Abstract
Allergen-induced airway inflammation may lead to allergic asthma, a chronic inflammatory disease of the respiratory system. Despite its high incidence, the majority of the world’s population is unaffected by allergic airway inflammation most likely due to innate protective mechanism(s) in the respiratory system. The mammalian airway epithelia constitutively express uteroglobin (UG), a protein with potent anti-inflammatory and anti-chemotactic properties. We report here that UG binds to FPR2, a G-protein coupled receptor, inhibits chemotaxis, down-regulates SOCS-3 gene expression and STAT-1 activation, which are critical for the differentiation of T-helper 2 (TH2) cells that secrete pro-inflammatory TH2 cytokines. We propose that UG suppresses allergen-mediated activation of TH2 response by down-regulating the expression of genes that are critical for TH2 differentiation.
Keywords: Uteroglobin, Airway Inflammation, Allergy
List of abbreviations: UG, uteroglobin; UG-KO, uteroglobin-knockout; CC10, Clara cell 10kDa protein; OVA, ovalbumin; SAA, serum amyloid A; FPR, formyl peptide receptor; TH2 cells, T-helper 2 cells; DCs, dendritic cells; SOCS-3, suppressor of cytokine signaling-3
1. Introduction
During the past two decades, the incidence of allergen-induced airway inflammation, one of the leading causes of allergic asthma, has nearly doubled [1]. Interestingly, despite this high incidence the majority of the World’s population manages to remain free of this chronic airway inflammatory disease. Innate mechanisms may exist to suppress allergen-induced inflammatory response in the airways and to maintain homeostasis in the respiratory system. Accumulating evidence indicates that a complex interplay of genetic and environmental factors contributes to the onset and maintenance of allergen-mediated airway inflammation in allergic asthma [2]. In this scenario, cytokines secreted by T helper-2 (TH2) cells, such as interleukin (IL)-4, IL-5 and IL-13, play critical pathogenic roles [2]. More specifically, it has been reported that the presence of IL-4 during the initiation phase of allergic inflammatory response leads to the differentiation and predominance of TH2 cell lineage that elaborate pro-inflammatory TH2 cytokines.
Dendritic cells (DCs), the major antigen presenting cells in the body, play pivotal roles in the differentiation of TH2 cell lineage from naïve CD4+ T cells [2]. Upon encountering antigen(s), the DCs process the antigen and migrate to the lymphatic system to present the processed antigen to naïve T cells and regulate the differentiation as well as activation of antigen-specific TH2 cells [3]. Recently, it has been shown that suppressor of cytokine signaling-3 (SOCS-3) plays critical regulatory roles in the initiation and maintenance of TH2-mediated allergic airway inflammatory responses [4], which are mediated by TH2 cytokines. Consistent with these findings, SOCS-3 over-expression has been shown to play a critical role in the differentiation of the TH2 cell lineage and the maintenance of TH2 predominance [4].
Uteroglobin (UG) or Clara cell 10kDa protein (CC10) is the founding member of the newly recognized Secretoglobin superfamily. It is a steroid-inducible secreted protein, which is constitutively expressed at a high level by the airway epithelial cells of all mammals and possesses potent anti-chemotactic and anti-inflammatory properties [5]. We recently reported that UG-knockout (UG-KO) mice [6], sensitized and challenged with a model allergen, ovalbumin (OVA), manifest exaggerated inflammatory response in the airways mediated by elevated levels of TH2 cytokines and eotaxin, which are characteristically found in allergic asthma [7]. Several years ago, we reported that UG inhibits chemotaxis of monocytes and neutrophils induced by a potent chemoattractant peptide, fMLP [8]. It is now known that chemoattraction by fMLP is mediated via a family of G-protein coupled receptors known as formyl peptide receptors (FPRs) [reviewed in 9]. Interestingly, among the two isoforms of FPR, fMLP binds to FPR with high affinity while it manifests low affinity for binding to FPR2 (FPRL1R in human) [10].
