Abstract
The purpose of this study was to compare the effects of amphetamine exposure on subsequent amphetamine-induced changes in behavior and dopamine (DA) release in the dorsal and ventral striatum, as a function of time following the discontinuation of repeated amphetamine treatment. Rats were pretreated with either saline or an escalating-dose amphetamine regimen, and then received a 0.5 mg/kg amphetamine “challenge” after either 3, 7, or 28 days of withdrawal. Animals tested after 28 days of withdrawal were hypersensitive (sensitized) to the locomotor-activating effects of amphetamine, and relative to control animals showed a significant enhancement in amphetamine-stimulated DA release in both the dorsal and ventral striatum, as revealed by in vivo microdialysis. Animals tested after only 3 or 7 days of withdrawal showed neither behavioral sensitization nor enhanced amphetamine-stimulated DA release. These results establish that time-dependent changes in behavioral sensitization to amphetamine are associated with time-dependent changes in amphetamine-stimulated DA release, and support the hypothesis that persistent sensitization-related changes in striatal DA neurotransmission contribute to the expression of behavioral sensitization.
Keywords: Nucleus accumbens, Caudate nucleus, Locomotion, Motor activity, Withdrawal, Amphetamine, Psychomotor stimulants
INTRODUCTION
Many effects of addictive drugs on behavior and cognitive function do not remain constant when drugs are administered repeatedly. Some drug effects show tolerance, that is, they get smaller and smaller with repeated drug administration (Jaffe, 1990, whereas other drug effects get larger and Larger, a phenomenon known as behavioral sensitization (Antelman, 1988; Kalivas and Stewart, 1991; Post and Contel, 1983; Robinson and Becker, 1986; Segal, 1975). The potential importance of sensitization in the development of addiction (Robinson and Berridge, 1993, and in stimulant-induced psychosis (Post and Contel, 1983; Robinson and Becker, 1986; Segal et al., 1981), has led to increasing interest in characterizing the nature of persistent drug-induced adaptations in the nervous system responsible for behavioral sensitization. Much of this research has focused on mesotelencephalic dopamine (DA) systems because (1) many addictive drugs are known to influence dopaminergic activity; (2) the specific drug effects that show sensitization (e.g., psychomotor activating and incentive motivational effects) are known to be mediated in large part by mesotelencephalic DA systems; (3) the activation of DA systems is known to be necessary for the induction and expression of psychomotor stimulant sensitization; and (4) the local activation of DA systems is sufficient to produce sensitization (Kalivas and Stewart, 1991; Robinson, 1991; Robinson and Berridge, 1993; White and Wolf, 1991).
This report concerns specifically the neural basis of amphetamine sensitization. A number of neural correlates of amphetamine sensitization have been reported, but probably the most consistent and persistent sensitization-related adaptation in DA neurotransmission is an enhancement in amphetamine-stimulated striatal DA “release”1 (Kalivas and Stewart, 1991; Robinson, 1991; Robinson and Berridge, 1993; White and Wolf, 1991). A sensitization-related enhancement in amphetamine-stimulated DA release has been reported in studies using in vitro preparations of caudate or nucleus accumbens tissue fragments (Castaneda et al., 1988; Kolta et al., 1985, 1989; Robinson and Becker, 1982; Robinson et al., 1982; Wilcox et al., 1986; Yamada et al., 1988, and in studies utilizing in vivo intracerebra1 microdialysis (Kazahaya et al., 1989; Patrick et al., 1991; Robinson, 1991; Robinson et al., 1988; Vezina, 1993; Wolf et al., 1993). There have been, however, negative experiments as well, in which behavioral sensitization was not associated with an enhancement in amphetamine-stimulated DA release (Kolta et al., 1985; Segal and Kuczenski, 1992; Wolf et al., 1993).
We hypothesize that some of the differences between studies with positive vs. negative outcomes may be related to the complex time course of sensitization-related changes in brain and behavior. For example, we reported previously that rats do not express behavioral sensitization for up to a week following the discontinuation of an escalating-dose amphetamine regimen, a regimen that maximizes amphetamine withdrawal symptoms (Paulson et al., 1991). Indeed, with this paradigm behavioral sensitization does not seem to “emerge” until withdrawal-related changes in behavior dissipate. This indicates that time following amphetamine pretreatment is a critical variable that must be considered in studying neural correlates of sensitization, a point that has been emphasized previously by a number of researchers (Antelman, 1988; Hirabayashi and Alam, 1981; Post, 1980). There is, however, little known about the relationship between time-dependent changes in behavior associated with the discontinuation of amphetamine treatment and changes in amphetamine-stimulated DA release. The two previous studies on this topic involved the quantification of amphetamine-stimulated DA release from striatal (mostly caudate) tissue in vitro (Kolta et al., 1985) or in vivo microdialysis in anaesthetized animals (Wolf et al., 1993), both of which precluded the simultaneous assessment of changes in behavior and DA neurochemistry.
