Abstract
Postoperative peritoneal adhesions can cause pelvic pain, infertility, and potentially lethal bowel obstruction. We have designed and synthesized injectable hydrogels that are formed by mixing hydrazide-modified hyaluronic acid (HA) with aldehyde-modified versions of cellulose derivatives such as carboxymethylcellulose (CMC), hydroxypropylmethyl cellulose (HPMC), and methyl cellulose (MC). Gelation of these hydrogels occurred in less than 1 min, and had higher shear moduli than that of HA-HA gel (HAX). Hydrogels degraded in the presence of hyaluronidase in vitro, with HA-MC and HA-HPMC degrading more slowly than HAX and HA-CMC. The aldehyde-modified cellulose derivatives showed dose-dependent mild-to-moderate cytotoxicity to mesothelial cells and macrophages in vitro, but all were biocompatible in the murine peritoneum, causing no adhesions for 3 weeks. All the cellulose-derived gels showed efficacy in reducing the area of adhesion formation in a rabbit sidewall defect-bowel abrasion model.
Keywords: Post-operative adhesion, Hydrogel, Hyaluronic acid, Carboxymethyl cellulose, Methyl cellulose, Hydroxylpropylmethyl cellulose, Rabbit sidewall defect-cecum abrasion model
1. Introduction
Postoperative peritoneal adhesions can cause pelvic pain, bowel obstruction and infertility [1]. A number of membranous barrier devices have been developed commercially, with varying degrees of success [1]. Gels that form in situ by simple mixing of two different polymers are appealing for this purpose as they are easy to handle at room temperature and do not require a radiant light source or toxic chemical cross-linkers [2–8]. They are generally easier to apply over the injured areas, especially if those are difficult to cover with simple sheets, or if the area is very large.
Hyaluronic acid (HA) is a good candidate material for such an application [2–4,6–8], because HA is well-known to be biocompatible in the peritoneum [1], and chemically cross-linked HA hydrogels (HAX) can prevent peritoneal adhesions in a rabbit model [6]. Hyaluronic acid is degraded by endogenous hyaluronidase [9] and by hydroxyl radicals [10,11]. We have shown that the HAX gels degraded substantially within a week, leaving a significantly reduced amount of gels in the peritoneum [6]. Depending on the severity or area of injury, however, it may be beneficial to design hydrogels that can last longer in the peritoneum. We hypothesized that hybridization of HA with other biocompatible polysaccharides that are not degraded enzymatically in humans could slow degradation while preserving HA’s excellent biocompatibility. Cellulose derivatives such as carboxymethylcellulose (CMC) [12–15] and hydroxypropylmethyl cellulose (HPMC) [14] are also known to have good biocompatibility in the peritoneum. The biocompatibility of methyl cellulose (MC) in the peritoneum is not known, but the mixture of MC and HA has been reported to be biocompatible in intrathecal injection [16].
Here we synthesized in-situ cross-linking injectable hydrogels composed of HA and cellulose derivatives such as CMC, HPMC and MC. We characterized these hydrogels in vitro, studied their cytotoxicity in cell culture, and their biocompatibility in the murine peritoneum. Finally, we studied their effectiveness in preventing peritoneal adhesions in a rabbit model.
2. Materials and methods
2.1. Synthesis of the polymers and hydrogels
Reagents
HA (Mw = 490 kDa and 1.4 MDa) was purchased from Genzyme (Cambridge, MA). CMC (Product No: C4888), HPMC (Product No: H9262), MC (Product No: M0387), hyaluronidase, adipic dihydrazide (ADH), 1-ethyl-3-[3-(dimethylamino)propyl]-carbodiimide (EDC), hydroxybenzotriazole (HOBt), sodium periodate, ethylene glycol, tert-butyl carbazate (t-BC), sodium bicarbonate, sodium chloride, and acetic acid were purchased from Sigma-Aldrich (St.Louis, MO). Pullulans purchased from Showa Denko (Japan) were used as standards for gel permeation chromatography (GPC).
2.1.1. Preparation of aldehyde polymers
1.4 MDa HA, CMC, HPMC, and MC were modified to aldehyde forms (HA-CHO, CMC-CHO, HPMC-CHO, and MC-CHO respectively), as shown in Fig.1. The protocol was similar as that previously used for HA-CHO [6–8]. Briefly, 1.5 g of HA, CMC, HPMC, or MC was dissolved in 150 ml water, then 802 mg sodium periodate were added, and stirred for 2 h. 200 μl ethylene glycol was added to stop the reaction, and the mixture was dialyzed immediately against water. The purified product was freeze dried and kept at 4 °C.
Fig. 1.
