Abstract
Human umbilical cord blood stem cells (hUCB) hold great promise for therapeutic repair after spinal cord injury (SCI). Here, we present our preliminary investigations on axonal remyelination of injured spinal cord by transplanted hUCB. Adult male rats were subjected to moderate SCI using NYU Impactor, and hUCB were grafted into the site of injury one week after SCI. Immunohistochemical data provides evidence of differentiation of hUCB into several neural phenotypes including neurons, oligodendrocytes and astrocytes. Ultrastructural analysis of axons reveals that hUCB form morphologically normal appearing myelin sheaths around axons in the injured areas of spinal cord. Colocalization studies prove that oligodendrocytes derived from hUCB secrete neurotrophic hormones neurotrophin-3 (NT3) and brain-derived neurotrophic factor (BDNF). Cord blood stem cells aid in the synthesis of myelin basic protein (MBP) and proteolipid protein (PLP) of myelin in the injured areas, thereby facilitating the process of remyelination. Elevated levels of mRNA expression were observed for NT3, BDNF, MBP and PLP in hUCB-treated rats as revealed by fluorescent in situ hybridization (FISH) analysis. Recovery of hind limb locomotor function was also significantly enhanced in the hUCB-treated rats based on Basso-Beattie-Bresnahan (BBB) scores assessed 14 d after transplantation. These findings demonstrate that hUCB, when transplanted into the spinal cord 7 days after weight-drop injury, survive for at least 2 weeks, differentiate into oligodendrocytes and neurons, and enable improved locomotor function. Therefore, hUCB facilitate functional recovery after moderate SCI and may prove to be a useful therapeutic strategy to repair the injured spinal cord.
Keywords: brain derived neurotrophic factor, myelin basic protein, neurotrophin-3, proteolipid protein, spinal cord injury, umbilical cord blood stem cells
INTRODUCTION
There have been many efforts to restore normal neuronal functions—and thus motor functions—after spinal cord injury (SCI), in which the myelin sheaths and/or myelinating cells (e.g., oligodendrocytes) are destroyed. Although some spontaneous remyelination occurs, this process is not consistent enough for complete repair (Franklin et al., 1997). This phenomenon depends on molecules (e.g., growth factors), most of which are still unidentified (Woodruff and Franklin, 1999). Since the natural capacity of the CNS to recover from injury is limited, most research into SCI focuses upon promotion of axonal growth, remyelination of demyelinating axons, and reduction of neuronal degeneration.
Demyelination contributes to the dysfunction of the traumatically injured spinal cord in both humans and experimental animals ( Waxman, 1989; Bunge et al., 1993; Cao et al., 2005b; Guest et al., 2005; Totoiu et al., 2005;). Remyelination of demyelinated, but otherwise intact, axons could be an important strategy for the treatment of spinal cord injury (Blight, 2002). At present, transplantation is the most promising approach for restoring lost myelin. Recent studies have focused on the use of transplanted oligodendrocyte precursor cells (OPC) or neural stem cells (NSC) after SCI ( Brustle et al., 1999; Keirstead et al., 1999; Liu et al., 2000; Cao et al., 2001; Ogawa et al., 2002; Bambakidis et al., 2004; Hill et al., 2004; Hofstetter et al., 2005). Transplantation of embryonic stem cell-derived NSC or OPC has led to partial functional improvement after SCI suggesting the feasibility of facilitating functional recovery from SCI by remyelination (Barres et al., 1994b; McDonald et al., 1999; Keirstead et al., 2005).
Human umbilical cord blood is a valuable source of stem cells that have the therapeutic potential to initiate and maintain tissue repair. This capability holds special promise for the treatment of neural diseases, for which no cure is currently available. In addition, therapies based on hUCB are attractive because the cells are readily available and less immunogenic as compared to other sources of stem cells, such as bone marrow. The therapeutic potential of hUCB may either be attributed to the inherent ability of stem cell populations to replace damaged tissues outright, or alternatively, to their ability to repair damaged tissues through neural protection and secretion of neurotrophic factors by various cell types within the graft (Sanberg et al., 2005). Perhaps more importantly, stem cells could promote axonal regeneration either by constituting a “bridge” through a lesion site capable of supporting axonal attachment and growth or by secreting diffuse molecules, such as growth factors, to attract injured axons. Previous studies have reported that hUCB are beneficial in reversing the behavioral effects of spinal cord injury, even when infused 5 days after injury (Saporta et al., 2003). Transplanted hUCB differentiate into various neural cells and induce motor function improvement in cord-injured rat models (Kuh et al., 2005). To date, three reports have utilized hUCB in SCI. More thorough experiments are needed to evaluate how hUCB modulates improvement after SCI and whether it possesses the potential of tissue plasticity (Enzmann et al., 2006). It is also unclear whether the enhanced functional recovery results from remyelination of demyelinated axons by engrafted cells or by trophic support to spare the white matter that would otherwise degenerate. Thus, the relationship between remyelination and functional recovery after traumatic SCI remains unresolved and mechanistic explanations are needed.
In this study, we grafted hUCB into the injured spinal cords of male rats to evaluate functional recovery in the hind limbs due to remyelination of the demyelinated axons. Our preliminary results evaluate the secretion of neurotrophic hormones by hUCB-differentiated oligodendrocytes and their role in remyelination.
METHODS
Study design
This study was designed to assay the differentiation of hUCB into different neural phenotypes in the injured spinal cord, functional improvement in motor control, and axonal remyelination and regeneration in spinal cord injured rats after transplantation of hUCB. Since human spinal cord trauma is primarily a disorder of males (Jackson et al., 2004), we used male rats for all of the following experiments.
Spinal cord injury (SCI) and post-surgical care
A total of 52 rats were used in this study and assigned to different groups as described in Table 1. Moderate spinal cord injury was induced using the weight drop device (NYU Impactor) developed at New York University (Gruner, 1992) and the injury protocol developed by a multicenter consortium [Multicenter Animal Spinal Cord Injury Study: (Basso et al., 1995; Basso et al., 1996a; Basso et al., 1996b)] as reported previously (Liu et al., 1997; Xu et al., 1998; Lee et al., 2003). Briefly, adult male rats (Lewis; 250–300 g) were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg; ip.) (both from Med-Vet International, Mettawa, IL). The dorsal aspect of the back was shaved and scrubbed with Betadine solution. A laminectomy was performed at the T9-T10 level exposing the cord beneath without disrupting the dura. The spinous processes of T8 and T11 were then clamped to stabilize the spine, and the exposed dorsal surface of the cord was subjected to a weight drop impact at T10 using a 10 g rod (2.5 mm in diameter) dropped at a height of 12.5 mm. After injury, the muscles and skin were closed in layers, and the rats were placed in a temperature and humidity-controlled chamber overnight. Cefazolin (25 mg/kg) (Fisher, Hanover Park, IL) was given to prevent urinary tract infection for 3 to 7 days. Manual expression of the urinary bladder was performed two times per day until reflex bladder emptying was established. For the sham-operated controls, the animals underwent a T10 laminectomy without weight-drop injury. All surgical interventions and post-operative animal care were approved by the Institutional Animal Care and Use Committee of the University of Illinois College of Medicine at Peoria.
Table 1.
Experimental design and description of groups.
| Group No. | Group Description | Designation | No. of animals studied | No. of animals used for each study
|
||||
|---|---|---|---|---|---|---|---|---|
| Electron microscopy | Immunohistochemistry | Western Blot | RT-PCR | Behavioral studies (BBB scoring) | ||||
| 1. | Control animals without laminectomy and spinal cord injury | Control | 10 | 2 | 3 | 3 | 2 | 10 |
| 2. | Control animals with laminectomy and PBS injected | Sham control | 10 | 2 | 3 | 3 | 2 | 10 |
| 3. | Spinal cord injured and untreated animals | Injured | 13 | 3 | 4 | 3 | 3 | 13 |
| 4. | Spinal cord injured and hUCB transplantation | hUCB treated | 14 | 3 | 4 | 4 | 3 | 14 |
| 5. | Spinal cord injured and cyclosporine treated | Cyclosporine treated | 5 | - | - | - | - | 5 |
Five animals died unexpectedly during the course of the experiment; two from group 3(died of hematuria), one from group 4 (died of hematuria). Early data from these animals were omitted from the analysis. All animals were sacrificed after 21d after SCI (i.e., 14 d after transplantation of hUCB).