The purpose of this study, therefore, was to use the technique of in vivo microdialysis in behaving animals to characterize the time course of changes in behavioral responsiveness to amphetamine following the discontinuation of repeated amphetamine treatment, and its relationship to the time course of regional changes in amphetamine-stimulated DA release. The two striatal regions selected for study were the dorsolateral caudate nucleus (dorsal striatum) and the nucleus accumbens (ventral striatum) because these represent the two most anatomically and functionally distinct subdivisions of the striatal complex (Nauta, 1989). We hypothesized that this comparison would maximize the probability of detecting any regional differences in the effects of amphetamine sensitization within the striatum, if any exist.
MATERIALS AND METHODS
Subjects
Adult male Holtzman rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 200-250 g at the start of the experiment were housed individually in wire-hanging cages in a temperature-controlled animal colony, on a normal light-dark cycle (14:lO h; lights on at 06:OO h). The animals had free access to food and water, and were allowed to acclimatize to the light cycle in the colony room for at least 2 weeks prior to beginning the experiment.
Amphetamine pretreatment regimen and groups
Animals were pretreated with either d-amphetamine sulfate or saline (i.p.) according to the schedule described by Robinson and Camp (1987) and Paulson et al. (1991). Briefly, amphetamine-pretreated rats received twice daily injections of amphetamine, approximately 8 h apart, in their home cage. Injections were given on weekdays, but not on weekends, to mimic the pattern of “runs” followed by “crashes” often seen in amphetamine addicts (Kramer et al., 1967). The dose of amphetamine was escalated over a 6 week period, from 1 to 10 mg/kg (weight of the salt), as illustrated in Figure 1 of Paulson et al. (1991). Control animals received 1 ml/kg of 0.9% saline. Approximately equal numbers of amphetamine-pretreated and control rats were prepared for microdialysis testing, which took place either 3,7, or 28 days after the discontinuation of pretreatment with amphetamine or saline. There were, therefore, three independent groups of amphetamine-pretreated animals (tested after 3,7, or 28 days of withdrawal) and three independent groups of control animals (tested 3,7, or 28 days after the last injection of saline). In addition, approximately half of the animals in each of these groups received a microdialysis probe in the ventral striatum (nucleus accumbens), and half received a probe in the dorsal striatum (dorsolateral caudate nucleus), so in total there were 12 independent groups of animals tested.
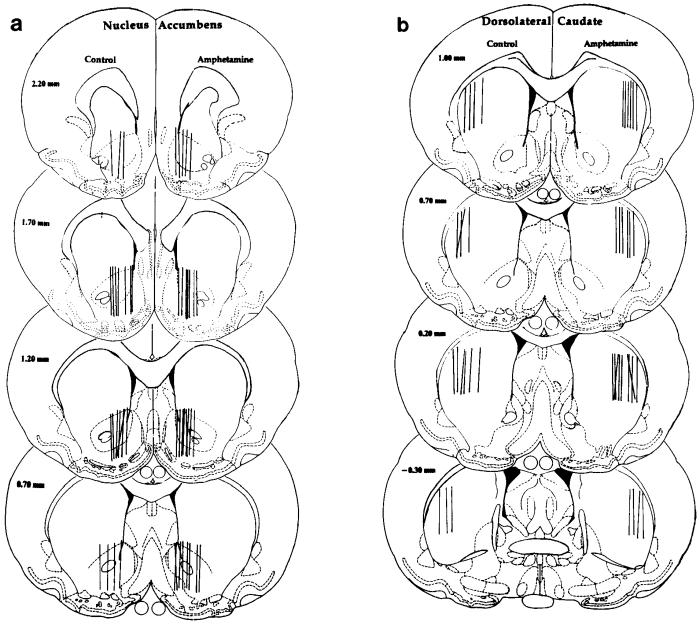
Fig. 1.
Schematic drawings of coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (1986), showing the location of the dialysis surface of probes in either the nucleus accumbens (ventral striatum; a) or dorsolateral caudate nucleus (dorsal striatum; b). Probe locations in control (saline-pretreated) animals are shown on the left-hand side of each section and those in amphetamine-pretreated animals on the right-hand side of each section.