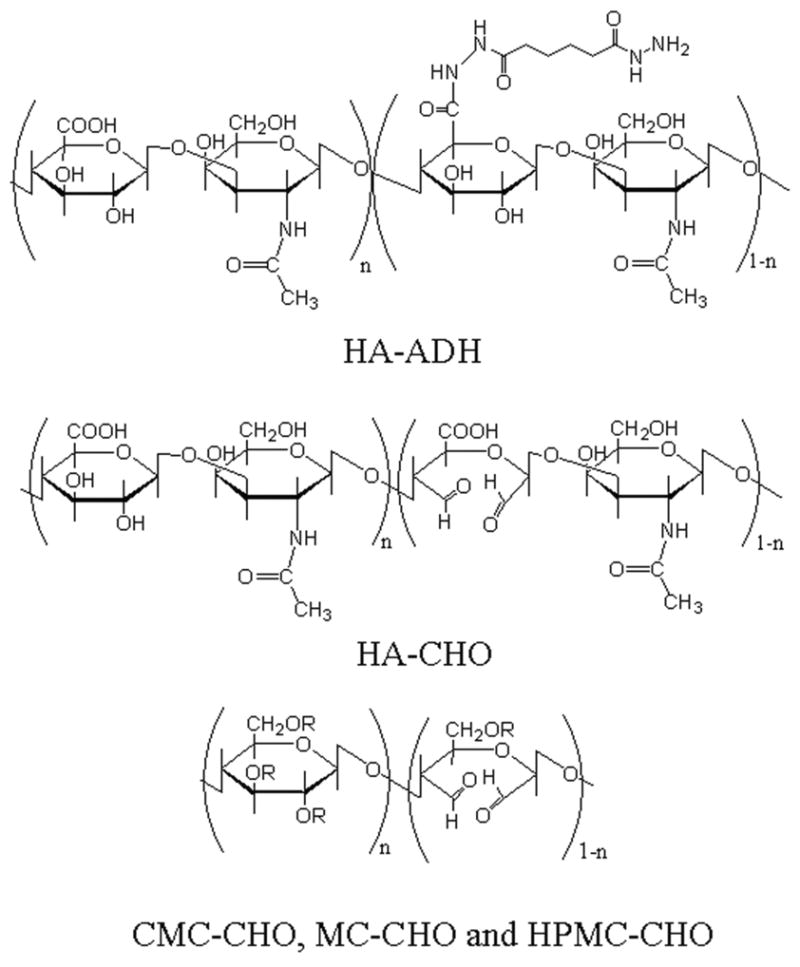
Chemical structure of polymers. Regarding the cellulose derivatives: for CMC-CHO: R is H or CH2COOH. For HPMC-CHO: R is H or (CH2CH(CH3)O)nH. For MC-CHO: R is H or CH3.
2.1.2. Preparation of hydrazide polymer
490 kDa HA was modified into adipic dihydrazide HA (HA-ADH) using a previously described method [6–8].
2.1.3. Preparation of disk hydrogels
2 wt% HA-ADH in PBS and 2 wt% HA-CHO, CMC-CHO, HPMC-CHO, or MC-CHO in PBS were injected into a rubber mold sandwiched between two glass slides using a double-barreled syringe (Baxter, Deerfield, IL). The diameter and the thickness of the prepared hydrogel were 1.2 cm and 3.5 mm, respectively. Below, these cross-linked hydrogels are termed HAX, HA-CMC, HA-HPMC, and HA-MC, respectively.
2.2. Characterization of polymers and hydrogels
2.2.1 Characterization of polymers
1H-NMR (Varian: Unity 300 spectrophotometer, Palo Alto, CA) spectroscopy of 10mg/ml solutions of HA-ADH, HA-CHO, CMC-CHO, HPMC-CHO and MC-CHO in D2O was performed. The aldehyde polymers (HA-CHO, CMC-CHO, HPMC-CHO, and MC-CHO) were analyzed after reacting with t-BC as described in [8]. 1H-NMR spectra of the polymers reacted to t-BC were measured in D2O.
The molecular weights of the polysaccharides were measured using GPC. The column was Ultrahydrogel Linear (Waters, Milford, MA), and refractive index (RI) was detected by refractometer (Wyatt Technology: OPTILAB DSP, Santa Barbara, CA) Mobile phase was the mixture of 0.05 M sodium and 0.2 M sodium chloride (pH = 6.7), and its flow rate was 0.8 ml/min. Pullulans (Shodex, Pullulan Standards P5-P800, Japan) were used as molecular weight standards.
2.2.2. Characterization of hydrogels
Gelation time was measured by the following protocol. One hundred microliters of aqueous HA-CHO, CMC-CHO, HPMC-CHO, or MC-CHO solution were added to 100 μl of aqueous HA-ADH solution which was mixed with a magnetic stir bar on a petri dish at 155 rpm using a hot plate/stirrer (Corning: Model PC-320, Corning, NY). The gelation time was the time until the mixture became a globule; it was measured 4 times per sample.
Shear moduli of the prepared disk gel were measured with a rheometer (TA Instruments: AR1000, New Castle, Delaware). The disk gels were immersed in PBS for 5 days and allowed to swell to equilibrium. Creep and relaxation tests were done at different shear stresses. Shear was applied for 3 min, followed by 3 min relaxation. The strain values reached constancy during the creep tests, and then returned to zero during the relaxation tests in each measurement. Shear modulus, G, was calculated from the slope of the linear relationship between stress and strain. R2 values of fitted lines between stress and strain were above 0.95.
The time course of swelling of the gel disks was measured gravimetrically in PBS at 37 ºC. The weight of hydrogel after gelation, Ws, was measured after immersion in PBS for 5 days. The swelling ratio, Q, of Ws to the initial weight of hydrogel right after the gelation, Wi, was calculated as Q= Ws/Wi.
Degradation kinetics was measured as follows: Four hydrogel discs were incubated in 10 unit/ml hyaluronidase in PBS at 37 °C. At each time point, the gel disks were weighed, and the hyaluronidase solution was replaced. Measurements were made over 14 days. The ratio of the volume of hydrogels at each time point to the initial volume was determined (volume of the hydrogel (%)).