Behavioral assessment after SCI
A behavioral test was performed to measure the functional recovery of the rats’ hind limbs following the procedure as described in Basso et al. (1995). The scale used for measuring hind-limb function with these procedures ranges from a score of 0, indicating no spontaneous movement, to a maximum score of 21, with an increasing score indicating the use of individual joints, coordinated joint movement, coordinated limb movement, weight-bearing, and other functions. Rats were first gently adapted to the open field used for the test. After a rat had walked continuously in the open field, two investigators conducted 4-minute testing sessions on each leg. Two individuals ‘blinded’ to rat treatment status performed the open field test at least once a week from day 1 post-SCI to 3 weeks post-SCI on all animals in the study. Behavioral outcomes and examples of specific BBB locomotor scores were recorded using digital video.
Intraspinal grafting of hUCB
BBB locomotor rating scores were obtained before transplantation and every week after SCI. Animals were re-anesthetized as described above, and the laminectomy site was re-exposed. Sham control group animals were injected 7 days after laminectomy with 5 μL of sterile PBS using a 10 μL Hamilton syringe. The hUCB-transplanted group was injected 7 days after injury, with 5 μL mononuclear cell layer of hUCB (5×105 cells/μL). These cells were delivered into the site of injury, at a rate of 0.5 μL/min using a 10 μL Hamilton syringe. Thus, a total of 2.5×106 cells were grafted into each injured spinal cord. The hUCB were previously labeled with DiL (1,1′-dioctadecyl-3, 3,3′, 3′tetramethyl-indocarbocyanine per chlorate) (Molecular Probes, OR) in order to facilitate identification of the cells within the subsequent histological specimens. Cyclosporine A (10 mg/kg) (Bedford Labs, Bedford, OH) was administered as an immunosuppressant for 7 days after transplantation of hUCB. The Cyclosporine-treated group rats received Cyclosporine A (10 mg/kg) for 7 days after SCI.
Culture and in vitro differentiation of hUCB
Human umbilical cord blood was collected from healthy volunteers with informed consent and according to a protocol approved by the Institutional Review Board. Human UCB were enriched by sequential Ficoll density gradient purification followed by selection of stem cells with the following markers: CD44+, CD133+ and CD34−. The cells were grown in Mesencult basal medium (Stem Cell Technologies, USA) supplemented with 20% heat inactivated FBS (Hyclone, Logan, UT) and 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA). Stem cells were incubated at 37°C in an incubator with 5% CO2 at saturating humidity. When cells reached 70% to 80% confluency, cells were detached with TrypLE Express (Invitrogen, Carlsbad, CA) and centrifuged at 250g for 3 min and re-plated. An acclimatization step was carried out 24 h prior to neural induction by replacing the growth medium with preinduction medium consisting of Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT), 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA), 1% 200 mM L-Glutamine (Mediatech Inc.-Fisher, Hanover Park, IL), 2% B27 (Invitrogen, Carlsbad, CA), 1% N2 (Invitrogen, Carlsbad, CA), bFGF (10 ng/mL, Invitrogen, Carlsbad, CA), β-NGF (10 ng/mL, Sigma, St. Louis, MO), BDNF (10 ng/mL, EMD Biosciences, San Diego, CA) and NT-3 (10 ng/mL, EMD Biosciences, San Diego, CA). Neural differentiation was then initiated the following day by incubating the cells in neurogenic medium (preinduction medium with 0.5 μM retinoid acid (Sigma, St. Louis, MO) and hEGF (10 ng/mL, Sigma, St. Louis, MO). The cells were observed for differentiation for 10 days.
Electron microscopic studies
To further characterize chronic histopathology, rats were anesthetized and perfused with 4% paraformaldehyde followed by a fixative solution (2% glutaraldehyde, 2% paraformaldehyde, and 2 mM CaCl2 in 0.1 M cacodylate buffer, pH 7.3). One μm sections were cut from the lesion epicenter with glass knives on an ultramicrotome, stained with toluidine blue, and examined under light microscopy. After fixation with 2.5% glutaraldehyde, the TEM samples were post-fixed with 1% osmium tetroxide, dehydrated, and flat embedded in Epon 812 epoxy resin (Tousimis, Rockville, MD). A Reichert OMU3 ultramicrotome (Austria) was used to prepare 600Å thin sections that were mounted on 200 mesh copper grids, stained with uranyl acetate and lead citrate. The sections were viewed under a JEOL (Tokyo, Japan) JEM 100C electron microscope. For SEM, after fixation with 2.5% glutaraldehyde, the samples were dehydrated, critical point dried (Denton Critical Point Apparatus, Cherry Hill, NJ), and sputter-coated (Commonwealth Scientific, Alexandria, VA) with 200Å gold. The samples were viewed under a JEOL (Tokyo, Japan) JSM35 electron microscope and were tilted and rotated for a cross-section view.
Subcellular fractionation and western blot analysis
Different protein levels in spinal cord tissue after SCI were compared with those in laminectomy controls and hUCB-treated samples. For western blot analysis, rats (n ≥ 3 per group) were euthanized, and 2 cm lengths of spinal cord centered on T10 (the injury site) were rapidly removed, weighed, and frozen at −70°C until used. Segments of spinal cord (5 mm) were isolated using the lesion site as the epicenter and the tissues were re-suspended in 0.2 mL of homogenization buffer (250 mM sucrose, 10 mM HEPES, 10 mM Tris-HCl, 10 mM KCl, 1% NP-40, 1 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM DTT, 0.5 mM PMSF plus protease inhibitors: 1 μg/mL pepstatin, 10 μg /mL leupeptin and 10 μg/mL aprotinin; pH 7.4) and homogenized in a Dounce homogenizer. Tissue homogenates were centrifuged at 14,000g for 20 min at 4°C. Protein levels in the supernatant were determined using the BCA assay (Pierce, Rockford, IL). Samples (50 μg of total protein per well) were subjected to 10%–14% SDS-PAGE (Laemmli and Favre, 1973) and transferred onto nitrocellulose filters and the reaction was detected with Hyperfilm-MP autoradiography film (Amersham, Piscataway, NJ). For western blot analysis, the following antibodies were used: rabbit anti-Neurotrophin-3 (1:500 dilution; Abcam, Cambridge, MA), mouse anti-MBP (1:5000 dilution; BD Biosciences, Franklin Lakes, NJ), goat anti-PLP (1:1000 dilution; Chemicon, Temecula, CA) and sheep anti-BDNF (1:500 dilution; Chemicon, Temecula, CA). The membranes were blocked with 5% nonfat skim milk in TBS for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C. The membranes were then processed with HRP-conjugated secondary antibodies. Immunoreactive bands were visualized using chemiluminescence ECL western blotting detection reagents (Amersham, Piscataway, NJ). Experiments were performed in triplicate to ensure reproducibility. Values for injured and control samples were compared using the Student's t test. A p value of less than 0.05 was considered significant.
Immunohistochemical assessment
To evaluate the cellular characteristics of transplanted cells in vivo, we performed immunohistochemical analysis. Three weeks after the induction of SCI, rats were perfused with PBS and 4% paraformaldehyde. The animals’ spinal cords were removed and fixed in 4% paraformaldehyde. After fixation for an additional hour, 1.5 cm lengths of spinal cord tissue centered at T10 (the injury site) were cryoprotected and frozen in blocks that contained both normal and injured tissue; and serial longitudinal and cross sections (5 μm thick) of the spinal cord were obtained with a microtome and cryostat. The specimens were then stored at −80°C for less than 1 month before further processing. The lesion was reconstructed by staining slides representing each millimeter of tissue with luxol blue, hematoxylin, and eosin. Additional slides representing the epicenter and levels 1 and 2 mm rostral and caudal to the lesion were used for immunocytochemistry. Following storage, the sections were rinsed with PBS for 20 min. The sections were then treated with blocking solution (1% BSA in 1X PBS) to prevent nonspecific staining, and were incubated with primary antibodies (1:100 dilution; 1:200 dilution for the secondary antibody) overnight at 4°C. Neuronal or glial markers were detected using fluorescent staining. We used the following primary antibodies: mouse anti-CD44 (Biomeda, Foster City, CA)/rabbit anti-CD44 (Abcam, Cambridge, MA), rabbit anti-GFAP (Abcam, Cambridge, MA), rabbit anti-neurofilament H 200kD (NF200) (Chemicon, Temecula, CA), rabbit anti-NT-3 (Abcam, Cambridge, MA), mouse anti-MBP (BD Biosciences, Franklin Lakes, NJ); goat anti-PLP (Chemicon, Temecula, CA), sheep anti-BDNF (Chemicon, Temecula, CA) and mouse anti-APC (Calbiochem). After staining with primary antibodies, the sections were washed three times in PBS (10 min/wash) and incubated in goat anti-mouse or anti-rabbit HRP-conjugated secondary antibodies. After 1 h, sections were washed three times in PBS (10 min/wash), incubated in DAB solution (Sigma, St. Louis, MO) until staining was evident microscopically. For immunofluorescence studies, the sections were washed three times in PBS (10 min per wash) and incubated in Texas Red conjugated anti-mouse secondary antibody or FITC-conjugated anti-rabbit secondary antibody for 1 h at room temperature. Sections were then washed three times in PBS (10 min per wash), counter stained with DAPI, cover slipped using fluorescent mounting medium (Dako, USA), and observed under both fluorescence microscope (IX71, Olympus, Melville, NY) and a confocal microscope (Olympus Fluoview, Olympus, Melville, NY). Negative controls (without primary antibody) were maintained for all the samples. Fluorescent images were captured using a fluorescence microscope (IX71 Olympus) and/or a confocal microscope (Olympus Fluoview) and counted using Image-Pro Discovery Analysis software (Media Cybernetics, Silver Spring, MD). Statistical analysis was performed by comparing groups using Student t-test (p< 0.05).