Procedures
All animals were prepared surgically with a chronic guide cannula using procedures described by Paulson and Robinson (1994). Animals to be eventually tested after 3 or 7 days of withdrawal had surgery on the Saturday of the last drug-free weekend, before the last week of pretreatment injections (i.e., 9-12 days before dialysis testing), and then were returned to their home cages. Animals to be tested after 28 days of withdrawal had surgery 12 days prior to their dialysis test session. A 26-gauge stainless steel guide cannula was placed unilaterally on the dural surface above either the dorsolateral caudate nucleus (0.5 mm anterior to bregma and 3.5 mm lateral to the saggital suture) or the nucleus accumbens (1.8 mm anterior, 1.2 mm lateral); half the animals had a cannula placed above the left hemisphere and the other half had it placed above the right hemisphere (Paxinos and Watson, 1986). In addition, an L-shaped length of stainless steel tubing was placed just above the skull surface near the back of the head, and approximately 5 mm of this extended vertically (to be used later for attachment of a tether). The entire assembly was fixed in place with skull screws and dental acrylic.
Six to 10 days after surgery each animal was placed in a habituation chamber located in the testing room. This chamber was identical to that described by Paulson and Robinson (1994), except it also had a semicircular opening located on one wall, which provided access to an 18 cm diameter stainless steel running wheel. Food and water were freely available. Each animal was left in a habituation chamber for 24 h, and the light-dark cycle was the same as in the animal colony. After the habituation period, each animal was lightly anesthetized with ether, and a dialysis probe (see below) was quickly lowered via the guide cannula and secured in place with cyanoacrylic adhesive. The animal was then placed into a test chamber identical in design to the habituation chamber. The animal was attached to a dual-channel liquid swivel (Instech) via a flexible steel tether made from model airplane control cable, which was fixed to the length of tubing protruding from the dental acrylic “cap” on the animal’s head. The perfusion medium (128.3 mM NaC1,1.35 mM CaCI, 2.6 mM KC1, 2.0 mM MgCl, 0.2 mM ascorbic acid, pH 7.3) was pumped into the probe at a rate of 1.5 μl/min via the side channel on the swivel and exited via the central channel of the swivel, where it passed through a 56 cm length of flexible fused silica capillary tubing. The animal was left undisturbed in the test chamber overnight, and no samples were collected.
The following morning timed samples of dialysate were collected into minivials and flow rate was checked by measuring their volumes. At least three of these dialysate samples were manually injected onto a high performance liquid chromatography (HPLC) system. In addition, sample containing the perfusion solution only, and three standards prepared in the perfusion medium, containing known concentrations of DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA1, were manually injected. The output from the probe (fused silica tubing) was then attached to a computer-controlled HPLC injection valve for “on-line” dialysis sampling (Wages et al., 1986), using procedures described previously (Paulson and Robinson, 1994).
Animals were then sampled over successive 20 min intervals for the next 22 h, during which time the animal was left undisturbed. The next morning the flow rate was checked again, as described above, and then the outlet from the probe reconnected to the automated injection value, and automated sample collection and injection were continued for 1 h (“baseline”). The animals then received an i.p. injection of 0.9% saline and three additional samples were collected. All animals then received an i.p. challenge injection of 0.5 mg/kg of d-amphetamine sulfate (weight of the salt), and automated sample collection and analysis continued for another 3 h. The animals were then removed from the chamber, and the exit line from the probe was disconnected from the HPLC injection valve. The dialysate flow rate was rechecked manually by again measuring the volume of timed samples, and additional standards were injected manually at this time. Dialysate was assayed for DA, DOPAC, HVA, and 5-HIAA using HPLC and electrochemical detection (Paulson and Robinson, 1994).
Probe design
The microdialysis probes were a modified version of the concentric-style probes described by Robinson and Camp (1991). The major difference between the probes described by Robinson and Camp (1991) and those used here was that the stainless steel shaft of these probes only extended to the cortical surface. Only the hollow fiber dialysis tubing extended into the brain. The proximal surface of this membrane was coated with cyanoacrylic to prevent diffusion, and only the distal surface, located in the target structure, was uncoated. For both caudate and accumbens probes, the exposed portion of the membrane was 2.0 mm long. Prior to use all probes were tested in vitro at 37°C to determine their ability to recover known concentrations of DA, DOPAC, HVA, and 5-HIAA.
Behavior
On the test day motor activity was quantified automatically over 5 min intervals by a device equipped with four photocells, located 90° apart, which was mounted on the liquid swivel, and was similar in design to that described by McFarlane et al. (1992). Essentially, this device divides the area of the test chamber diagonally into four equal quadrants and registers movements from one quadrant to another. These “counts” correlate very highly with “crossovers” (locomotion) from one side of the cage to the other, as determined by visually scoring videotapes (r = 0.95; Paulson and Robinson, 1994). In addition, revolutions of the running wheel were recorded.