2.3 Cytotoxicity Assay
In vitro cell viability in the presence of HA-CHO, CMC-CHO, MC-CHO, and HPMC-CHO were investigated by the MTT assay (Promega, Madison, WI) using a human mesothelial cell line (ATCC: CRL-9444, Manassas, VA) and macrophage cell line J774.A1 (ATCC: TIB-67TM).
Mesothelial cells were grown and maintained in a complete growth medium (GIBCO: Medium199 with Earle's BSS, 0.75 mM L-glutamine and 1.25 g/L sodium bicarbonate supplemented with with 3.3 nM epidermal growth factor, 400 nM hydrocortisone, 870 nM insulin, 20 mM HEPES and 10% fetal bovine serum) at 37ºC in 5% CO2. Macrophages were grown and maintained in DMEM media (GIBCO: DMEM Cat # 10569–010 with 10% fetal bovine serum). 5×104 cells were placed in each well of a 24-well plate, and incubated at 37 ºC in 5% CO2 overnight, then media were replaced with media containing different concentration of HA-B, CMC-B, HPMC-B, and MC-B. On the third day after adding those materials in the case of mesothelial cells, or the second day in the case of J774.A1cells, MTT assays were performed. One hundred μl of tetrazolium salt solution was added into each well and incubated at 37 °C for 4 h. The purple formazan produced by active mitochondria was solubilized using 1 ml detergent solution and then read at 570 nm by plate reader (Molecular Devices: SpectraMax 384, Union City, CA). The absorbance values were normalized to wells in which cells were not treated with polymers.
2.4. In vivo experiments
All the animals were cared for in compliance with protocols approved by the Animal Care and Use Committee at the Massachusetts Institute of Technology, and the Principles of Laboratory Animal Care (NIH publication #85–23, revised 1985).
2.4.1. Injections of hydrogels into mouse peritoneum
SV129 mice weighing 25g were purchased from Taconic (Hudson, NY), and housed in groups in a 6 AM-6 PM light-dark cycle.
The polymers were sterilized by UV irradiation for 2 hours, then dissolved in saline at 2 wt% concentration. Anesthesia was induced with 50 mg/kg ketamine and 10 mg/kg xylazine, and a 5 mm skin incision was made, revealing the translucent abdominal wall. A 24 gauge catheter (Terumo: Surflash I.V. Catheter, Japan) was placed through the abdominal wall, and 0.3 ml of air was insufflated to confirm positioning. The catheter was then advanced 1 cm, and 0.5 ml aldehyde polysaccharide (HA-CHO, CMC-CHO, HPMC-CHO, or MC-CHO) and 0.5 ml HA-ADH were injected using a dual syringe applicator (Baxter: Deerfield, IL).
The mice were sacrificed after 4 days, 1 week, 2 weeks and 3 weeks after the injections, and the presence of residue and adhesions were evaluated. The dissector was blinded as to which treatment individual mice had received. Abdominal contents were sampled as needed were sampled, fixed in 10% formalin, and processed for histology (hematoxylin-eosin stained slides) using standard techniques.
2.4.2. Evaluation of peritoneal adhesion-preventing effect by a rabbit sidewall defect-bowel abrasion model
Peritoneal adhesions were induced as described [6]. Female albino rabbits (Oryctolagus cuniculus; New Zealand White, Covance, Hazleton, PA) (3 ± 0.5 kg) were anesthetized using ketamine (35 mg/kg i.m.) and xylazine (5 mg/kg i.m.); maintenance was achieved using 1–3% isoflurane in balance oxygen. A 10 cm long midline incision was made along the linea alba, and the peritoneum was opened. Peritoneal adhesions were induced by making a 3 × 4 cm defect on the right lateral abdominal wall and abrading seven haustra of the cecum until a bleeding surface was obtained.
Four animals were assigned randomly to each experimental group: (i) saline; (ii) covering the excised abdominal wall and abraded cecal surface with 10 ml of cross-linked HA-CMC, HA-HPMC, or HA-MC. Prior to application, the materials were sterilized by germicidal UV illumination for 2 hours and dissolved in sterile saline. The gel precursor solutions (5 ml of HA-ADH (20 mg/ml) and 5 ml of CMC-CHO, HPMC-CHO or MC-CHO (20 mg/ml)) were placed in separate sterile 10 ml syringes, which were connected to a dual syringe applicator, and co-extruded through a 15 gauge needle. The liquid precursors started to gel instantly, conforming to the shape of the target area.
Post-surgical animal care was delivered as described previously [6]. One week after the procedure, animals were euthanized with sodium pentobarbital 100 mg/kg IV. Adhesions were scored using a reported method [6]: Score 0 = no adhesion, score 1 = tissue adherence that would separate with gravity, score 2 = tissue adherence separable by blunt dissection, score 3 = adhesion requiring sharp dissection. The area of adhesions with scores of 2 and 3 were also measured. Tissues of interest were sampled and prepared for histology as described above.
2.5. Statistical analysis
Data were analyzed by Student t-tests preceded by ANOVAs. Wilcoxon rank-sum tests were done for adhesion scores between each hydrogel and control. Statistical tests were done with KaleidaGraph® (Synergy Software). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Synthesis and characterization of HA-ADH, HA-CHO, CMC-CHO, HPMC-CHO, and MC-CHO
Synthesis of HA-ADH was confirmed by the methylene protons of the adipic dihydrazide (singlet peak at 1.62 ppm and doublet peak at 2.25 ppm and 2.38 ppm) [6–8]. The degree of modification was calculated from the ratio of the area of the peak for N-acetyl-D-glucosamine residue of HA (singlet peak at 2.0 ppm) to that for the methylene protons of the adipic dihydrozide at 1.62 ppm; the degree of modification was 48.4%.