RNA extraction and RT-PCR of neurotrophic factors
Total RNA from the epicenter of the spinal cords of sham control, injured and hUCB-treated rats were isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA concentrations were determined spectrophotometrically. One μg of total RNA was reverse transcribed into cDNA in reverse transcription reaction with SuperScript One-Step RT-PCR System with Platinum Taq (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control. We used the following sequences for the forward and reverse primers:
for BDNF, 5′GTGATGACCATCCTTTTCCTT3′ (forward) and 5′CCACTATCTTCCCCTTTTAATGGT3′ (reverse);
for NT-3, 5′GTGACCATGTCCATCTTGT3′ (forward) and 5′ GCCAATTCATGTTCTTCCGAT3′ (reverse);
for MBP, 5′ GTGATGGCATCACAGAAGAGA3′ (forward) and 5′CTCTCAGCGTCTTGCCATGGGAGA3′ (reverse);
for PLP, 5′GCCAAAGACATGGGTTTGTTAGAGT3′ (forward) and 5′GGGAGATCAGAACTTGGTGCCT3′ (reverse).
for GAPDH, 5′CCACCCATGGCAAATTCC3′ (forward) and 5′CAGGAGGCATTGCTGATGAT3′ (reverse);
The housekeeping gene GAPDH was used for normalization of BDNF, NT-3, MBP and PLP mRNA expression. Optimum annealing temperatures, cycle numbers, and RT input were empirically determined by amplification of a single PCR product at the appropriate molecular weight for each target cDNA. Samples were subjected to 25–35 cycles at 95°C for 30 sec, 58–60°C for 30 sec, and 72°C for 1 min on GeneAmp PCR System 9700 (Perkin Elmer, Boston, MA) in 25 μL reaction volumes. After amplification, RT-PCR products were separated on a 1% agarose gel containing 0.5 mg/mL ethidium bromide. The amplified cDNA fragments were visualized under ultraviolet light. Densitometry readings of gel bands were performed using a Chemi-Imager Model 2.1.C (Alpha Innotech Co., San Leandro, CA). Experiments were performed in triplicate and the values obtained for the relative intensity were subjected to statistical analysis.
Fluorescent in situ hybridization (FISH) analysis
For mRNA in situ hybridization we followed the method of Wrathall et al., (1998) with slight modifications. At 21 d after SCI, spinal cords from injured rats, hUCB-treated rats and controls (n ≥ 3) were rapidly removed. The tissue was frozen in blocks that contained one uninjured control and one or more injured and hUCB-treated spinal cords. Serial 5 μm cross-sections were prepared on a cryostat, thaw mounted on slides coated with 3-aminopropyltriethoxysilane (Sigma, St. Louis, MO) and stored frozen until they were used for in situ hybridization. Oligonucleotide antisense sequences with a length of 48 bases were used as probes for the following genes: Neurotrophic hormones NT3 and BDNF; PLP, a major protein of CNS myelin and MBP, a major component of CNS myelin.
NT3 Sense:
GCCAGGCCAGTCAAAAACGGTTGCAGGGGGATTGATGACAAACACTGG
NT3 Antisense:
CCAGTGTTTGTCATCAATCCCCCTGCAACCGTTTTTGACTGGCCTGGC
BDNF Sense:
AGGAAGGCTGCAGGGGCATAGACAAAAGGCACTGGAACTCGCAATGCC
BDNF Antisense
GGCATTGCGAGTTCCAGTGCCTTTTGTCTATGCCCCTGCAGCCTTCCT
PLP Sense
TCCAGAGGCCAACATCAAGCTCATTCTTTGGAGCGGGTGTGTCATTGT
PLP Antisense
ACAATGACACACCCGCTCCAAAGAATGAGCTTGATGTTGGCCTCTGGA
MBP Sense
ATGGCATCACAGAAGAGACCCTCACAGCGACACGGATCCAAGTACTTG
MBP Antisense
CAAGTACTTGGATCCGTGTCGCTGTGAGGGTCTCTTCTGTGATGCCAT
The oligonucleotides were labeled with FITC at 3’ ends (Sigma-Genosys, The Woodlands, TX). For in situ hybridizations, slides were post-fixed with 4% formaldehyde in PBS, pH 7.4, for 10 min, acetylated (0.25% acetic anhydride in 0.1 M triethanolamine HCl, pH 8, for 10 min), and dehydrated with graded alcohols and chloroform. They were then incubated overnight at 37°C with hybridization buffer [50% formamide, 5X SSC, 5X Denhardt’s solution (1% BSA, 1% Ficoll and 1% Polyvinyl pyrrolidone), 0.025% bakers yeast tRNA (Sigma, St. Louis, MO) and 0.05% herring sperm DNA (Sigma, St. Louis, MO)] containing 200ng/ml of each oligonucleotide probe. The next day, slides were washed sequentially with 2X SSC (0.15 M NaCl, and 15 mM sodium citrate, pH 7.0) for 5 min at room temperature, 0.2X SSC (1h at 72°C in shaking water bath) and 0.2X SSC (5 min at room temperature) and then allowed for detection of fluorescent-labeled probes using ELF 97 mRNA in situ hybridization kit (Invitrogen, Carlsbad, CA). Finally, the slides were counterstained with Hoechst 333342 (for visualization of cell nuclei) and mounted using mounting medium. Visualization of FISH signal was done with a fluorescence microscope (IX71 Olympus) and/or a confocal microscope (Olympus Fluoview). Sections stained with sense probes served as controls, which do not show any signal.
Statistical analysis
Quantitative data from open field locomotor scores were evaluated for statistical significance by one-way ANOVA with replications; data from RT-PCR and western blot analyses were also evaluated for statistical significance using one-way ANOVA. Results were considered statistically significant at p<0.05. All data points represent group mean ± SEM.
RESULTS
In vitro differentiation of stem cells to neural phenotypes
In order to establish the differentiation potential of hUCB before intraspinal grafting, we assessed the trans-differentiation of these stem cells to neural phenotypes under in vitro conditions. Human UCB can be induced to differentiate and express neural-specific antigens. When exposed to hEGF/RA, hUCB morphologically appear to take on some of the features of neural cells in culture, including long bipolar extensions and branching ends. We observed neural differentiation of hUCB after 10 days in culture. After neural culture, cells from hUCB expressed the neural antigens found in neurons (NF-200) (Fig.1A), astrocytes (GFAP) (Fig. 1B) and oligodendrocytes (APC) (Fig. 1C). Among the differentiated cells, neurons comprised the major population followed by oligodendrocytes and astrocytes (Table 2). The stem cells expressed these markers only after culture in the neurogenic differentiation media.
Figure 1. Transdifferentiation of hUCB into neural phenotypes in vitro.
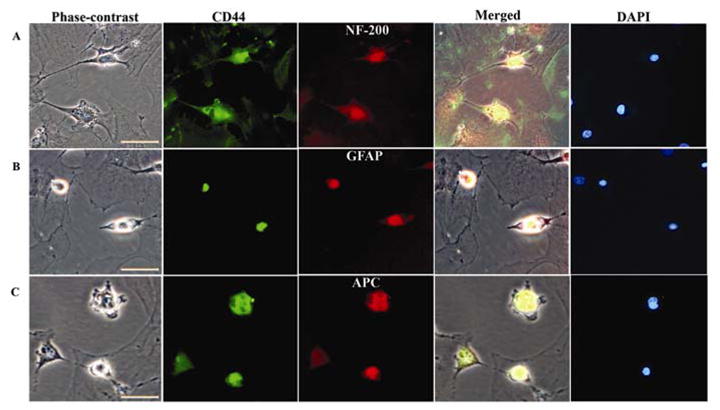
RA-treated CD44+ cells were fixed, incubated with primary antibodies directed against NF-200 (A), GFAP (B) or APC (C), followed by incubation with fluorescein-conjugated or Texas-red conjugated secondary antibodies. After immunostaining, cells were counterstained with DAPI. Neural cell markers and phase-contrast images were merged. Extreme left panel shows bright field images and extreme right panel shows nuclei of the cells stained with DAPI. Scale bar = 50μm. (A) Cells expressing NF-200 and displaying neuron like morphology with long axonal projections. (B) Cells immunostained with the anti-GFAP antibody. Some of the cells are round and relatively small, whereas others contain long projections with immunoreactive filamentous structures that are visible in the cytoplasm. (C) APC-immunoreactive cells displaying morphology characteristic of oligodendrocytes, with flat cell body and short or long branched projections. Smaller, round immunoreactive cells are also occasionally present. All these cells exhibit CD44 markers specific for hUCB. A subpopulation of hUCB-derived cells growing in a monolayer before clone formation was found to be negative for all investigated antigens. The results are expressed as the mean ± SE of cell number from nine independent cultures (three parallel experiments from three separate cord blood preparations).