Antigen (allergen) exposure to the respiratory system stimulates the expression of acute phase proteins such as serum amyloid A (SAA) [11]. It has been reported that dendritic cells (DCs), which are the major antigen presenting cells (APCs), express FPR2 [12] that promotes SAA-binding on these cells [13]. Thus, interaction of SAA with FPR2 on DCs may stimulate chemotactic migration of these APCs to the antigen site facilitating the processing and presentation of antigens. Recently, it has been shown that the direction of TH2 differentiation, which requires the input from the DCs, is determined by the cytokine environment at the site of initial antigenic (allergic) activation [4].
To determine the molecular mechanism(s) by which UG suppresses allergen-induced TH2 cytokine production that causes airway inflammation, we used UG-KO mice and studied the expression of genes that are critically important in the differentiation of TH2 cell lineage. Our results demonstrate that UG binds to FPR2 inhibits chemotaxis, down-regulates SOCS-3 gene expression and STAT-1 activation, which are reported to play critical roles in the differentiation of TH2 cells.
2. Materials and Methods
2.1. Materials
Chicken OVA (grade V), protease inhibitor mixture, phosphatase inhibitor mixture were from Sigma; purified human serum amyloid A (SAA) from Peprotech Inc. (Rocky Hill, NJ); Pre-cast SDS-polyacrylamide (4–15%) gels from Bio-Rad; antibodies to SOCS-3 (Novus Biologicals); STAT-1, phospho-Ser-STAT-1, phospho-Tyr-STAT-1 (Cell Signaling) and horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG are from Santa Cruz.
2.2. Mice
UG-KO mice were generated as described previously [6]. Both UG-KO and WT mice were maintained under germ-free conditions and all experiments were performed according to a protocol approved by the NICHD Animal Care and Use Committee. Airway inflammation by OVA is induced as previously reported [7].
2.3.Cytokine array
Cytokine levels in sera from both WT and UG-KO mice were determined using Mouse Cytokine ArrayII from Ray Biotech, Inc. (Norcross, GA) following the vendor’s protocol.
2.4 Isolation of RNA and real-time PCR
Total RNA from the lungs was isolated by TriZol (In Vitrogen) method and real-time PCR was performed using ABI Prism 7000 Sequence Detection System (Applied Biosystems) as described previously [7]. The primers used are: SOCS-3 (sense 5’TTG TCT CTC CTA TGT GGG GC and antisense 5’TGT GTT TGG CTC CTT GTG TG), STAT-1 (sense 5’CTT GAC GAC CCT AAG CGA AC and antisense 5’ GCA GGT TGT CTG TGG TCT GA), FPR-2 (sense 5’TGG CTG GTT CCT GTG TAA AT and antisense 5’ CCA AGG CAA TGA GAG CAA TC), SAA-3 (sense 5’ GAA GCC TTC CAT TGC CAT CA and antisense 5’ CCCT TGA CCA GCT TCT TTC ATG) and β-Actin (sense 5’ ACG GCC AGG TCA TCA CTA TTG, and antisense 5’ TGG AAA AGA GCC TCA GGG C). The data from each PCR run were analyzed using ABI Prism Software version 1.01 (Applied Bios stems). The final data were normalized to β-Actin and presented as fold increase compared with the expression level in WT mice. Quantization was performed using three independent total RNA samples for each treatment group. The results are presented as the mean (n=3) + S.D.
2.5. Western Blot analysis
Lung lysates (20–30μg total protein) were resolved by SDS-PAGE and analyzed by Western blotting as previously described [7] using 1:1000 dilution of antibodies to the following proteins: SOCS-3; STAT-1, phospho-S-STAT-1and phospho-Y- STAT-1. The secondary antibody, HRP-conjugated donkey anti-rabbit IgG, was diluted 1:10,000. Chemiluminescent protein bands were detected by an ECL detection system (Amersham Biosciences) according to the manufacturer's protocol.
