35 |
| Right Pyramidal Tract | 26, −25, 40 | 0.43 | 0.30 | 0.45 |
| Right Occipito‐Parietal | 23, −41, 25 | 0.44 | 0.41 | 0.39 |
Shown are partial correlations of mean FA with Full‐Scale IQ (R Full), mean FA with Performance IQ (R Perf.), and mean FA with Verbal IQ (R Verbal), with age and gender used as covariates.
RESULTS
Regions of statistically significant positive correlations of FA (Figs. 1, 2) and negative correlations of MD (Fig. 3) with the WISC Full‐Scale IQ scores were found. Significant positive correlations of FA with IQ (Figs. 1, 2) were found mainly in association fibers bilaterally, including frontal areas, and occipito‐parietal and occipito‐temporo‐parietal areas encompassing the posterior aspects of the arcuate fasciculus. In addition, correlations were found in the right pyramidal tract and in a more anterior portion of the left parietal lobe encompassing a more medial portion of the arcuate fasciculus. The only region with a significant negative correlation of MD was found in the right frontal lobe (Fig. 3). No regions were found with statistically significant negative correlations of FA or positive correlations of MD with WISC‐IQ scores. (The double‐tailed statistical significance was corrected for multiple voxel comparisons via Monte Carlo simulations as described in Subjects and Methods. The significance, however, was not corrected for the two dependent variables [FA and MD] tested.)
For the regions displaying positive correlations of Full‐Scale IQ with FA, the correlations were repeated with the Performance and Verbal scales (Table II). All correlations were significant at a nominal P < 0.05 level (R > 0.3). A Fisher's z‐transformation was used to test for significant differences between the correlation values for the Performance and Verbal IQ scores; none of the differences were significant at a P < 0.05 level.
DISCUSSION
The subject population is skewed toward the higher end of the IQ range and contains significantly more girls than boys. We chose to include all available data for purposes of increasing the sensitivity; our results must be interpreted within the constraints of this limitation. However, since all subjects were specifically recruited for the investigation of normal brain development and their neurological, psychological, and structural measures were all within the normal range, the data do not have the potential problem of transferability of results [Rivkin,2000] for subjects clinically referred for MR examinations and only retrospectively classified as “normal.” In addition, since there does not appear to be sufficient power (Table II) with the sample size used (n = 47) to assess differences between Verbal and Performance IQ relationships to FA, our discussion will focus on the correlations found with Full‐Scale IQ, although certainly future larger‐scale studies will investigate not only the relationship of Verbal and Performance IQ, but also possibly specific subtests of the WISC with white matter diffusion properties.
Our observed changes in FA and MD are due almost completely to a faster‐diffusing component, as the relatively low b‐value used in this experiment of 710 s/mm2 will minimize any significant contribution from a slower‐diffusing component [Clark and Le Bihan2000]. The fraction of the fast diffusion component has been shown to be larger in newborns than in adults [Mulkern et al.,2001], a result attributed to cellular development and myelination. However, since the lower age limit of our study is 5 years old, at which time gross myelination is virtually complete [Nakagawa et al.,1998], we do not expect there to be significant differences in the fast diffusion fraction. Still, further research will be necessary in order to confirm this hypothesis, especially with regard to evidence of ongoing white matter tract development in older children [Schmithorst et al.,2002].
With regard to development, it has previously been proposed [Schmithorst et al.,2002] that there are at least two processes contributing to the maturation of white matter in the normal pediatric population: 1) increasingly dense and ordered packing of the fiber tracts, resulting in more directionally restricted extracellular space; and 2) changes in the intracellular compartment including a greater concentration of membranes and a greater membrane surface‐to‐cell volume ratio. Process 1) should lead to a marked increase in diffusion anisotropy as well as a possible decrease in MD, due to the increasingly restricted diffusion perpendicular to the axon direction; process 2) should lead only to a decrease in MD, as suggested by Baratti et al. [1999] in a detailed longitudinal study investigating maturation of the cat brain.