Histology
At the end of each experiment the animal was given an overdose of sodium pentobarbital and perfused through the heart with 0.9% saline, followed by 10% formalin. The brain was removed and stored in 10% formalin until it was sectioned using a frozen technique, stained with cresyl violet, and examined to determine the exact location of the dialysis probe.
Data analysis
Dialysate values expressed in pg/μ1 of dialysate are corrected for probe recovery. There were no significant group differences in probe recovery. For all probes the average (± SEM) recovery values were as follows: caudate probes, DA, 13.12 ± 0.49%; DOPAC, 13.67 ± 0.56%; HVA, 13.42 ± 0.54%; 5-HIAA, 12.96 ± 0.63%; accumbens probes, 12.64 ± 0.52%) 12.79 ± 0.61%, 13.04 ± 0.51%, 13.21 ± 0.46%, respectively. The group means for motor activity and the dialysate concentrations of neurochemicals were analyzed using parametric statistics, including analyses of variance and Fisher’s least significant difference (LSD) tests for follow-up pairwise comparisons (Statview®, Abacus Concepts, Inc.). The results of all statistical analyses are presented in the figure captions to make the text easier to read. All statements regarding group differences made in the results section are supported by the outcome of the relevant statistical tests.
RESULTS
Criteria for inclusion
There were two criteria for inclusion of data in this experiment: (1) chromatographic--basal peaks had to be at least three times greater than background noise; and (2) histological—the probe had to be located in either the dorsolateral caudate nucleus or the nucleus accumbens. In addition, data from some animals was lost due to equipment malfunction (e.g., fluid leaks, power loss, etc.).
Probe placements
Figure 1 illustrates the location of the dialysis surface of probes located in either the dorsolateral caudate nucleus (dorsal striatum) or the nucleus accumbens (ventral striatum) of animals used in this study.
Behavior
The animals showed only low and highly variable levels of activity in the running wheel, confining nearly all their activity to the main portion of the test chamber, and therefore, only the latter data (90° movements) are reported here. In addition, there were no differences between animals with probes in the nucleus accumbens vs. caudate nucleus, and therefore, these groups were pooled for analysis and presentation of the behavioral data.
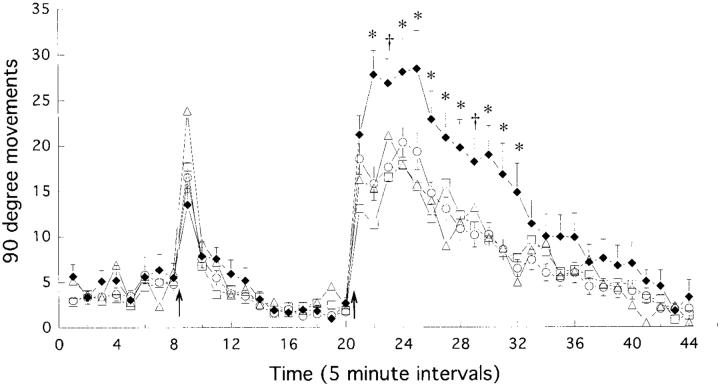
Figure 2 shows the effects of a challenge injection of first saline, and then 1 h later, 0.5 mg/kg of amphetamine, on motor activity (90° movements) in the four groups: control animals and animals tested after either 3, 7, or 28 days of withdrawal from amphetamine. There was no effect of amphetamine pretreatment on basal motor activity (the eight 5 min intervals prior to saline), although this was very low because the animals appeared to mainly sleep at this time. An injection of saline produced a brief increase in motor activity, lasting only 5-10 min, and there were no significant group differences. Amphetamine produced a large increase in motor activity in all groups, lasting for about 90 min, but there were significant group differences. Animals given an amphetamine challenge 28 days after the discontinuation of amphetamine pretreatment showed a significantly greater increase in motor activity (sensitization) than did control animals, or animals tested after only 3 or 7 days of withdrawal. These latter two groups did not differ from the control group, or from each other.
Fig. 2.