For analysis of aldehyde groups formed by the oxidation reaction, the aldehyde polymers were reacted with t-BC prior to 1H-NMR analysis. In each of the aldehyde-modified polysaccharides, the chemical shifts of t-butyl groups appeared (single peak at 1.20 ppm and single peak at 1.43 ppm), indicating the successful syntheses of HA-CHO, CMC-CHO, HPMC-CHO, and MC-CHO. The Mw and Mw/Mn of HA were 1432 kDa and 5.2 [6]. The Mws of CMC, HPMC, and MC were > 1MDa. The Mws of the aldehyde polymers were between 109 and 239 kDa, which were lower than that of HA-ADH (Table 1).
Table 1.
Molecular weights of modified polymers
| Mw (kDa) | Mn (kDa) | Mw/Mn | |
|---|---|---|---|
| HA-ADH* | 1502 | 108 | 13.9 |
| HA-CHO | 239 | 65 | 3.7 |
| CMC-CHO | 128 | 46 | 2.8 |
| HPMC-CHO | 109 | 23 | 4.8 |
| MC-CHO | 162 | 33 | 4.9 |
Previously reported [6]
Mw: weight-averaged molecular weight, Mn: number-averaged molecular weight
3.2. In vitro physicochemical properties of in- situ hydrogels
HA-ADH and the aldehyde-modified cellulose derivatives all formed gels within an acceptably brief time frame (Table 2). The gelation time of HA-CMC was significantly longer than the rest (p < 0.0001 between HA-CMC and other aldehyde polysaccharides).
Table 2.
Physical properties of cross-linked hydrogels
| Gelation time (sec) | Swelling ratio* (%) | G* (Pa) | |
|---|---|---|---|
| HAX | 3.5±1.0 | 206±14 | 32.4±17.2 |
| HA-CMC | 18.5±1.7 | 225±10 | 91.7±19.3 |
| HA-HPMC | 4.0±1.2 | 123±13 | 291.7±108.6 |
| HA-MC | 5.8±2.9 | 152±4 | 296.8±40.7 |
Measured on day 5 of immersion in phosphate buffered saline
Date are averages ± standard deviations (n=4)
The hydrogels swelled, reached equilibrium one day after immersion in PBS, and remained constant for the following 4 days (data not shown). Throughout, the swelling ratios (Table 2) were ordered as follows: HAX, HA-CMC > HA-MC (p < 0.001) >HA-HPMC (p = 0.0053).
The shear modulus (Table 2), G, of HAX was lower than those of HA-CMC, HA-HPMC, or HA-MC (p < 0.05). The shear modulus of HA-CMC was lower than those of HA-HPMC or HA-MC (p < 0.05). There was no statistical difference in G between HA-MC and HPMC (p = 0.93).
3.3. Degradation kinetics in hyaluronidase solution
There were differences in the degradation kinetics of the hydrogels in hyaluronidase solution (Fig. 2). HAX degraded the most rapidly (p < 0.001 vs. HA-CMC on days 1 and 2). HAX and HA-CMC were completely degraded by the 4th and 5th days, respectively. In contrast, HA-MC and HA-HPMC did not degrade completely for 2 weeks. One possible reason for these differences is that HA-MC and HA-HPMC are more cross-linked than HAX. Another reason could be that hyaluronidase did not diffuse as effectively into HA-MC and HA-HPMC because they did not swell as much as HAX and HA-CMC.
Fig. 2.
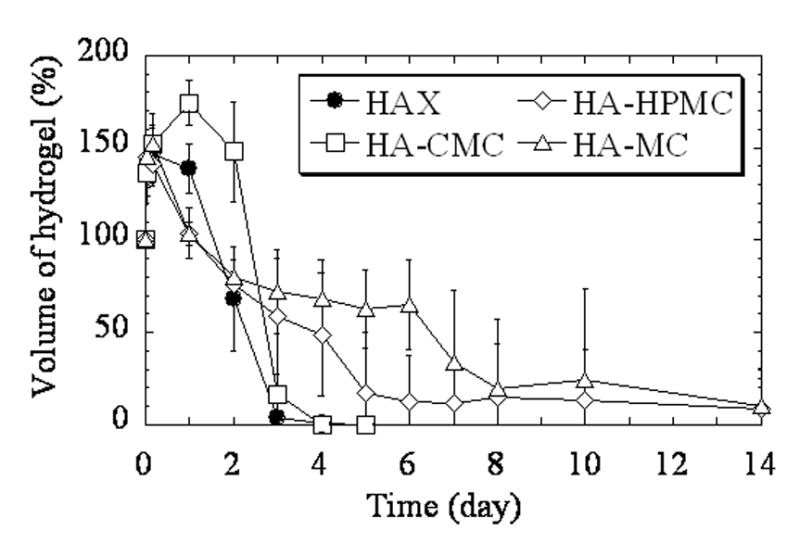
Degradation kinetics of the hydrogels in 10 unit/ml hyaluronidase in PBS at 37 ºC. Volume of the hydrogel (%) is the ratio of the volume of hydrogel at each time point to the initial volume, expressed as a percentage. Data are averages ± standard deviations (n=4).