Thus, based on the diffusion properties of white matter laid out above, the areas exhibiting increases in FA with increasing cognitive abilities reflect an overall correlation of fiber organization and/or density with IQ. These results are in agreement with an earlier study [Schmithorst et al.,2002] investigating normal development of white matter in the pediatric population displaying regionally specific increases in FA, and indicate that efficient organization of white matter association fibers is essential for optimal cognitive performance. Only one frontal region displayed a correlation with MD (Fig. 3), and this region substantially overlaps a similar region containing a correlation with FA. Thus, while preliminary, our results support the view that fiber organization is a much more important developmental process as far as cognitive performance than intracellular maturation.
Our findings show a striking spatial overlap with earlier findings of Klingberg et al. [2000] on a small group of reading impaired and normal adults. They found a positive correlation of FA in a left temporo‐parietal area with higher reading skills, and the spatial location of that region is extremely close to a left parietal region exhibiting increases in FA with general cognitive ability in the current study (Table II; Talairach coordinates −32, −13, 25). Reading is a task that heavily relies on highly specialized brain areas in frontal, temporo‐parietal, and occipito‐temporal regions and their fast and efficient connection [Habib,2000; Pugh et al.,2000]. In fact, it has been hypothesized that in reading‐impaired individuals, “temporo‐parietal difficulties disrupt this developmental trajectory” [Pugh et al.,2001, p. 487], which is consistent with neuroimaging studies demonstrating pathological patterns of posterior activation in reading‐impaired subjects, with a compensatory shift involving stronger activation in frontal areas [Pugh et al.,2001]. The DTI results in the reading‐impaired (adult) individuals thus reflect the disruption of a normal (anatomical and functional) connection between cooperating brain regions. Our results reflect normal variations in healthy children that correlate with their individual, overall cognitive abilities as measured by a broad range of tasks in a test of “general” intelligence (which is normal in most dyslexic individuals [Habib,2000]). However, the partial correlation (Table II) exhibited with Verbal IQ (R = 0.57) is greater than that with Performance IQ (R = 0.33); and, although this difference is not statistically significant, the data are consistent with the hypothesis that the majority of the FA differences in this area may be traced to verbal proficiency.
The results also match well with a recent voxel‐based morphometry study investigating correlations of brain structure with intelligence in the normal pediatric population [Wilke et al.,2003]. Gray matter volume positively correlated with cognitive function in anterior brain regions and in posterior temporal/inferior parietal regions. Our findings of increased FA in adjoining white matter areas are compatible with a shift of cognitive functions to frontal and temporal regions during normal development [Schlaggar et al.,2002; Wilke et al.,2003]. Therefore, our findings could be related and secondary to gray matter developmental processes. However, more research addressing this issue will be necessary, especially with regard to questions relating to causality.
The areas in frontal white matter found to exhibit correlations of FA with cognitive function (at Z = +25 bilaterally; Table II) in this voxelwise study are virtually identical to those previously found in a small‐scale study [Adler et al.,2004] investigating abnormal white matter tracts in patients with bipolar disorder using a region‐of‐interest based approach. The results were interpreted as a loss of bundle coherence in patients suffering from bipolar disorder. In particular, it was hypothesized that “…while disorganization of white matter tracts may be a neuropathic phenomenon, a loss of bundle coherence may be developmental in origin. White matter formation and development begins during the prenatal period and extends through early adulthood, suggesting the presence of neuropathologic changes prior to the onset of affective symptomatology marking the overt appearance of bipolar disorder” [Adler et al.,2004, p. 201]. Our results, relating increased FA in frontal white matter with improved cognitive function in normal, healthy children, are consistent with the hypothesis of Adler et al. [2004] of a developmental basis for white matter abnormalities linked to bipolar and possibly other disorders, although further research involving pathologic populations is needed.
The anatomical locations displaying correlations of FA with IQ also overlap significantly with those found in previous 1H MRS studies of cognitive function in normal adults. NAA concentration in the left occipitoparietal white matter was found to be positively correlated with IQ [Jung et al.,1999], while choline concentration was negatively correlated with IQ. Increased NAA concentration in the frontal white matter was found to be associated with greater executive and attentional abilities [Valenzuela et al.,2000]. NAA has been suggested to be a neuronal marker, although at least one case with a complete absence of NAA in the brain has been reported [Martin et al.,2001]. Its biological function is highly disputed, although recent evidence suggests a possible role as a molecular water pump [Baslow,2002] facilitating movement of intracellular water across a hydrophobic myelin sheath during neuronal firing. Using both methods (MRS and DTI) in the same population would be a promising way to further investigate the relationship of the findings reported here and earlier results using MRS. Until such data is available, it seems premature to draw definitive conclusions from a possible link between these findings.