The mean (± S.E.M.) number of 90° movements per 5 min interval in control animals (○, open circles; N = 42) and animals tested after 3 days (□, open squares; N = 20), 7 days (△, open triangles; N = 18), or 28 days (◆, closed diamonds; N = 20) of withdrawal from escalating-dose amphetamine treatment. The first eight intervals represent basal activity. The animals received an injection of saline at the time indicated by the first arrow and then were left undisturbed for 12 intervals (1 h), at which time they received an injection of 0.5 mg/kg of amphetamine (second arrow). There were significant group differences in the response to amphetamine (two-way ANOVA with repeated measures on one factor, effect of treatment group, F = 5.47, P < 0.002; effect of time, F = 74.8, P < 0.0001; group by time interaction, F = 2.11, P < 0,0001). Subsequent factorial one-way ANOVAs (followed by Fisher’s PLSD tests if significant) at each point in time after amphetamine administration revealed that the group tested after 28 days of withdrawal differed from the other three groups at the times indicated by asterisks. The control, 3 day, and 7 day withdrawal groups did not differ from one another at any point in time. * 28 days differs from control, 3 day, and 7 day groups, P < 0.05, † 28 days differs from control and 3 day groups.
Microdialysis
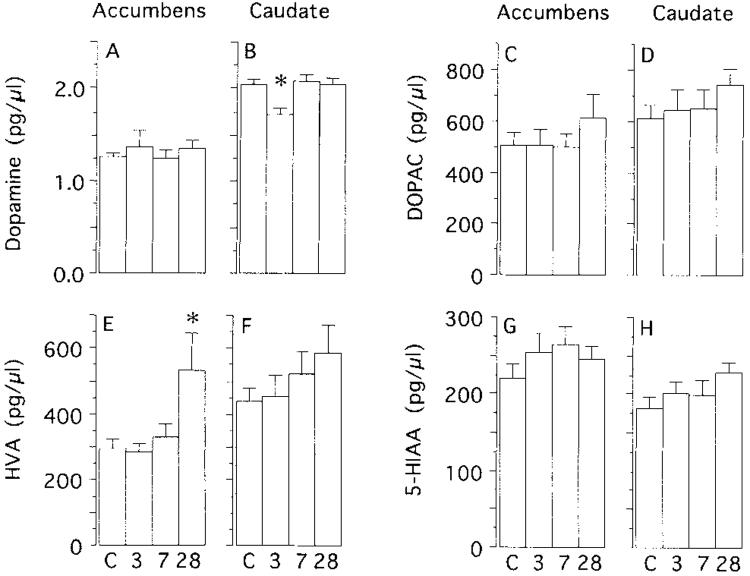
Figure 3 shows the basal concentrations of DA, DOPAC, HVA, and 5-HIM in the nucleus accumbens and caudate for each of the four groups. There was no significant effect of amphetamine withdrawal on the basal concentration of DA in the nucleus accumbens. There was, however, a significant decrease in basal DA in the dorsolateral caudate nucleus of animals tested after 3 days of withdrawal (but not in animals tested after 7 or 28 days of withdrawal). The only significant effect of amphetamine pretreatment on the basal concentration of DA metabolites on dialysate was in animals tested after 28 days of withdrawal. Relative to controls, the basal concentration of HVA was elevated significantly in the nucleus accumbens of animals tested after 28 days of withdrawal. There was no effect of amphetamine pretreatment on basal 5-HIAA levels.
Fig. 3.
The mean (+S.E.M.) basal concentrations of DA (A,B), dihydroxyphenylacetic acid (DOPAC; C,D), homovanillic acid (HVA; E,F), and 5-hydroxyindoleacetic acid (5-HIAA; G,H) in the nucleus accumbens (accumbens) and dorsolateral caudate nucleus (caudate) of control animals (C) and animals tested after 3, 7, or 28 days of withdrawal. The data were analyzed by one-way ANOVAs, and if significant, followed by Fisher’s PLSD tests. There was no effect of amphetamine withdrawal on the basal concentration of DA in the accumbens (F = 0.62), DOPAC in the accumbens (F = 0.52), DOPAC in the caudate (F = 0.861, HVA in the caudate (F = 1.29), 5-HIAA in the accumbens (F = 0.92), or 5-HIAA in the caudate (F = 1.79). There was a significant effect of amphetamine withdrawal on DA in the caudate (F = 4.62, P < 0.01) and HVA in the accumbens (F = 4.16, P = 0.012). The asterisk in panel B indicates that there was a significant (P < 0.05) decrease in basal DA in animals tested after 3 days of withdrawal relative to all other groups (which did not differ from one another). The asterisk in panel E indicates there was a significant (P < 0.05) increase in basal HVA in animals tested after 28 days of withdrawal, relative to all other groups (which did not differ from one another). Group Ns: accumbens control, N = 18-20; caudate control, N = 15-17; accumbens 3 day, N = 8-11; 7 day, N = 9-10; 28 day, N = 4-7; caudate 3 day, N = 6-7; 7 day, N = 8; 28 day, N = 9. Group Ns vary because both DA and metabolite data were not available for every animal.