2.6. Migration of mL4-7 cells in response to SAA
mL4-7 cells were generated by stably transfecting HEC-293 cells with a mouse FPR-2-cDNA construct as previously described [11]. Migration of mL4-7 cells were performed using BD Biocoat Matrigel Invasion chambers, following the manufacturer’s protocol. Briefly, 0.5 ml of 5X104 cells/ml were added in absence and presence of 10nM to 200nM of UG to the insert and 1 ml of cell culture medium containing 1μM SAA was added to each well. Myoglobin (MG; 200nM) was used as a non-specific protein control for UG and the cells were cultured for 72 hrs at 37° C and cells were stained as per standard protocol.
2.7. Binding Studies
Binding of 3H-UG to HEK-293 and mL-47 cells was carried out according to a protocol described previously [14]. The data were analyzed by using graph Pad Prism version 4.1 to determine the dissociation constant (Kd) for each ligand.
3. Results
3.1. Allergen exposure induces elevated serum amyloid A expression UG-KO lungs
Since DCs are the major antigen (allergen)-presenting cells and SAA is reported to be a chemoattractant for DCs, we first sought to determine whether antigenic exposure to UG-KO mice induces elevated levels of SAA-mRNA in the lungs. Accordingly, we sensitized and challenged UG-KO mice and their WT littermates with OVA. The results show that the basal levels of SAA-mRNA in the lungs of the UG-KO mice are appreciably higher than those in the lungs of WT littermates (Fig. 1A). However, while OVA-sensitization and challenge stimulates SAA-mRNA expression in both groups of mice the level of expression in the UG-KO lungs is still markedly higher (Fig. 1B). We also determined the FPR2-mRNA levels by real time PCR using total RNA from the lungs of UG-KO mice and from those of their WT littermates. The results show that the FPR2-mRNA expression in the lungs of UG-KO mice is markedly higher compared with that of their WT littermates (data not shown). Taken together, these results indicate that UG-KO mice may be more susceptible to allergen-induced production of SAA, which has been reported to provide chemotactic signal for the migration of the DCs.
Figure 1.

A. Expression of SAA-3 mRNA in non-sensitized and un-challenged WT (bar1) and non-sensitized and un-challenged UG-KO (bar 2) mice. Real time PCR using total RNA from the lungs was used to determine the mRNA levels; B. Expression of SAA-mRNA in WT (bar 1) and UG-KO (bar 2) mice sensitized and challenged with OVA by real time quantitative PCR. Note the level of SAA-mRNA in OVA-sensitized and challenged UG-KO mice is significantly higher (p<0.05) compared with that of the WT mice (n=3).
3.2. UG binds to cells expressing FPR2 with high affinity and specificity
Several years ago, we reported that UG is a potent inhibitor of fMLP-induced chemotaxis of monocytes and neutrophils [8]. However, at that time fMLP receptors were not identified by molecular cloning and characterization. Since SAA binds to FPR2 on DCs [12] and stimulates migration of these cells, we sought to determine whether UG may interact with FPR2 and compete with SAA for binding to this receptor, thereby inhibiting the migration of the DCs to the antigen site essential for antigen processing. Because pure cultures of DCs are difficult to obtain, we performed binding assays using [125I]-SAA and [3H]-UG on mL4-7 cells [13], which are generated by stably transfecting HEK-293 cells with a FPR2-cDNA construct. The results show that while HEK-293 cells, without FPR2-cDNA transfection (control), do not bind SAA or UG (data not shown), mL-4-7 cells manifest saturable binding of both ligands (Fig. 2A and 2B). However, while the dissociation constant (Kd) for SAA binding to FPR2 is 292 nM (Fig. 2A) that of UG is 33 nM (Fig. 2B) suggesting a slightly higher affinity of FPR2 for UG. We then tested the specificity of UG-binding on FPR2 by conducting a [3H]-UG binding assay in which non-radioactive UG was used as the competing ligand. The results show that while HEK-293 cells do not interact with 3H-UG, the mL4-7 cells readily bind this ligand and in a competition assay, it is displaced by non-radioactive UG in a dose-dependent manner (data not shown). Taken together, these results suggest that UG binds to FPR2 with high specificity and it appears to have a slightly higher affinity for binding to FPR2 than that of SAA. Thus, UG may have the potential to effectively compete with SAA, a known chemoattractant for DCs [12]. It should be noted here that there are many known ligands of FPR2 [15], however, UG is one of the few ligands that binds to this receptor with high affinity. Most interestingly, while most ligands of FPR2 are agonists, UG appears to be an antagonist of this receptor.