The changes in FA could conceivably be influenced by mechanisms other than increased fiber organization, such as changes in axonal size or use‐dependent changes in the degree of myelination. However, apart from the strong positive correlation of FA with age in the corticospinal tract observed earlier [Schmithorst et al.,2002], not much is known about the extent and regional specificity of such processes. At this point, it can only be speculated as to how far such modulating influences play a role in the effects observed in our data. As to the interesting question of whether the observed white matter changes reflect differences in specific cognitive functions, since the majority of our changes are found at the intersection of large fiber bundles, drawing inferences about the origin and/or target of these fibers is quite difficult. A further difficulty is that only very few neuropsychologically defined cognitive functions have been mapped reliably to specific brain regions [Cabeza and Nyberg,2000]. Much further research will therefore be necessary to link changes in white matter microstructure to differences in specific aspects of cognitive function.
Since our analysis was, by its nature, exploratory, we chose to use a voxelwise approach rather than a region‐of‐interest based approach for the analysis. A well‐known statistical software package (SPM99) [Friston et al.,1995] was utilized to remove gray matter and CSF voxels from further analysis. Since we desired to investigate changes in white matter microstructure rather than gross morphological differences related to intelligence, we chose to use a rather strict threshold in order to safeguard against partial‐volume effects, despite the fact that that might result in the discarding of some voxels actually in white matter and a subsequent loss of sensitivity. This was necessary due to the rather low acquired spatial resolution (2 × 3 × 5 mm), which limits detectability of smaller fiber tracts, and due to the affine spatial normalization, which, while shown to be fairly reliable for subjects in our age range [Muzik et al.,2000; Wilke et al.,2002], is nevertheless not as robust as it is for adult subjects. Due to these limitations, it is likely that some white matter areas actually related to intelligence and cognitive function were not found in our analysis. The interpretability of our data is further limited by the fact that the sample was biased towards females, although gender was incorporated as a covariate in all analyses. While gender differences have recently been demonstrated relating to function–structure relationships [Pfleiderer et al.,2004], further speculation on potential gender differences in the maturational processes described here is difficult. However, future work, ideally combining different methodological approaches in the same participants, will help in elucidating further the mechanisms responsible for the effects seen in these data. MRS or chemical shift imaging would seem to be particularly helpful in this respect, along with structural image analyses as done before for gray matter [Wilke et al.,2003].
CONCLUSION
DTI was performed on 47 normal children ages 5–18. Statistically significant positive correlations with IQ were seen for FA in specific white matter association areas, indicating that cognitive function correlates with greater fiber organization in the pediatric population.
Acknowledgements
We thank Dr. Anna Byars, Ph.D., for assistance in the administration of the Wechsler Full‐Scale IQ tests, Dr. Richard Strawsburg for performing the neurological examinations, and Dr. William Ball for reading the structural scans.