3.4. The effect of polymers on the viability of mesothelial cells and macrophages
Mesothelial cells were cultured in the presence of a range of concentrations of aldehyde polymers. There was a dose-dependent reduction in cell viability for all polymers. Cell viability was not reduced by HA-CHO and MC-CHO at 0.3 % (w/v) (p > 0.05) (Fig.3(A)), while HPMC-CHO (p = 0.0011) and CMC-CHO (p = 0.044) caused a small reduction in cell viability. At higher concentrations, HA-CHO showed a small decrease in cell viability, while the cellulose derivatives showed more: the rank order of cell viability after 3 days of incubation was HA-CHO > CMC-CHO >MC-CHO >HPMC-CHO (p < 0.01 at any pair at 0.9% (w/v)).
Fig. 3.
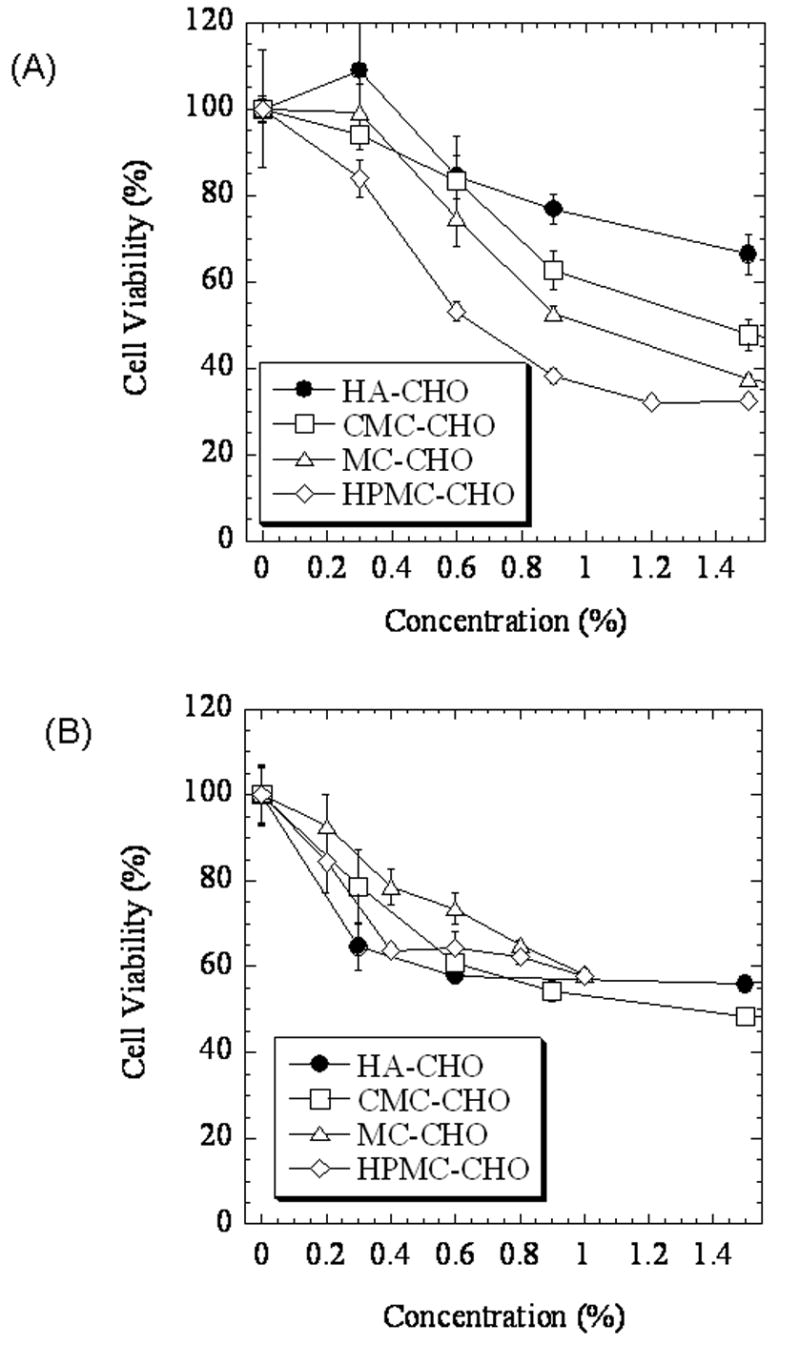
Effect of aldehyde polymers on cell viability measured by the MTT assay. (A) Mesothelial cells after 3 days incubation with polymers. (B) Macrophages (J774.A1 cell line), after 2 days incubation with polymers. Data are averages ± standard deviations (n=4).
Aldehyde-modified polymers also showed a dose-dependent effect on cell viability in macrophages (Fig.3(B)). Here, the difference in cell viability between HA and cellulose derivatives was not seen. Although there were some statistical differences between compounds at some concentrations, on the whole cell viability was similar between groups.
3.5. Biocompatibility of the in-situ hydrogels in the mouse peritoneum
Mice (n = 4 to 6) were injected with 1 ml of gel precursors. Animals were sacrificed at predetermined intervals over the next three weeks to assess adhesion formation (Table 3). In all twenty animals, there was only one adhesion between the bladder and other viscera. Given the presence of scar and clot at the site of the adhesion, it was felt to be due to direct trauma during the injection of gel.
Table 3.