Figure 2.

Saturation isotherms of [125I]-SAA (A) and [3H]-UG (B) on mL4-7 cells. Note a slightly higher dissociation constant (Kd) for 125I-SAA compared to that of 3H-UG.
3.3. UG inhibits SAA mediated migration of cells expressing FPR2
To determine whether the binding of UG with FPR2 may prevent SAA-mediated chemotactic migration of mL4-7 cells, we performed migration assays using SAA as the chemoattractant and UG as the competing ligand. The non-transfected HEK-293 cells, which do not express FPR2, served as controls. The results show that while SAA does not stimulate the chemotactic migration of HEK-293 cells, it readily induces migration of mL4-7 cells (Fig. 3, upper row). Most importantly, UG appears to strongly inhibit SAA-mediated chemotactic migration of mL4-7 cells in a dose-dependent manner (Fig. 3, upper & lower rows). We used myoglobin (MG) as a non-specific protein control for UG and the results show that it has no inhibitory effect on SAA-mediated chemotactic migration of mL4-7 cells (Fig. 3, lower row). Moreover, while 1μM SAA mediates strong chemotactic migration of mL4-7 cells, such migration is inhibited by nM concentrations of UG in a dose-dependent manner. Since DCs express FPR2 and SAA mediates chemotactic migration of the DCs our results imply that UG-mediated suppression of SAA-induced migration of these cells may be due to the competition between the two protein ligands for FPR2. Thus, UG-mediated inhibition of migration is likely to interfere both with the recruitment of DCs to the antigen site as well as with antigen presentation to naïve T cells, thereby suppressing the differentiation of TH2 cells.
Figure 3.
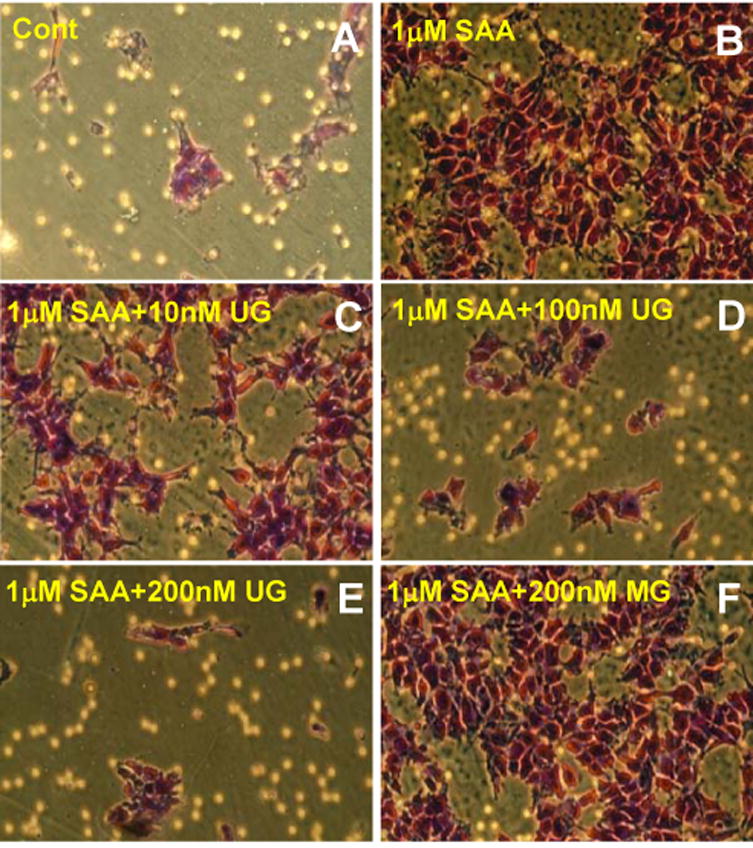
Inhibition of SAA-induced migration of mL4-7 cells by UG. Note while UG inhibits SAA-induced migration of mL4-7 cells in a dose-dependent manner and myoglobin (MG), even at a concentration of 200nM, does not appear to inhibit migration of these cells.