REFERENCES
- Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM (2004): Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord 6: 197–203. [DOI] [PubMed] [Google Scholar]
- Baratti C, Barnett AS, Pierpaoli C (1999): Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology 210: 133–142. [DOI] [PubMed] [Google Scholar]
- Baslow MH (2002): Evidence supporting a role for N‐acetyl‐L‐aspartate as a molecular water pump in myelinated neurons in the central nervous system. An analytical review. Neurochem Int 40: 295–300. [DOI] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E (2002): Practical aspects of conducting large‐scale functional magnetic resonance imaging studies in children. J Child Neurol 17: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition. II. An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Clark CA, Le Bihan D (2000): Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med 44: 852–859. [DOI] [PubMed] [Google Scholar]
- Duewll S, Wolff SD, Wen H, Balaban RS, Jezzard P (1996): MR imaging contrast in human brain tissue: assessment and optimization at 4T. Radiology 199: 780–786. [DOI] [PubMed] [Google Scholar]
- Edwards SG, Liu C, Blumhardt LD (2001): Cognitive correlates of supratentorial atrophy on MRI in multiple sclerosis. Acta Neurol Scand 104: 214–223. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Habib M (2000): The neurological basis of developmental dyslexia: an overview and working hypothesis. Brain 123(Pt 12): 2373–2399. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A (1999): Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42: 515–525. [PubMed] [Google Scholar]
- Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL Jr (1999): Biochemical markers of intelligence: a proton MR spectroscopy study of normal human brain. Proc R Soc Lond B Biol Sci 266: 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA (2000): Microstructure of temporo‐parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron 25: 493–500. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE (1998): Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage 8: 113–128. [DOI] [PubMed] [Google Scholar]
- Martin E, Capone A, Schneider J, Hennig J, Thiel T (2001): Absence of N‐acetylaspartate in the human brain: impact on neurospectroscopy? Ann Neurol 49: 518–521. [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor J, Langston J, Gajjar A (2001): Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol 19: 472–479. [DOI] [PubMed] [Google Scholar]
- Mulkern RV, Vajapeyam S, Robertson RL, Caruso PA, Rivkin MJ, Maier SE (2001): Biexponential apparent diffusion coefficient parametrization in adult vs newborn brain. Magn Reson Imaging 19: 659–668. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT (2000): Statistical parametric mapping: assessment of application in children. Neuroimage 12: 538–549. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Iwasaki S, Kichikawa K, Fukusumi A, Taoka T, Ohishi H, Uchida H (1998): Normal myelination of anatomic nerve fiber bundles: MR analysis. AJNR Am J Neuroradiol 19: 1129–1136. [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001a): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57: 632–638. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS (2001b): Normal‐appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57: 2307–2310. [DOI] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Houston GC, Smith JM, Smith MI, James MF, Parsons AA, Huang CL, Hall LD, Carpenter TA (1999): A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging 17: 881–892. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (2001): Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54: 255–266. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, Michael N (2004): N‐acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience 123: 1053–1058. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA (2000): Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6: 207–213. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA (2001): Neurobiological studies of reading and reading disability. J Commun Disord 34: 479–492. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ (2000): Developmental neuroimaging of children using magnetic resonance techniques. Ment Retard Dev Disabil Res Rev 6: 68–80. [DOI] [PubMed] [Google Scholar]
- Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM (2000): Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry 69: 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Falautano M, Possa F, Martinelli V, Comi G, Filippi M (2002): Cognitive dysfunction in patients with mildly disabling relapsing‐remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci 195: 103–109. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002): Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science 296: 1476–1479. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ (2002): Automatic gradient preemphasis adjustment: a 15‐minute journey to improved diffusion‐weighted echo‐planar imaging. Magn Reson Med 47: 208–212. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M (2002): Differences in white matter architecture between musicians and non‐musicians: a diffusion tensor imaging study. Neurosci Lett 321: 57–60. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK (2001): Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging 20: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2002): Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross‐sectional diffusion‐tensor MR imaging study. Radiology 222: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Bosworth HB, Provenzale JM, MacFall JR (2002): Subcortical white matter lesions and functional impairment in geriatric depression. Depress Anxiety 15: 23–28. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planar stereotaxic atlas of the human brain. Rayport M, translator. New York: Thieme Medical. [Google Scholar]
- Valenzuela MJ, Sachdev PS, Wen W, Shnier R, Brodaty H, Gillies D (2000): Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: subcortical‐frontal axonal N‐acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage 12: 747–756. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK (2002): Assessment of spatial normalization of whole‐brain magnetic resonance images in children. Hum Brain Mapp 17: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK (2003): Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage 20: 202–215. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J‐H, Lancaster JL, Fox PT (1995): Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp 3: 287–301. [Google Scholar]
- Yeo RA, Hill D, Campbell R, Vigil J, Brooks WM (2000): Developmental instability and working memory ability in children: a magnetic resonance spectroscopy investigation. Dev Neuropsychol 17: 143–159. [DOI] [PubMed] [Google Scholar]