Adhesions following intraperitoneal injection of hydrogels in mice
| Days to dissection post-surgery
|
|||||
|---|---|---|---|---|---|
| Hydrogel | 4 | 7 | 14 | 21 | Total |
| HAX | 0/2 | 0/1 | 0/1 | 0/1 | 0/5 |
| HA-CMC | 0/3 | 0/1 | 0/1 | 0/1 | 0/6 |
| HA-HPMC | 0/2 | 0/1 | 1/1 | 0/1 | 1/5 |
| HA-MC | 0/2 | 0/1 | 0/1 | - | 0/4 |
It was not possible to quantitate the amount of residual gel in the abdominal cavity. On gross examination, there appeared to be much more hydrogel residue in animals injected with HA-MC than in the others (Fig. 4). After 3 weeks, HAX had completely disappeared, and a small volume of HA-HPMC was found as a discrete gel. HA-CMC persisted only as a thin layer covering the viscera. Histology of the peritoneum and viscera was normal in all samples.
Fig. 4.
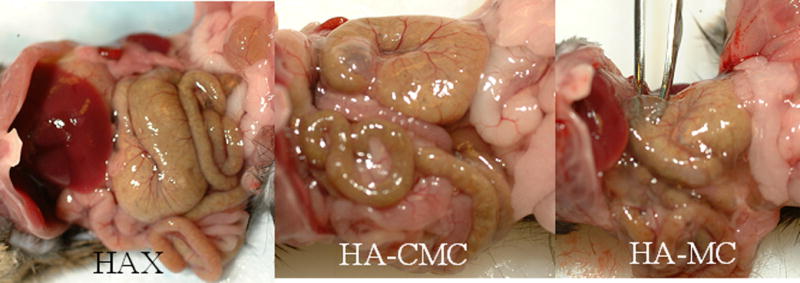
Peritoneums of mice 1 week after injection of hydrogels. (A) HAX: no residue. (B) HA-CMC: note the thin coating of gel-like material. (C) HA-MC: note the increased amount of residual material, demonstrated the forceps submerged beneath it.
3.6. Prevention of peritoneal adhesions
Adhesions were induced in rabbits by abrasion of the cecum and excision of a section of adjacent abdominal wall (Table 4). In control animals, saline was applied instead. All animals in the saline group developed adhesions over a large area (Fig.5(B)). The area of adhesions was greatly reduced in groups treated with HA-CMC (p = 0.0011), HA-MC (p < 0.0001), and HA-HPMC (p < 0.0001). There was no statistically significant difference between the gels in that parameter. Statistical significance of the decrease in adhesion scores could only be shown with HA-MC (p=0.023) (Fig.5(C)). Of note, in rabbits treated with HA-HPMC two of the score 3 adhesions formed on the incision line on the abdominal midline i.e. outside of the area where the gel was applied. We have described the effectiveness of HAX in preventing peritoneal adhesions elsewhere [6].
Table 4.
Effectiveness of hydrogels in preventing peritoneal adhesions in the rabbit
| HA-CMC | HA-HPMC | HA-MC | Control (Saline) | |
|---|---|---|---|---|
| Animal weight loss postoperatively (%) | 6.5±3.6 | 2.8±2.6 | 8.4±3.3 | 11.7±2.4 |
| Score 3 | 1 | 2 | 0 | 3 |
| Score 2 | 1 | 0 | 0 | 1 |
| Score 1 | 0 | 0 | 1 | 0 |
| No adhesion | 2 | 2 | 3 | 0 |
| Median adhesion score | 1 | 2 | 0 | 3 |
| Adhesion area (cm2) | 2.2±3.3 | 0.3±0.6 | 0.0±0.0 | 13.1±1.9 |
Animal weight loss refers to loss in the week following surgery. Adhesion area is the total area of adhesions with scores of 2 and 3. Weight change and adhesion area are expressed as average ± standard deviation (n=4 per group).
Fig. 5.
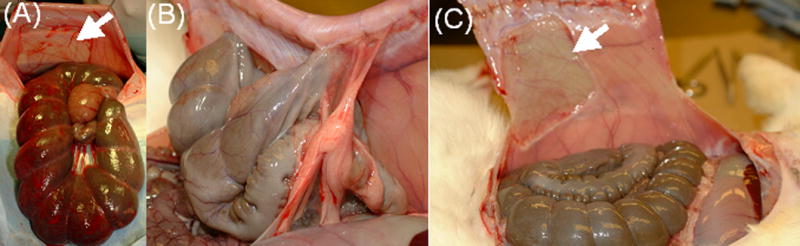
Prevention of peritoneal adhesions in a rabbit abrasion model. A. Induction of adhesions. Note the abdominal wall defect (arrow), and the bleeding surface of the cecum. B. Adhesions seen on dissection after 1 week in an animal treated with saline. C. Absence of adhesions after 1 week in an animal treated with HA-MC.
Histological analysis of the adhesion site in the saline-treated animals showed fibroblasts and inflammatory cells in the tissue connecting the cecum and abdominal wall (Fig.6(A)). In animals treated with cross-linkable gels, neutrophils and macrophages were found in the hydrogel residues (Fig.6(B)). Where adhesions were prevented, the site of injury was re-epithelialized, (Fig.6(C)), although fibroblasts were still prominent in the subjacent layers compared to the normal abdominal wall (Fig.6(D)). Similar results were observed with all three cellulose derivatives.
Fig. 6.