Table 2.
Differentiation of hUCB in vitro and in vivo.
| Marker | in vitro (%) | in vivo (%) |
|---|---|---|
| NF-200 | 46.31 ± 0.81 | 37.83 ± 0.68 |
| GFAP | 17.21 ± 0.29 | 15.98 ± 0.49 |
| APC | 36.48 ± 0.47 | 46.19 ± 0.71 |
Quantification of the extent of NF-200, GFAP and APC expressing cells in neurogenic induction medium in vitro and in the injured spinal cord in vivo. For in vitro experiments, the results are expressed as the mean ± SE of cell number from nine independent cultures [three parallel experiments from three separate cord blood preparations (hUCB)]. For in vivo experiments, the results are expressed as the mean ± SE of cell number from three independent sections 2 mm caudal from the injury epicenter from hUCB treated group (n ≥ 3).
Survival and differentiation of hUCB in vivo in the injured spinal cord
One week after SCI, hUCB were transplanted into the injury site (referred to as the hUCB-treated group). Two weeks after transplantation, robust survival of transplanted hUCB was observed in the spinal cords of treated rats, with cells distributed around the cavities throughout the injury site. The differentiation of these hUCB into several neural phenotypes in the spinal cord could be traced by immunoflourescence analysis (Fig. 2). Surviving hUCB labeled with antibodies against markers specific for stem cells (CD 44) colocalized with NF-200 (a neurofilament protein) (Fig. 2A), oligodendrocytes (APC) (Fig. 2B) and astrocytes (GFAP) (Fig. 2C) could be identified distinctly. Most surviving hUCB were oligodendrocytes (46.19% were APC-labeled) followed by neurons, with some hUCB-derived astrocytes present in the dorsal region of the cord (Table 2). We observed differentiation of hUCB up to 2mm rostrocaudally to the injury epicenter. Many of the hUCB-derived oligodendrocytes were also immunoreactive for MBP and PLP, which are integral components of myelin.
Figure 2. Survival and differentiation of hUCB in rat spinal cords.
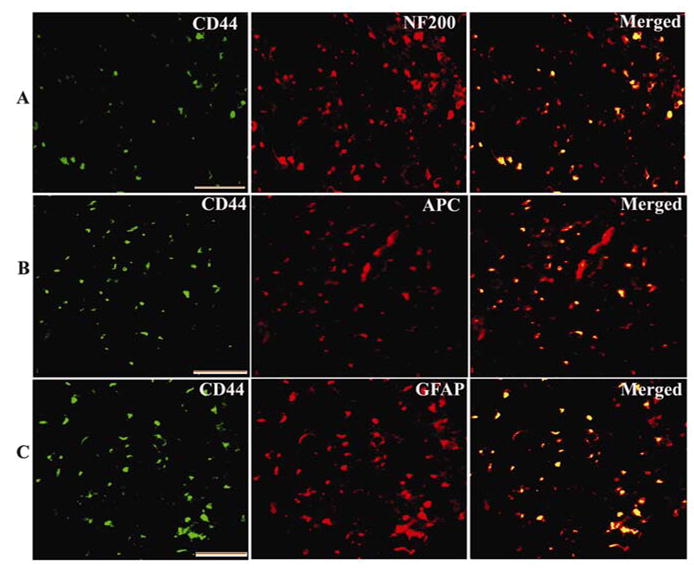
Differentiation of hUCB in injured spinal cords showing specific antigens: CD44 (hUCB marker) colocalized with (A) NF-200 (a neurofilament protein), (B) APC (oligodendrocyte marker) and (C) GFAP (an astrocyte marker). The differentiation of hUCB in vivo was observed after intraspinal grafting into injured spinal cords 7 days post-SCI. NF-200-, GFAP-, and APC-positive cells occurred in the vicinity of the injury site. Scale Bar = 100 μm. Results are from three independent sections 2 mm caudal from the injury epicenter (n ≥ 3).
Demyelination due to spinal cord injury
We next evaluated the extent of axonal demyelination and survival after SCI. Degenerative changes in the spinal cord were observed at three weeks post-SCI. In SCI rats, loss of large numbers of oligodendrocytes was evident around the injury epicenter (Fig. 3A). In contrast, in hUCB-treated rats, colocalization studies confirmed the presence of hUCB-differentiated oligodendrocytes widely distributed around the injury epicenter, towards the dorsal white matter (Fig. 3B). Mostly, we observed hUCB-differentiated oligodendrocytes towards the caudal region of the injury epicenter than rostral region. Hence, we confined our study to the dorsal white matter, caudal to the injury epicenter. Myelination was found throughout the dorsal white matter and along the margins of the lesion zone of hUCB-treated group. An important change in the myelin was the presence of vacuoles as the myelin layers separated as revealed by electron microscopic studies (Fig. 3C). Many axons showed degeneration, and extracellular space increased (Fig. 3D). Many demyelinated axons appeared morphologically normal, although some showed axoplasmic organelle condensation, suggesting axonal degeneration. However, many healthy-appearing demyelinated axons, which were undergoing remyelination, were evident in the hUCB-treated rats (Fig. 3E). Scanning electronic microscopic studies reveal that the myelin around the axons and surrounding the growth cones of axons was damaged in injured spinal cords (Fig. 3F), whereas in the hUCB-treated groups, remyelination aided in the development and navigation of growth cones (Fig. 3G). Some axons were wrapped by thin myelin relative to their axonal diameters in hUCB-treated spinal cords, thereby suggesting remyelination. In conclusion, ultrastructural analysis indicated that demyelination occurred after contusive SCI with some of the demyelinated axons going through the process of remyelination after treatment with hUCB.
Figure 3. Remyelination due to intraspinal grafting of hUCB.
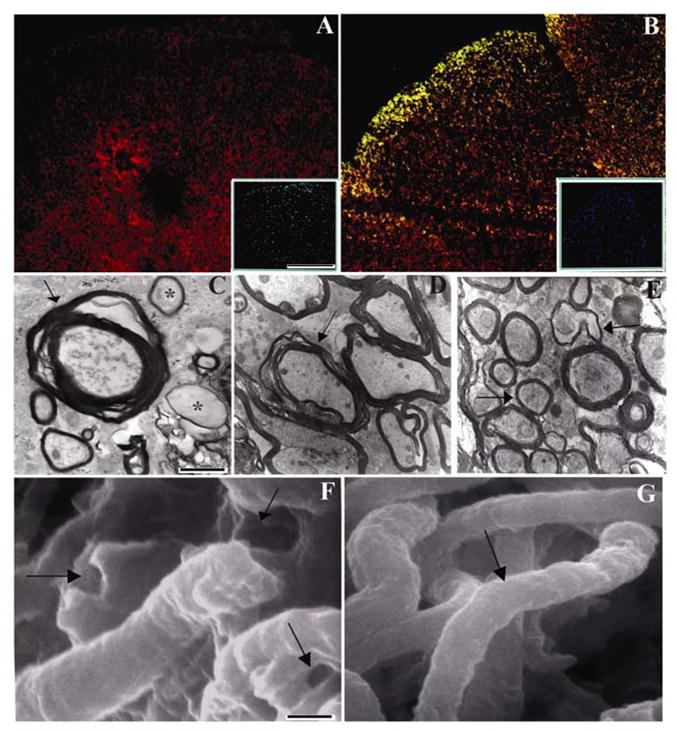
Section from injured rats showing loss of oligodendrocytes around the injury epicenter (A). The section is stained with Texas-Red conjugated APC antibody. (B) Greater preservation of oligodendrocytes in hUCB-treated sections. Merged image of section immunostained with Texas-Red conjugated APC antibody and FITC-conjugated CD44 antibody. For both A and B inset shows DAPI images. Transmission electron micrographs showing deformation of myelin sheath and axons in contused spinal cords (↑) (C & D). Myelin is thin and fragmented in many axons (*). In contrast, hUCB-treated sections showing normal myelin with several layers (E) (↑) indicates demyelinated axons undergoing remyelination. Scanning electron micrographs showing ruptured myelin (↑) in injured (F) and smooth myelin sheath (↑) in hUCB-treated (G) spinal cords. Magnification shown at 35000X for TEM and 15000X for SEM. (n ≥ 2).