3.4. UG inhibits SOCS-3 expression
Recently, it has been reported that suppressor of cytokine signaling-3 (SOCS-3) regulates the initiation and maintenance of TH2-mediated allergic responses [4]. We previously demonstrated that the basal levels of TH2 cytokines in UG-KO mice are higher compared with those of their WT littermates [7]. Moreover, OVA-sensitization and challenge of the UG-KO mice augment these levels even further. Thus, we sought to determine the levels of SOCS-3 gene expression in the lungs of UG-KO mice and in those of their WT littermates with or without OVA-sensitization and challenge. The results show that while low levels of SOCS-3 mRNA (Fig. 4A, bar 1) and SOCS-3 protein (Fig. 4B, lane 1) are expressed in the lungs of WT mice, these levels are appreciably higher in their UG-KO littermates (Fig. 4A, bar 3 & 4B, lane 3). Moreover, OVA-sensitization and challenge elevated the expression of SOCS-3-mRNA albeit slightly (Fig 4A, bar 2) and SOCS-3-protein (Fig. 4B, lane 2) in the lungs of WT littermates. Most importantly, in the lungs of OVA-sensitized and challenged UG-KO mice the SOCS-3 mRNA (Fig. 4A, bar 4) and SOCS-3 protein (Fig. 4B, lane 4) levels are markedly augmented. Notably, these high levels of SOCS-3 mRNA and protein are drastically suppressed when OVA-sensitized UG-KO mice are treated with purified recombinant UG prior to OVA-challenge (Fig. 4A, bar 5 & 4B, lane 5). These results strongly suggest that UG suppresses OVA-induced stimulation of SOCS-3 expression in the lungs, and most likely, down-regulates this critical step in the pathway of allergen-mediated differentiation of TH2 cells [4].
Figure 4.

A. Expression of SOCS-3 was determined by quantitative real time PCR using total RNA from the lungs of WT (bar 1), OVA-sensitized and challenged WT (bar 2), UG-KO (bar 3), OVA-sensitized and challenged UG-KO (bar 4), and , OVA-sensitized UG-KO mice pretreated with recombinant UG (rUG) prior to OVA challenge (bar 5); B. Detection of SOCS-3 protein by Western blot analysis using total protein extracted from the lungs of WT (lane 1), OVA sensitized and challenged WT (lane 2), UG-KO ( lane3), OVA sensitized and challenged UG-KO (lane 4), and OVA sensitized UG-KO mice pretreated with rUG before OVA challenge (lane 5); C. UG inhibits OVA-induced expression and activation of STAT-1. Expression of STAT-1 mRNA was determined by quantitative real time RT-PCR using total RNA from lungs of WT(bar 1), OVA sensitized and challenged WT (bar 2), UG-KO (bar 3), OVA sensitized and challenged UG-KO (bar 4), and , OVA sensitized UG-KO mice pretreated with rUG before OVA challenge (bar 5). D. Detection of STAT-1 protein and phosphorylation of tyrosine on STAT-1 (p-Y--STAT-1) and phosphorylation of serine on STAT-1 (p-S--STAT-1) by Western blot analysis using total protein extracted from WT (lane1), OVA sensitized and challenged WT (lane 2), UG-KO (lane 3), OVA-sensitized and challenged UG-KO (lane 4), and OVA-sensitized UG-KO mice pretreated with rUG before OVA challenge (lane 5).