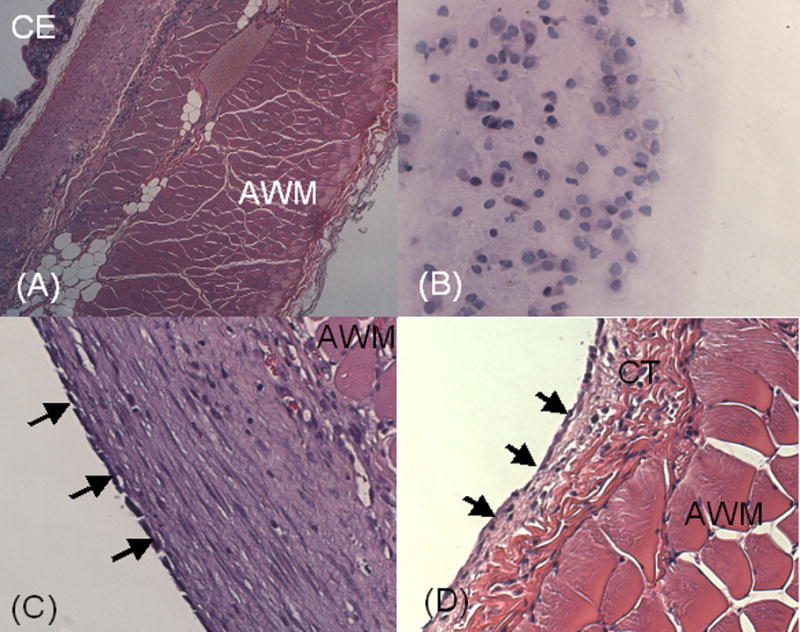
Photomicrographs of tissues recovered 1 week after injury in the rabbit sidewall defect-bowel abrasion model. (A) Cross-section of an abrasion in a saline-treated animal. The cecal lumen (CE) is in the left upper corner of the picture. The cecal smooth muscle is fused to the striated muscle of abdominal musculature (AM). Magnification 100 X. (B) Hydrogel recovered from an animal treated with HA-MC, with inflammatory cells (predominantly macrophages and lymphocytes). Magnification 100 X. (C) Site of abdominal wall defect in an animal treated with HA-MC. The defect has been re-epithelialized (arrows), with a subjacent layer of healing tissue (predominantly fibroblasts). Magnification 400 X. (D) Normal untreated parietal peritoneum. The mesothelium (arrows) overlies connective tissue (CT) and abdominal muscle.
4. Discussion
The hybrid HA-cellulose derivative hydrogels presented here were suitable for use in the peritoneum. Their physicochemical properties including gelation time, mechanical strength, water content, swelling kinetics, and degradation kinetics were appropriate to the anticipated use. This was confirmed by good handling properties during surgery, and by biological outcomes. Although the precursor polymers showed some cytotoxicity in vitro, there was no apparent local toxicity in vivo. One possible explanation is that the rapid cross-linking leaves little free precursor. The benign nature of these formulations was shown by their biocompatibility in the murine and rabbit models, although long-term safety and efficacy remain to be demonstrated. Finally, the hydrogels showed a marked effect in reducing adhesion formation. We note that many of the adhesions that occurred in the rabbits were located outside of the areas in which the treatments were applied. Therefore, an important unanswered question is whether these materials are best applied in a manner restricted to the site of injury, as done here, or more broadly, or throughout the peritoneum.
There are commercially available materials for the prevention of peritoneal adhesions that have related compositions of matter. Seprafilm® (Genzyme) is a preformed hydrogel sheet of HA and CMC crosslinked with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride [17,18]. Interceed® (Johnson & Johnson) is a preformed sheet of oxidized regenerative cellulose [19]. There are differences between those devices and ours in the chemistry of cross-linking, the molecules released by that cross-linking (Seprafilm® releases carbodiimide, while the materials described here release water), and composition of matter. However, the most significant difference is that these materials must be applied as solid sheets, while the formulations presented here form by in situ gelation without using cross-linking agents.
Hydrogels that cross-link in situ have advantages over conventional barrier devices in terms of ease of applicability, the types of devices through which they can be applied, and perhaps the type of surface that can be treated. We have previously shown the effectiveness of one such system, even without added therapeutic compounds [6]. However, it is not yet known what the ideal properties of such a system would be. The materials presented here have a range of physicochemical properties. It is reasonable to expect that these properties could be further tuned by modifying the concentrations of the polymers, their relative proportions, their molecular weights, and other parameters. Furthermore, the use of hydrazide versions of the cellulose derivatives (instead of HA-ADH) would further affect properties, e.g. by making the resulting gels even more resistant to enzymatic hydrolysis. We also note that there is a considerable difference in cost between the cellulose derivatives and hyaluronic acid.
Of the gels tested here, HA-MC gel was the most effective in preventing peritoneal adhesions. This could be related to the fact that HA-MC degraded more slowly than HA-CMC and HAX in vitro in hyaluronidase, an enzyme present in peritoneum, and thus had a more prolonged barrier effect. Differences in the effectiveness of the various hydrogels preventing adhesions could be due to differences in unsuspected intrinsic biological activities, as may be the case for HA [6]. Effectiveness in preventing adhesions could be changed by further optimizing the physicochemical properties of the gels.
We examined physicochemical properties of the hydrogels, including gelation time, mechanical strength, water content, swelling kinetics, and degradation kinetics. These properties are interrelated, and depend in large part on the properties of the pre-polymers, such as viscosity, electric charge, conformation, solubility, degree of modification, concentration in solution, ease of mixing, and others. Difference in performance between the various polymers probably are due to differences in these parameters, but our results do not allow us to discern the mechanism.