Survival of differentiated oligodendrocytes and secretion of neurotrophic hormones in the spinal cord
Next, we addressed whether axonal remyelination is mediated by hUCB-derived oligodendrocytes or due to endogenous repair mechanisms of the injured spinal cord. Two weeks after transplantation, the number of cells that had survived and differentiated into several neural phenotypes after transplantation was evaluated by immunoreactivity. Oligodendrocyte survival after SCI was evaluated using APC immunolabeling. The number of APC-positive cells was greatly reduced after SCI. However, we observed good recovery of oligodendrocytes in the hUCB-treated group, in which APC-positive cells were distributed along the dorsal regions of the white matter.
Three weeks post-SCI, axons were present in differing degrees within all hUCB-transplanted spinal cords. This suggests that neuritogenesis at the lesion site can be enhanced by the presence of growth-promoting substrates. The extent of axon growth can be influenced by growth factor expression. Once we confirmed that most surviving hUCB were oligodendrocytes, we evaluated the secretion of neurotrophic hormones NT3 and BDNF and myelin components MBP and PLP in the spinal cord using DAB immunochemistry (Fig. 4). The increased expression of these two neurotrophic hormones and the synthesis of myelin components by hUCB establish the prominent role of stem cells in the remyelination of axons.
Figure 4. Stem cell-mediated secretion of neurotrophic factors and synthesis of myelin proteins in treated rats.
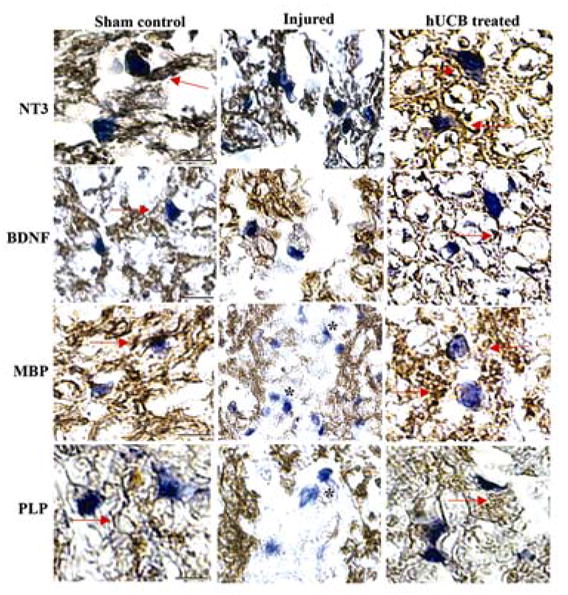
Immunohistochemical comparison of uninjured sham control, injured and hUCB-treated spinal cord sections was performed to analyze the secretion of neurotrophic hormones (NT3 and BDNF) and synthesis of myelin proteins (MBP and PLP). Paraffin sections from spinal cord blocks adjacent to the epicenter were probed with respective antibodies using DAB immunohistochemistry and counterstained with hematoxylin to stain the live nuclei and then photographed using bright-field microscope. Arrows indicate the stained portions with respective antibodies. Note the presence of demyelinated axons in the injured sections are indicated by *. Scale Bar = 200 μm. Results are from three independent sections caudal from the injury epicenter (n ≥ 3).
To determine the maturation state of the transplanted hUCB, cells were double immunostained with NT3, BDNF and a mature oligodendrocyte marker, APC. As shown in Figure 5, transplanted hUCB-differentiated oligodendrocytes expressed NT3 and BDNF respectively, apposing the longitudinal axons in the white matter, suggesting that some transplanted hUCB formed mature myelin (Fig 5A, B). Although APC has been reported previously to label Schwann cells in addition to oligodendrocytes after SCI (McTigue et al., 1998), we did not observe colabeling with APC and the Schwann cell marker. Quantitative analysis indicates that higher numbers of oligodendrocytes, which secrete NT3 and BDNF, were present in hUCB-treated spinal cords as compared to injured spinal cords (Fig. 5C, 5E). A significant proportion of NT3-secreting hUCB-derived oligodendrocytes (11.41 cells/section) (Fig. 5D) and BDNF-secreting hUCB-derived oligodendrocytes (9.66 cells/section (Fig. 5F) were observed in treated rats. The hUCB-derived oligodendrocytes constitute a significant proportion of oligodendrocytes apart from the endogenous population suggesting the role of hUCB-derived oligodendrocytes in the secretion of NT3 and BDNF.
Figure 5. Confocal scanning microscope images demonstrate the secretion of neurotrophic hormones in spinal cords of rats.
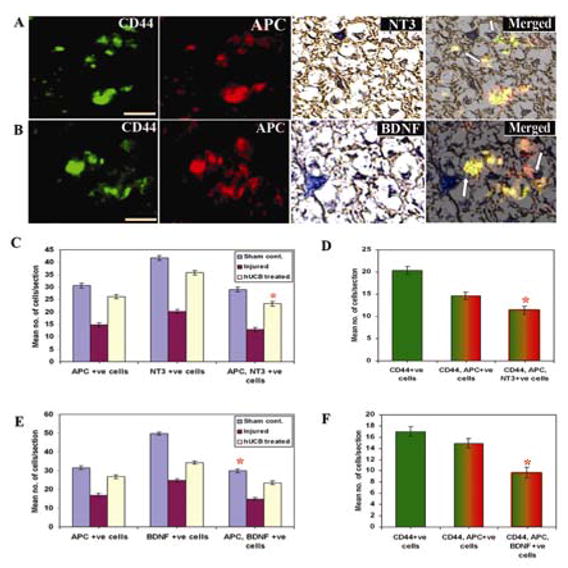
Cryosections from spinal cord blocks adjacent to the epicenter were processed for immunoflourescence studies as described in Materials and Methods. Sections were immunostained with FITC-conjugated CD44 and Texas-red conjugated APC antibodies. Further, they were DAB-stained with NT3 and BDNF antibodies. All the sections were stained with DAPI for showing nuclear localization. In hUCB-treated sections, remyelination was established by co-localization of APC with NT3 and BDNF (A & B). Arrows indicate NT3 and BDNF secreting hUCB-derived oligodendrocytes. Scale Bar = 200 μm. Quantitative estimation from sham control, injured and hUCB treated sections for NT3(C) and BDNF (E). Figs. D and F show quantitation of hUCB-derived oligodendrocytes secreting NT3 and BDNF respectively. Results are from three independent sections caudal from the injury epicenter (n ≥ 3). (Error bars indicate SEM. * Significant at p <0.05).
Similarly, we also evaluated the immunoreactivity of MBP and PLP proteins, which are constituent proteins of the myelin sheath. Co-localization studies with three antibodies established the role of hUCB-derived oligodendrocytes in the synthesis of MBP and PLP proteins (Fig. 6A, B). Quantitative analysis confirmed the presence of higher numbers of APC, MBP-positive cells (Fig. 6C) and APC, PLP-positive cells (Fig. 6E) in hUCB-treated spinal cords. Similar to the neurotrophic hormones, hUCB-derived oligodendrocyte synthesized MBP and PLP cells were 8.74 (Fig. 6D) and 10.74 (Fig. 6F) respectively, per each section analyzed.
Figure 6. Confocal scanning microscope images demonstrate the synthesis of myelin proteins in spinal cords of rats.
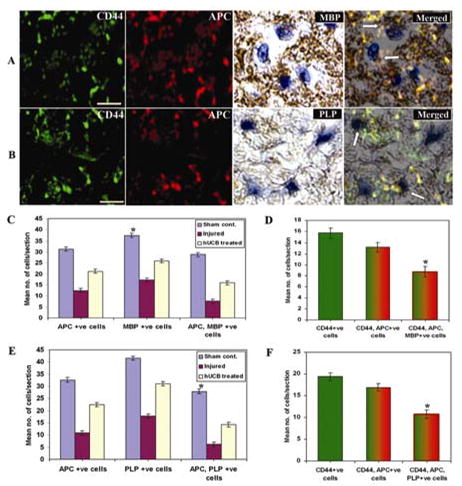
Cryosections from spinal cord blocks adjacent to the epicenter were processed for immunoflourescence studies as described in Methods. Sections were immunostained with FITC-conjugated CD44 and Texas-red conjugated APC antibodies. Further, they were DAB-stained with MBP and PLP antibodies. All the sections were stained with DAPI for showing nuclear localization. In hUCB-treated sections, remyelination was established by co-localization of APC with MBP and PLP (A & B). Arrows indicate MBP and PLP synthesizing hUCB-derived oligodendrocytes. Scale Bar = 200 μm. Quantitative estimation from sham control, injured and hUCB treated sections for MBP(C) and PLP (E). Figs. D and F show quantitation of hUCB-derived oligodendrocytes synthesizing MBP and PLP respectively. Results are from three independent sections caudal from the injury epicenter (n ≥ 3). (Error bars indicate SEM. * Significant at p <0.05).