3.5. UG suppresses allergen-induced SOCS-3 expression by inhibiting STAT-1 activation
How might UG suppress SOCS-3 expression? It has been reported that the 5’-promoter region of the SOCS-3 gene contains binding motifs for signal transducer and activator of transcription-1 (STAT-1) and STAT-1 activation has been shown to play a critical role in SOCS-3 gene expression [4]. Accordingly, we determined the expression of STAT-1 in the lungs of WT and UG-KO mice before and after OVA-sensitization and challenge. The results show that there is virtually no difference in the basal levels of STAT-1 mRNA in the lungs of WT (Fig. 4C, bar 1) and UG-KO mice (Fig. 4C, bar 3). However, while in the WT mice OVA-sensitization and challenge made virtually no difference in the STAT-1 mRNA level (Fig. 4C, bar 2), in the UG-KO mice OVA-sensitization and challenge strikingly increased the level of STAT-1 mRNA (Fig. 4C, bar 4). Most interestingly, the level of STAT-1 mRNA in the lungs of OVA-sensitized UG-KO mice is dramatically reduced when they were treated with recombinant UG prior to OVA-challenge (Fig. 4C, bar 5). These results suggest that UG is an effective inhibitor of OVA-induced STAT-1 mRNA expression.
Phosphorylation of serine and tyrosine residues on STAT-1 plays critical roles in its activation. Thus, we first sought to determine the effect of OVA-sensitization and challenge on STAT-1 phosphorylation in the lungs of UG-KO mice and their WT littermates and the effect of UG treatment of UG-KO mice on STAT-1 phosphorylation. Accordingly, we first performed Western blot analyses of lung lysates from WT and UG-KO mice before and after OVA-sensitization and challenge using phosphoserine- and phosphotyrosine-antibodies. We also determined the effect of UG-treatment on STAT-1 phosphorylation. Our results show that there is virtually no change in the levels of serine- and tyrosine-phosphorylation of STAT-1 in the lungs of control WT and UG-KO mice (Fig. 4D, lanes 1 & 3). However, while a slightly increased level of serine- as well as tyrosine-phosphorylation on STAT-1 is appreciable in OVA-sensitized and challenged WT mouse lungs (Fig. 4D, lane 2), such levels are markedly elevated in those of the UG-KO mice sensitized and challenged with OVA (Fig. 4D, lane 4). Most importantly, these high levels of phosphorylated STAT-1 were dramatically reduced when OVA-sensitized UG-KO mice were treated with recombinant UG prior to OVA-challenge (Fig. 4D, lane 5). These results strongly suggest that UG suppresses SOCS-3 gene expression by down-regulating allergen-induced stimulation of expression and phosphorylation (activation) of STAT-1.
4. Discussion
We previously reported that UG-KO mice manifest OVA-induced exaggerated airway inflammatory response mediated by TH2 cytokines, which are produced by TH2 cells. However, the molecular mechanism(s) by which UG suppresses the predominance of TH2 cells until now remained unclear. In this study we show that UG binds to FPR2, a G-protein coupled receptor known to be expressed on antigen-presenting DCs, inhibits chemotaxis, down-regulates SOCS-3 gene expression and STAT-1 activation, all of which play critical roles in TH2 differentiation. These results imply that UG promotes allergen-induced TH2 predominance by suppressing the expression of genes that are known to play critical roles in TH2 differentiation and predominance.