Given the relationship between swelling ratio, polymer concentration and shear modulus [20,21], the physicochemical properties studied above suggest that HA-HPMC and HA-MC might be more highly crosslinked than HAX and HA-CMC. The higher cross-linking density may have resulted in part from the fact that MC-CHO and HPMC-CHO are not anionic, so that there was less electrostatic repulsion between the hydrazide polymer and the aldehyde polymer than in those with HA-CHO and CMC-CHO. This interpretation is consistent with the rapid gelation of HA-MC and HA-HPMC as compared to HA-CMC.
5. Conclusion
The hybrid hydrogels of HA and cellulose derivatives described here could be applied via a double barreled syringe and cross-linked rapidly, suggesting ease of application in the clinical setting, with both open surgery and laparoscopy. Although the aldehyde-modified cellulose derivatives showed some cytotoxicity in vitro, there was good biocompatibility in the murine peritoneum. These formulations were effective in preventing peritoneal adhesions in a rabbit cecal injury-side wall defect model.
Acknowledgments
Financial support was provided by the DuPont/MIT Alliance (to RL, DSK) and GM073626 (to DSK).
References
- 1.DiZerega GS. Peritoneal Surgery. New York: Springer; 1999. [Google Scholar]
- 2.Johns DB, Rodgers KE, Donahue WD, Kiorpes TC, diZerega GS. Reduction of adhesion formation by postoperative administration of ionically cross-linked hyaluronic acid. Fertil Steril. 1997;68(1):37–42. doi: 10.1016/s0015-0282(97)81472-0. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Liu YC, Shu XZ, Gray SD, Prestwich GD. Synthesis and biological evaluation of a cross-linked hyaluronan-mitomycin C hydrogel. Biomacromolecules. 2004;5(3):895–902. doi: 10.1021/bm034463j. [DOI] [PubMed] [Google Scholar]
- 4.Liu YC, Li H, Shu XZ, Gray SD, Prestwich GD. Crosslinked hyaluronan hydrogels containing mitomycin C reduce postoperative abdominal adhesions. Fertil Steril. 2005;83:1275–1283. doi: 10.1016/j.fertnstert.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Oh SH, Kim JK, Song KS, Noh SM, Ghil SH, Yuk SH, Lee JH. Prevention of postsurgical tissue adhesion by anti-inflammatory drug-loaded pluronic mixtures with sol-gel transition behavior. J Biomed Mater Res A. 2005;72(3):306–16. doi: 10.1002/jbm.a.30239. [DOI] [PubMed] [Google Scholar]
- 6.Yeo Y, Highley C, Bellas E, Ito T, Marini R, Langer R, Kohane D. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27:4698–4705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47(2):152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Jia XQ, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25(19):4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Knepper PA, Farbman AI, Telser AG. Exogenous Hyaluronidases And Degradation Of Hyaluronic-Acid In The Rabbit Eye. Investigative Ophthalmology and Visual Science. 1984;25(3):286–293. [PubMed] [Google Scholar]
- 10.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7(3):659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 11.Yui N, Okano T, Sakurai Y. Inflammation Responsive Degradation Of Cross-Linked Hyaluronic-Acid Gels. J Control Rel. 1992;22(2):105–116. [Google Scholar]
- 12.Elkins TE, Bury RJ, Ritter JL, Ling FW, Ahokas RA, Homsey CA, Malinak LR. Adhesion Prevention By Solutions Of Sodium Carboxymethylcellulose In The Rat.1. Fertil Steril. 1984;41(6):926–928. doi: 10.1016/s0015-0282(16)47909-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu LS, Berg RA. Adhesion barriers of carboxymethylcellulose and polyethylene oxide composite gels. J Biomed Mater Res. 2002;63(3):326–332. doi: 10.1002/jbm.10211. [DOI] [PubMed] [Google Scholar]
- 14.Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. Invitro Evaluation Of Mucoadhesive Properties Of Chitosan And Some Other Natural Polymers. International Journal of Pharmaceutics. 1992;78(1):43–48. [Google Scholar]
- 15.Leach RE, Burns JW, Dawe EJ, SmithBarbour MD, Diamond MP. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil Steril. 1998;69(3):415–418. doi: 10.1016/s0015-0282(97)00573-6. [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Diamond MP, Bieber E, Coddington C, Franklin R, Grunert G, Gunn D, Lotze E, Rowe G, Grainger D, Tjaden B, Holtz G, Patton G, Johns DA. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): A blinded, prospective, randomized, multicenter clinical study. Fertility And Sterility. 1996;66(6):904–910. [PubMed] [Google Scholar]
- 18.Kling J. Genzyme's Seprafilm gets FDA marketing nod. Nature Biotechnology. 1996;14(5):572–572. doi: 10.1038/nbt0596-572a. [DOI] [PubMed] [Google Scholar]
- 19.Pagidas K, Tulandi T. Effects Of Ringer Lactate, Interceed(Tc7) And Gore-Tex Surgical Membrane On Postsurgical Adhesion Formation. Fertility And Sterility. 1992;57(1):199–201. doi: 10.1016/s0015-0282(16)54801-8. [DOI] [PubMed] [Google Scholar]
- 20.Anseth KS, Bowman CN, BrannonPeppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17(17):1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 21.Peppas NA, Merrill EW. Crosslinked Polyvinyl-Alcohol) Hydrogels As Swollen Elastic Networks. J Appl Polym Sci. 1977;21(7):1763–1770. [Google Scholar]