Figure 4 shows the effects of challenge injections of saline and amphetamine on the concentration of DA in dialysate obtained from either the nucleus accumbens or dorsolateral caudate nucleus. There was no effect of a saline injection on DA concentrations, and no group differences. Amphetamine produced a significant increase in the concentration of DA in dialysate in all groups, but there were also significant group differences. Amphetamine induced significantly greater DA release in both the nucleus accumbens and caudate nucleus of animals tested after 28 days of withdrawal, relative to control animals or animals tested after only 3 or 7 days of withdrawal, and these latter three groups did not differ from one another.
Fig. 4.
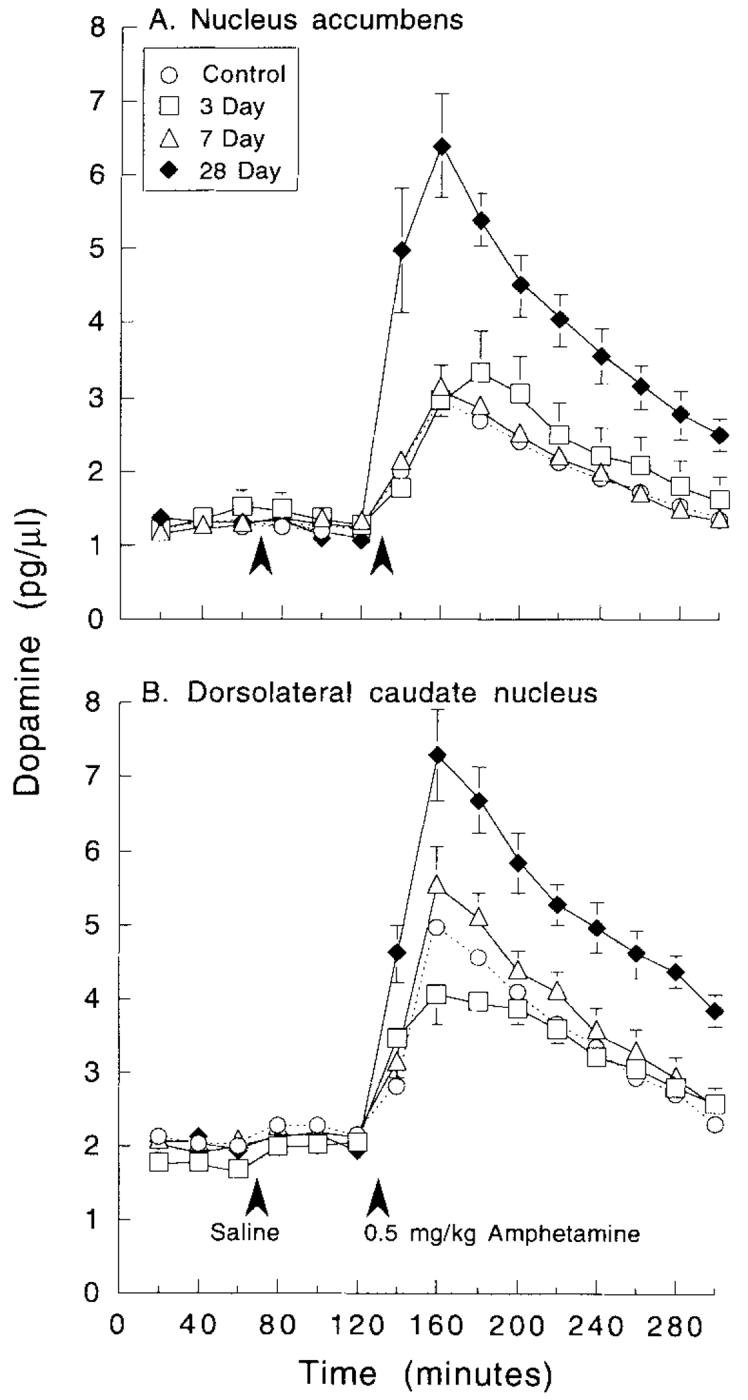
The mean (± S.E.M.) concentration (pg/μl) of DA in 20 min dialysate samples obtained from the nucleus accumbens or dorsolatera1 caudate nucleus in control animals (open circles) and animals tested 3 (open squares), 7 (open triangles), or 28 (closed diamonds) days after the discontinuation of amphetamine pretreatment. The first three samples represent the basal concentration of DA, and the animals were given an injection of saline at the time indicated by the first arrowhead. After 1 h the animals received an injection of 0.5 mg/kg of amphetamine (where indicated by the second arrowhead) and an additional nine samples collected. There was a significant effect of amphetamine withdrawal on the response to an amphetamine challenge in both the nucleus accumbens and caudate nucleus, as indicated by two-way ANOVAs with repeated measures on one factor: Accumbens (A) effect of treatment group, F = 10.4, P < 0.0001, effect of time, F = 98.7, P < 0.0001, group by time interaction, F = 9.3, P < 0,0001. Caudate (B) group, F = 11.15, P < 0.0001, time, F = 140.4, P < 0.0001, interaction, F = 5.85, P < 0.0001. Subsequent one-way ANOVAs (followed by Fisher’s PLSD tests if significant) for each interval after amphetamine administration revealed that the group tested after 28 days of withdrawal differed significantly from all other groups, at all points in time after amphetamine (Ps range from 0.03 to <0.0001), in both structures, but the control, 3 day, and 7 day groups did not differ from each other at any point in time after amphetamine administration. Group Ns: accumbens control, N = 18; caudate control, N = 15; accumbens 3 day, N = 8; accumbens 7 day, N = 9; accumbens 28 day, N = 7; caudate 3 day, N = 6; caudate 7 day, N = 8; caudate 28 day, N = 9.