Fluorescent in situ hybridization (FISH) analysis
To establish the loss of neurotrophic hormones and myelin genes after SCI, we determined the mRNA levels of neurotrophic hormones NT3, BDNF and MBP, PLP genes using FISH technique. The level of mRNA expression of all the above genes was decreased significantly in the injured sections, as compared with the corresponding segments of sham control rats (Fig. 7). Treatment of SCI rats with hUCB restored the transcription of all the genes of the present study. Co-immunofluorescence studies with hUCB-specific antibodies illustrates that hUCB are involved in the synthesis of neurotrophic hormones and myelination genes. This would augment myelin formation by the oligodendrocytes and improve locomotor function after SCI. These results support the ultra structural studies of remyelination by hUCB.
Figure 7. Pattern of mRNA expression of neurotrophic factors and myelin proteins in spinal cords of rats.
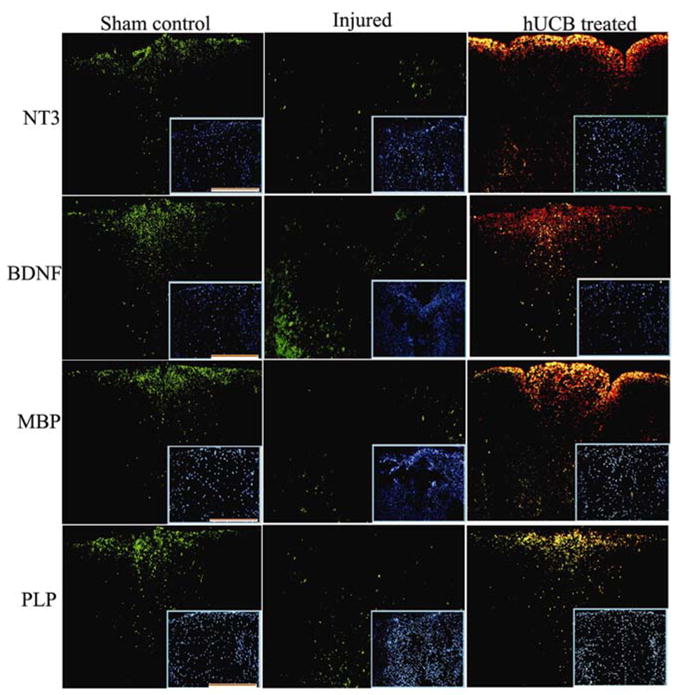
FISH analysis of neurotrophic factors and myelin proteins depicting sham control, injured, and hUCB-treated samples in the dorsal white matter region. Sequential serial sections hybridized with FITC-conjugated oligonucleotide antisense probes for NT3, BDNF, MBP and PLP were photographed using confocal microscope as described in Methods section. hUCB treated sections show colocalization (yellow) with Texas-red conjugated CD44 antibody, specific for hUCB. Inset shows representative Hoechst -33342 stained images. Scale Bar = 100 μm. Results are from three independent sections caudal from the injury epicenter (n ≥ 3).
Western blot and RT-PCR analyses
To further confirm the secretion of neurotrophic hormones and the synthesis of myelin proteins by hUCB-derived oligodendrocytes at the transcription and translation levels, we used RT-PCR and western blot analyses. The change in the mRNA levels after SCI was determined using standardized RT-PCR analysis. There was significant upregulation of NT3, BDNF, MBP and PLP genes (Fig. 8A, B) in hUCB-treated rats as compared to injured rats. Similar results were obtained at the protein level (Fig. 8C, D) also. Western blot analysis indicated reduced bands of neurotrophic factors in the injured cords in comparison to the hUCB-treated spinal cords. There were no significant differences observed in control and sham control rats. These data were consistent with the immunohistochemistry results and suggested that NT3 and BDNF enhanced the survival, differentiation, and myelination of hUCB-derived oligodendrocytes in vivo.
Figure 8. Expression of neurotrophic factors and myelin proteins in injured and treated spinal cords of rats.
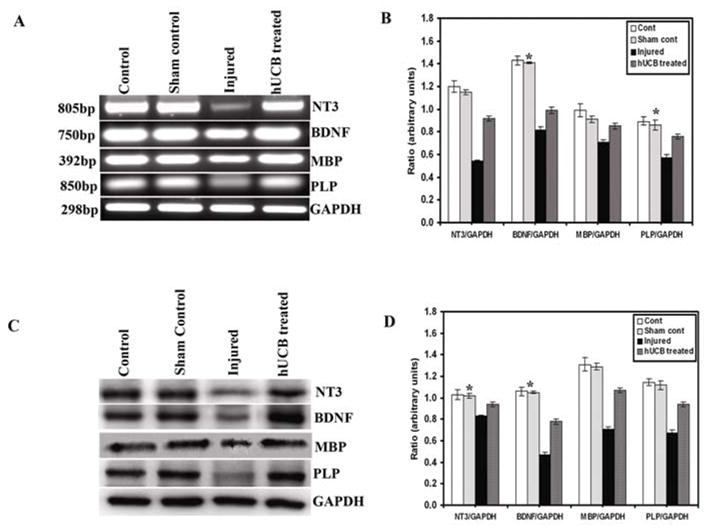
RT-PCR analysis of neurotrophic factors and myelin proteins depicting control, sham control, injured, and hUCB-treated samples (A). House keeping gene GAPDH was used as loading control. Quantitative data showing pixel density of RT-PCR bands (n ≥ 2). (B). Western blot analysis of changes in the levels of neurotrophic factors and myelin proteins following spinal cord injury and hUCB treatment (C) and their corresponding quantitative analysis of bands using Image Pro software (D). There was no significant difference between control and sham controls. GAPDH was used as loading control. This figure shows representative gels and blots obtained in one experiment that was repeated three times with similar results (n ≥ 3). (Error bars indicate SEM. * Significant at p <0.05).
Locomotor functional recovery after transplantation of hUCB
Finally, we checked whether the transplantation of hUCB, which helped in remyelination of axons, could restore hind limb locomotor function after SCI. Hind limb locomotor performance was tested in all rats using the BBB open-field procedure described in Materials and Methods. All animals were subjected to BBB testing at 1, 7, 14 and 21 days post-SCI and before transplantation. Animals with a low score and equally dysfunctional hind limbs were selected for transplantation with hUCB. Performance in open field locomotion was enhanced by transplantation of hUCB. In contrast to the inability of the injured group to support weight with their hind limbs, rats transplanted with hUCB demonstrated partial weight-supported ambulation (Fig. 9). A statistical difference in BBB scores was achieved 2 weeks after transplantation. The sham-operated group showed almost normal function throughout the observation period. The injured group had BBB scores of 0 for both legs at 1 day post-SCI, which then gradually increased to final scores of 6.54 ± 0.21 at 3 weeks post-SCI (Fig. 9A). The hUCB-transplanted group showed significantly improved hind limb performance at 2 weeks post-transplantation as compared to the injured groups (p < 0.05), with BBB scores of 15.78 ± 0.15. At 2 weeks post-transplantation, the hUCB-treated group showed consistent plantar stepping, forelimb-hind limb coordination and no toe-drag during walking (Fig. 9B). In contrast, the injured group exhibited no consistent plantar stepping, no toe clearance, and dragging of body weight. Thus, the hUCB-transplanted group showed significantly greater functional recovery than the injured group. However, cyclosporine-treated rats showed some improvement over injured rats (BBB average score of 9.15 ± 0.31).
Figure 9. Hind limb functional recovery after spinal cord contusion.