Recently, it has been reported that IL-12p40 is required for the migration of DCs [16], which plays critical roles in the differentiation, antigen presentation and maintenance of TH2 predominance. It has also been reported that while IL-12p70 promotes the differentiation of TH1 cells, IL-12 p40 in the presence of IL-4 acts as an antagonist of IL-12p70. As a result, elevated IL-12p40 production suppresses TH1 differentiation, thereby allowing TH2 predominance. We have previously reported that UG-KO mice have elevated levels of IL-4 expression [7]. Therefore, we determined IL-12p40 and IL-12p70 levels in the sera of UG-KO and WT mice, respectively, by serum protein microarray analysis. The results show that while in the sera of UG-KO mice IL-12p40 is readily detectable; it is virtually undetectable in those of the WT littermates (data not shown). Interestingly, no appreciable difference in the serum levels of IL-12p70 between UG-KO and WT mice were found (data not shown). Since IL-12p40 inhibits the differentiation of TH1 cells and it is present in the sera of UG-KO mice but not in those of their WT littermates, our results suggest that under physiological conditions one of the functions of UG is to suppress TH2 predominance and maintain homeostasis in the respiratory system.
Allergen-mediated airway inflammation leads to allergic asthma, a chronic airway inflammatory disease, the incidence of which continues to rise throughout the industrial world. In the US alone, more than 6000 deaths occur annually from complications of asthma. Insight into the molecular mechanisms of pathogenesis of allergen-mediated airway inflammation may facilitate the development of novel and rational therapeutic approaches to this common disease. The results of our present study, demonstrate for the first time that one of the important roles of UG is to down-regulate some of the molecular events critical for the differentiation of TH2 cells. These results also provide the proof of principle that recombinant UG is a potential drug target for the development of novel therapeutic approaches to allergen-mediated inflammatory diseases.
Acknowledgments
We thank Drs. P.M. Murphy, J. Y. Chou, I. Owens and S.W. Levin for critical review of the manuscript and helpful suggestions. This investigation was supported by the Intramural Research Program of the NIH [NICHD].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–12. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Cytokine modulators for allergic diseases. Curr Opin Allergy Clin Immunol. 2001;1:555–60. doi: 10.1097/00130832-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Oriss TB, Ostroukhova M, Seguin-Devaux C, Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P, Ray A. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol. 2005;174:854–63. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 4.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee AB, Kundu GC, Mantile-Selvaggi G, Yuan CJ, Mandal AK, Chattopadhyay S, Zheng F, Pattabiraman N, Zhang Z. Uteroglobin: a novel cytokine? Cell Mol Life Sci. 1999;55:771–87. doi: 10.1007/s000180050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Kundu GC, Yuan CJ, Ward JM, Lee EJ, DeMayo F, Westphal H, Mukherjee AB. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–12. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- 7.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J Exp Med. 2004;199:1317–30. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasanthakumar G, Manjunath R, Mukherjee AB, Warabi H, Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem Pharmacol. 1988;37:389–94. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 9.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–8. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 10.Liang TS, Wang JM, Murphy PM, Gao JL. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem Biophys Res Commun. 2000;270:331–5. doi: 10.1006/bbrc.2000.2416. [DOI] [PubMed] [Google Scholar]
- 11.Buyukozturk S, Gelincik AA, Genc S, Kocak H, Oneriyidogan Y, Erden S, Dal M, Colakoglu B. Acute phase reactants in allergic airway disease. Tohoku J Exp Med. 2004;204:209–13. doi: 10.1620/tjem.204.209. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Kang HK, Jo EJ, Kim JI, Lee YN, Lee SH, Park YM, Ryu SH, Kwak JY, Bae YS. Trp-Lys-Tyr-Met-Val-Met stimulates phagocytosis via phospho-lipase D-dependent signaling in mouse dendritic cells. Exp Mol Med. 2004;36:135–44. doi: 10.1038/emm.2004.20. [DOI] [PubMed] [Google Scholar]
- 13.Gao JL, Murphy PM. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–401. [PubMed] [Google Scholar]
- 14.Zhang Z, Kim SJ, Chowdhury B, Wang J, Lee YC, Tsai PC, Choi M, Mukherjee AB. Interaction of uteroglobin with lipocalin-1 receptor suppresses cancer cell motility and invasion. Gene. 2006;369:66–71. doi: 10.1016/j.gene.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190:741–7. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]