DISCUSSION
Animals were pretreated with escalating doses (1 → 10 mg/kg) of d-amphetamine over a 6 week period and then withdrawn for 3, 7, or 28 days before the effects of a 0.5 mg/kg challenge injection of amphetamine on behavior and DA neurotransmission were assessed. The major finding was that animals showed parallel time-dependent sensitization of behavior and amphetamine-stimulated DA release; that is, animals tested 28 days after the discontinuation of amphetamine pretreatment were hypersensitive (sensitized) to the psychomotor activating effects of an amphetamine challenge, and also showed a significant enhancement in amphetamine-stimulated DA release in both the nucleus accumbens and dorsolateral caudate nucleus, relative to control. In contrast, animals tested after only 3 or 7 days of withdrawal did not express behavioral sensitization, or sensitization-related changes in amphetamine-stimulated DA release.
This time-dependent “emergence” of behavioral sensitization is consistent with previous studies. For example, Paulson et al. (1991) employed the same amphetamine pretreatment regimen as used here and challenged animals with 2.6 mg/kg of amphetamine 3, 7, 14, 28, 90, 180, or 365 days after the discontinuation of amphetamine pretreatment. A measure of locomotor activity (cage “crossovers”) did not reveal evidence of sensitization after 3 or 7 days of withdrawal, but animals were markedly hypersensitive to an amphetamine challenge by 14 days of withdrawal, and behavioral sensitization then persisted undiminished for at least a year. Time-dependent behavioral sensitization has been reported by a number of researchers using quite different amphetamine treatment regimens and other drugs (Antelman, 1988; Antelman and Chiodo, 1981; Hirabayashi and Alam, 1981; Nelson and Ellison, 1978; Post, 1980; Robinson, 1984). It appears, therefore, that time dependence is a fundamental feature of the sensitization phenomenon (Antelman, 1988; Post, 1980). Indeed, if drug injections are given too close together in time, tolerance rather than sensitization may occur.
A sensitization-related enhancement in amphetamine-stimulated DA release in vivo, similar to that seen here after 28 days of withdrawal, has been reported previously (Kazahaya et al., 1989; Patrick et al., 1991; Robinson, 1991; Robinson et al., 1988; Vezina, 1993; Wolf et al., 1993). On the other hand, others have reported that sensitization to amphetamine is not always associated with an increase in amphetamine-stimulated DA release (Kolta et al., 1985; Segal and Kuczenski, 1992; Wolf et al., 1993). The present study suggests a reason for the apparent discrepancy between these studies may be related to the time-dependent nature of sensitization-related changes in amphetamine-stimulated DA release. Although animals tested after 28 days of withdrawal showed enhanced amphetamine-stimulated DA release, animals tested after only 3 or 7 days of withdrawal did not.
To explore this hypothesis further, we summarized all of the microdialysis studies published to date on the effects of amphetamine sensitization on amphetamine-stimulated DA release (Table I). Table I shows that there have been seven experiments published in which a sensitization-related enhancement in amphetamine-stimulated DA release was found (positive experiments), and four experiments in which amphetamine pretreatment produced either no change or a decrease in amphetamine-stimulated DA release, including the present study (negative experiments). It is obvious from examination of Table I that neither differences in the amphetamine pretreatment regimen, nor the challenge dose of amphetamine, account for differences between the positive and negative experiments. There is, however, essentially no overlap between positive experiments and negative experiments in the duration of withdrawal from amphetamine before animals here given a challenge injection of amphetamine.
TABLE I.