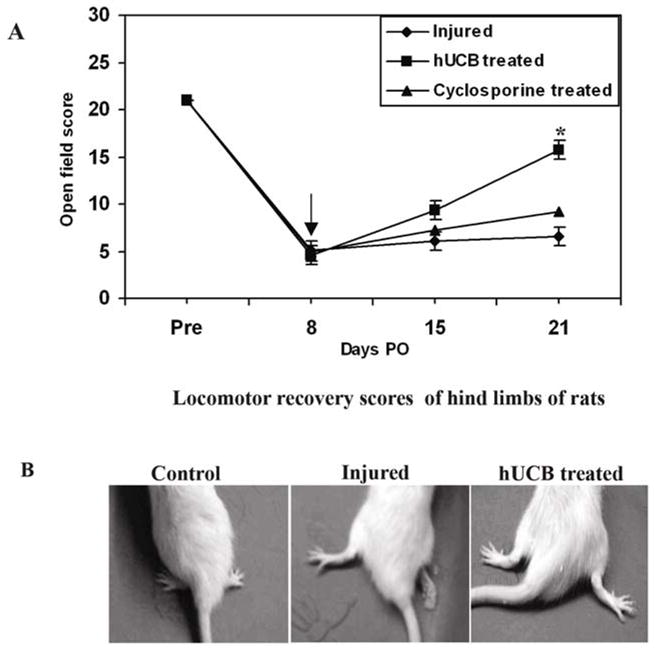
The BBB locomotor rating scale showed functional recovery of all hUCB-treated animals until day 21 post-SCI. The score indicating a gait characterized by no hind limb weight bearing and no coordinated hind limb movement is 0, whereas the score indicating a gait characterized by partial hind limb weight bearing and partial hind limb coordination is 13. The locomotor recovery scores averaged across hind limbs for weekly testing of rats with moderate contusion using NYU impactor (A). Each point represents the highest locomotor score achieved each day. Error bars indicate SEM ((n ≥ 5 per group). Video images of hind limb movements in rats (B). Spinal cord injury with NYU impactor resulted in paraplegia of hind limbs of rats when compared to normal rats. The hind limbs are internally rotated and the tail is not supporting the body weight. In hUCB-treated rats, hind limbs are recovered from the injury and are externally rotated. Pre = pre-operative; PO = post-operative. ↓ indicates transplantation point. (Error bars indicate SEM. * Significant at p <0.05).
DISCUSSION
Demyelination results in the loss of motor functions subsequent to CNS injury. Restoring myelin through the transplantation of myelin-producing cells may offer a logical approach to recover optimal neurological functions. In addition to replacing lost cells, transplantation appears to modify the host environment to promote endogenous remyelination. Thus, remyelination appears to be one of the most feasible restoration strategies (McDonald and Belegu, 2006). It has been reported that stem cells transplanted into the injured lesion were able to differentiate into oligodendrocytes and astrocytes, integrate into axonal pathways, and regenerate and remyelinate the injured axons (Ishii et al., 2001; McDonald and Howard, 2002; Murakami et al., 2003; Vroemen et al., 2003). Human cord blood stem cells are more pluripotent and genetically flexible than bone marrow neural stem cells and are more easily obtained. Various cell types within the graft may promote neural repair by delivering neural protection and secretion of neurotrophic factors (Sanberg et al., 2005). Cord blood stem cells have been implicated in neurological and functional improvements in injured spinal cord rats (Kuh et al., 2005). Previous studies by Saporta et al., (2003) have shown that hUCB are beneficial in reversing the behavioral effects of spinal cord injury, even when infused 5 days after injury. Hence, we hypothesized that 7 day post-injury would be the peak time for grafting hUCB and evaluating their remyelination potential of axons and functional improvement of hind limbs after SCI. Since we used the neurogenic medium that promotes differentiation of a mixed culture of neuronal population in the present study, trans-differentiation of stem cells to neurons, oligodendrocytes and astrocytes was observed in vitro, neurons being the major population. In contrast, in the injured spinal cord, stem cells differentiated mostly to oligodendrocytes than neurons. This is not surprising because, in the injured spinal cord, stem cells probably are more involved in the regeneration of lost oligodendrocytes in the injured areas and also in the remyelination of injured fiber tracts. However, the molecular mechanisms of trans-differentiation of stem cells and their survival in vivo for longer periods are being studied.
Destruction of the myelinated long tracts of the spinal cord is believed to be a critical factor in determining the extent of functional impairment (Banik et al., 1980). Balentine (1978) observed vesicular degeneration and intramyelinic vacuolization after SCI in rats. We have shown that many demyelinated, but otherwise intact, axons exist in the spinal cord after contusive SCI and that the demyelinated axons survive for at least 3 weeks after the injury. These results are consistent with previous histological studies showing that there is chronic demyelination of axons after traumatic SCI in experimental animals (Balentine, 1978; Banik et al., 1980; Bambakidis et al., 2004; Cao et al., 2005a; Cao et al., 2005b; Totoiu et al., 2005). Also, these results are in conformity with Bresnahan (1978), who observed ultrastructural details of many swollen axons, dark axons, empty myelin sheaths and myelin sheath with debris inside in spinal cords of SCI monkeys after three weeks.
Since massive oligodendrocyte death attributable to apoptosis occurs acutely after SCI, it is likely that endogenous oligodendrocyte precursor cells are unable to completely restore lost myelin in the injured spinal cord. Increasing the number of cells with the ability to differentiate into oligodendrocytes by transplantation may be a very important method for replacing lost myelin. In this study, we observed reduced levels of MBP and PLP, both at the mRNA and proteins levels in the injured spinal cord, as revealed by FISH, RT-PCR and Western blot analyses. These results are in agreement with Wrathal et al., (1998) and Ray et al., (2003). We observed that many transplanted-hUCB differentiated into oligodendrocytes as compared to astrocytes or neurons. Both oligodendrocytes and myelinated axons were elevated within the hUCB-transplanted group. These data suggest that hUCB differentiated to oligodendrocytes, and the neurotrophins (NT3 and BDNF) secreted by these oligodendrocytes enhanced myelinogenesis. Ultrastructural analysis showed that the hUCB formed morphologically normal-appearing sheaths around the axons in the injured areas. This is consistent with the rapidity of observed locomotor improvement (2 weeks) and the observation that most hUCB-derived cells were oligodendrocytes, many immunoreactive for myelin basic protein and proteolipid protein. Transplantation of oligodendrocytes or oligodendrocyte progenitors into demyelinating chemical lesions can be associated with remyelination and improved axonal conduction (Waxman, 1992). Other possibilities include the reduction of delayed oligodendrocyte death, or the enhancement of host axonal regeneration. We suggest that this enhancement of locomotion underlies the accelerated axonal growth and, hence, functional recovery.
Traumatic spinal cord injury results in loss of tissue, including important myelinated fiber tracts carrying descending motor and ascending sensory information. Reduced myelination could result from loss of myelinating cells and/or reduced myelin synthesis by surviving oligodendrocytes. Both NT3 and BDNF regulate neuronal development and axonal regeneration (Xu et al., 1995). They are also important mediators of myelination. NT3 enhances the survival and proliferation of OPCs in vitro (Barres et al., 1994a; Kumar et al., 1998; Yan et al., 2000; Franklin et al., 2001) and in vivo (Barres et al., 1994b). Myelination by oligodendrocytes is also enhanced by NT3 in both cultures of neurons and the injured CNS (McTigue et al., 1998; Yan et al., 2000; Jean et al., 2003; Bambakidis et al., 2004). BDNF is important for myelin formation in peripheral nerve during development because inactivation of BDNF signaling by deleting trkB receptors causes myelin deficits both in vivo and in vitro (Barres et al., 1993; Cosgaya et al., 2002). The augmented myelination due to neurotrophic hormones NT3 and BDNF and myelin genes MBP and PLP may have been caused by direct action of the hUCB-transformed oligodendrocytes or their precursors.
Although the suggestion has been made that mature oligodendrocytes can divide and contribute to remyelination (Wood et al., 1991), the majority of research has focused on and supported the hypothesis that endogenous oligodendrocyte progenitors are present within the CNS, which can differentiate into mature cells capable of myelinating bare axons (Norton, 1996). Axons of the mature mammalian CNS have an intrinsic capacity to regenerate, but they can do so for an extended distance when supported by a matrix that arises spontaneously at the injury site (West et al., 2001). However, co-localization studies suggest that the source of the new oligodendrocytes in the injured spinal cord was a population of hUCB, and that these oligodendrocytes secrete NT3 and BDNF. These NT3 and BDNF may, in turn, enhance proliferation and survival of oligodendrocyte precursors. A more in-depth analysis of the formation of new myelin is needed to examine this hypothesis. Another possibility is that proliferative oligodendrocyte progenitors are known to be present in the adult CNS. Also, precursor cells in the subcortical white matter differentiated in response to chemical demyelination and subsequently remyelinated the lesion area (Gensert et al., 1997). Growth factors can increase the proliferation and survival of oligodendrocyte progenitors (Barres et al., 1993; Barres et al., 1994b; McMorris et al., 1996). The present study reveals that the presence of NT3 and BDNF in the injured spinal cord induced the formation of new oligodendrocytes. Furthermore, hUCB producing these neurotrophins promoted neuritogenesis and myelination of the in-growing axons.
These results suggest that umbilical cord blood stem cells are beneficial in reversing the behavioral effects of spinal cord injury, even when infused 7 days post-SCI. Further, hUCB-derived cells were observed in injured areas, but not in non-injured areas of rat spinal cords. Behavioral recovery similar in magnitude to that shown here has previously been shown in acute injury models (Saporta et al., 2003). In the present study, we maintained a cyclosporine-treated group as another control to check the potential of hUCB in promoting functional recovery in SCI rats. It is apparent that the cyclosporine may have some synergistic effect with hUCB in improving significant functional recovery of hUCB-treated rats. The results are consistent with the hypothesis that hUCB-derived stem cells migrate to and participate in the healing of neurological defects caused by traumatic insult. We continue to study the long-term survival and effects of hUCB on remyelination.
Acknowledgments
We thank Noorjehan Ali and Robert Caughey for their technical assistance. We thank Shellee Abraham for manuscript preparation and Diana Meister and Sushma Jasti for manuscript review.
Footnotes
This research was supported by National Cancer Institute grant CA 75557, CA 92393, CA 95058, CA 116708 and N.I.N.D.S. NS47699, NS57529 and Caterpillar, Inc., OSF Saint Francis, Inc., Peoria, IL (to J.S.R.).
References
- BALENTINE JD. Pathology of experimental spinal cord trauma II. Ultrastructure of axons and myelin. Lab Investigation. 1978;39:254–266. [PubMed] [Google Scholar]
- BAMBAKIDIS NC, MILLER RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- BANIK NL, POWERS JM, HOGAN EL. The effects of spinal cord trauma on myelin. J Neuropathol Exp Neurol. 1980;39:232–244. doi: 10.1097/00005072-198005000-00002. [DOI] [PubMed] [Google Scholar]
- BARRES BA, RAFF MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994a;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- BARRES BA, RAFF MC, GAESE F, BARTKE I, DECHANT G, BARDE YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994b;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- BARRES BA, SCHMID R, SENDNTER M, RAFF MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- BASSO M, BEATTIE MS, BRESNAHAN JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- BASSO DM, BEATTIE MS, BRESNAHAN JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996a;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- BASSO DM, BEATTIE MS, BRESNAHAN JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996b;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- BLIGHT AR. Miracles and molecules--progress in spinal cord repair. Nat Neurosci. 2002;5(Suppl):1051–1054. doi: 10.1038/nn939. [DOI] [PubMed] [Google Scholar]
- BRESNAHAN JC. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta) J Neurol Sci. 1978;37:59–82. doi: 10.1016/0022-510x(78)90228-9. [DOI] [PubMed] [Google Scholar]
- BRUSTLE O, JONES KN, LEARISH RD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- BUNGE RP, PUCKETT WR, BECERRA JL, MARCILLO A, QUENCER RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- CAO Q, XU XM, DEVRIES WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005a;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO Q, ZHANG YP, IANNOTTI C, et al. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005b;191:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- CAO QL, ZHANG YP, HOWARD RM, WALTERS WM, TSOULFAS P, WHITTEMORE SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- COSGAYA JM, CHAN JR, SHOOTER EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- ENZMANN GU, BENTON RL, TALBOTT JF, CAO Q, WHITTEMORE SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23:479–495. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- FRANKLIN RJ, GILSON JM, BLAKEMORE WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50:337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- FRANKLIN RJ, HINKS GL, WOODRUFF RH, O'LEARY MT. What roles do growth factors play in CNS remyelination? Prog Brain Res. 2001;132:185–193. doi: 10.1016/S0079-6123(01)32075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENSERT JM, GOLDMAN JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- GRUNER JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- GUEST JD, HIESTER ED, BUNGE RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- HILL CE, PROSCHEL C, NOBLE M, et al. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- HOFSTETTER CP, HOLMSTROM NA, LILJA JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- ISHII K, TODA M, NAKAI Y, et al. Increase of oligodendrocyte progenitor cells after spinal cord injury. J Neurosci Res. 2001;65:500–507. doi: 10.1002/jnr.1180. [DOI] [PubMed] [Google Scholar]
- JACKSON AB, DIJKERS M, DEVIVO MJ, POCZATEK RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil. 2004;85:1740–1748. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- JEAN I, LAVIALLE C, BARTHELAIX-POUPLARD A, FRESSINAUD C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 2003;972:110–118. doi: 10.1016/s0006-8993(03)02510-1. [DOI] [PubMed] [Google Scholar]
- KEIRSTEAD HS, BEN-HUR T, ROGISTER B, O'LEARY MT, DUBOIS-DALCQ M, BLAKEMORE WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19:7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEIRSTEAD HS, NISTOR G, BERNAL G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUH SU, CHO YE, YOON DH, KIM KN, HA Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien) 2005;147:985–992. doi: 10.1007/s00701-005-0538-y. [DOI] [PubMed] [Google Scholar]
- KUMAR S, KAHN MA, DINH L, DE VJ. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J Neurosci Res. 1998;54:754–765. doi: 10.1002/(SICI)1097-4547(19981215)54:6<754::AID-JNR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- LAEMMLI UK, FAVRE M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- LEE SM, YUNE TY, KIM SJ, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- LIU S, QU Y, STEWART TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU XZ, XU XM, HU R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD JW, BELEGU V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- MCDONALD JW, HOWARD MJ. Repairing the damaged spinal cord: a summary of our early success with embryonic stem cell transplantation and remyelination. Prog Brain Res. 2002;137:299–309. doi: 10.1016/s0079-6123(02)37023-7. [DOI] [PubMed] [Google Scholar]
- MCDONALD JW, LIU XZ, QU Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- MCMORRIS FA, MCKINNON RD. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6:313–329. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- MCTIGUE DM, HORNER PJ, STOKES BT, GAGE FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAKAMI T, FUJIMOTO Y, YASUNAGA Y, et al. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974:17–24. doi: 10.1016/s0006-8993(03)02539-3. [DOI] [PubMed] [Google Scholar]
- NORTON WT. Do oligodendrocytes divide? Neurochem Res. 1996;21:495–503. doi: 10.1007/BF02527715. [DOI] [PubMed] [Google Scholar]
- OGAWA Y, SAWAMOTO K, MIYATA T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- RAY SK, MATZELLE DD, SRIBNICK EA, GUYTON MK, WINGRAVE JM, BANIK NL. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J Che Neuroanat. 2003;26:119–124. doi: 10.1016/s0891-0618(03)00044-9. [DOI] [PubMed] [Google Scholar]
- SANBERG PR, WILLING AE, GARBUZOVA-DAVIS S, et al. Umbilical cord blood-derived stem cells and brain repair. Ann N Y Acad Sci. 2005;1049:67–83. 67–83. doi: 10.1196/annals.1334.008. [DOI] [PubMed] [Google Scholar]
- SAPORTA S, KIM JJ, WILLING AE, FU ES, DAVIS CD, SANBERG PR. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. J Hematother Stem Cell Res. 2003;12:271–278. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- TOTOIU MO, KEIRSTEAD HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- VROEMEN M, AIGNER L, WINKLER J, WEIDNER N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- WAXMAN SG. Demyelination in spinal cord injury. J Neurol Sci. 1989;91:1–14. doi: 10.1016/0022-510x(89)90072-5. [DOI] [PubMed] [Google Scholar]
- WAXMAN SG. Demyelination in spinal cord injury and multiple sclerosis: what can we do to enhance functional recovery? J Neurotrauma. 1992;9:S105–S117. [PubMed] [Google Scholar]
- WEST NR, LEBLANC V, COLLINS GH. Support of axonal regrowth by endogenous mechanisms following spinal cord injury in adult rats. Neuropathology. 2001;21:188–202. doi: 10.1046/j.1440-1789.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- WOOD PM, BUNGE RP. The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- WOODRUFF RH, FRANKLIN RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25:216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- WRATHALL JR, LI W, HUDSON LD. Myelin gene expression after experimental contusive spinal cord injury. J Neurosci. 1998;18:8780–8793. doi: 10.1523/JNEUROSCI.18-21-08780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, FAN G, CHEN S, WU Y, XU XM, HSU CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- XU XM, GUENARD V, KLEITMAN N, AEBISCHER P, BUNGE MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- YAN H, WOOD PM. NT-3 weakly stimulates proliferation of adult rat O1(−)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro. J Neurosci Res. 2000;62:329–335. doi: 10.1002/1097-4547(20001101)62:3<329::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]