Microdialysis studies of amphetamine-stimulated DA release in amphetamine-sensitized rats
| Amphetamine treatment | Withdrawal (days) | Challenge (mgkg) | Effect | Reference |
|---|---|---|---|---|
| Escalatine, 1 → 10 mdke 2/d × 6 weekl,d-AMP | 30-60 | 1.5 | ↑ (Acc&Cau) | Robinson, 1991 |
| Escalating, 1→ 10 mgkg 2/d → 6 weeks, d-AMP | 28 | 0.5 | ↑ (Acc & Cau) | Present study |
| Escalating, 1 → 10 mgkg 2/d ×6 weeks, d-AMP | 15-2 1 | 2.0 | ↑ (Acc) | Robinson et al., 1988 |
| 5/mgkg 2/d → 5d, d-AMP | 15 | 0.5 | ↑(Cau) | Patrick et al., 1991 |
| 2.5 μg × 3 (intra-acc d-AMP) | 14 | 1.0 (i.p.) | ↑(Acc) | Vezina, 1993 |
| 1 mgkg l/d × 10d, d-AMP | 10-14 | 0.5 | ↑ (Acc) | Wolf et al., 1993 |
| 4 mgkg l/d × 14d, meth-AMP | 7 | 4.0 | ↑(Cau) | Kazahaya et al., 1989 |
| Escalating, 1 → 10 mgkg 2/d × 6 weeks, d-AMP | 7 | 0.5 | --(Acc & Cau) | Present study |
| 2.5-3.0 mgkg l/d × 4-6d, d-AMP | 2-6 | 2.5 | ↓ (Acc & Cau) | Segal and Kuczenski, 1992 |
| 1 mgkg l/d × 10d, d-AMP | 3-4 | 0.5 | --(Act) | Wolf et al., 1993 |
| Escalating, 1 → 10 mgkg 2/d × 6 weeks, d-AMP | 3 | 0.5 | --(ACC & Cau) | Present study |
In the negative experiments an amphetamine challenge was given after only 2-7 days of withdrawal, and in the positive experiments an amphetamine challenge was given after 7-60 days of withdrawal. This analysis suggests that a sensitization-related enhancement in amphetamine-stimulated DA release occurs only after a relatively extended period of withdrawal. Similar findings have been reported recently for cocaine sensitization (Kalivas and Duffy, 1993). Exactly how much time must elapse before the “emergence” of a sensitization-related enhancement in amphetamine-stimulated DA release is probably dependent on many factors, including how aggressive the pretreatment regime is, and thus, the magnitude and persistence of withdrawal-related symptoms and neuroadaptations. For example, the persistent withdrawal syndrome seen following the discontinuation of the escalating dose regimen used here may explain why amphetamine-stimulated DA release was not withdrawal but was enhanced after only 7 days of withdrawal in the study by Kazahaya et al. (1989).
In the present study there was excellent correspondence between the presence or absence of behavioral sensitization and sensitization-related changes in amphetamine-stimulated DA release in vivo; that is, neither behavioral nor neurochemical sensitization were evident at 3 or 7 days of withdrawal, and both behavioral and neurochemical sensitization were eviden by 28 days of withdrawal. We need to emphasize, however, that behavioral sensitization can be dissociated from an enhancement in amphetamine-stimulated DA release under some conditions. In particular, with some treatment regimens behavioral sensitization has been observed soon after withdrawal, when changes in amphetamine-stimulated DA release were not evident (Kolta et al., 1985; Segal and Kuczenski, 1992; Wolf et al., 1993). It is possible that even with the escalating-dose regimen used here, such a dissociation would occur if, for example, animals were tested at times between 7 and 28 days of withdrawal.
There are a number of reasons why behavioral sensitization may, under some conditions, become dissociated from a sensitization-related enhancement in amphetamine-stimulated DA release. Of course, one possibility is that there is no causal relationship between sensitization-related changes in amphetaminestimulated DA release and behavioral sensitization. There is, however, considerable evidence to suggest that the expression of behavioral sensitizatio is caused, at least in part, by neuroadaptations in DA systems leading to enhanced amphetamine-stimulated DA release (Akiyama et al., 1991; Kalivas and Stewart, 1991; Robinson and Becker, 1986; White and Wolf, 1991). A more likely possibility is that during and following amphetamine treatment there are multiple, complex, time-dependent changes in DA systems, and in other neurotransmitter systems, all of which interact to determine the behavioral outcome (Kalivas and Duffy, 1993; Kalivas and Stewart, 1991; Robinson and Becker, 1986; White and Wolf, 1991).
Some of these neuroadaptations may be relatively transient, and related to withdrawal syndromes or the induction of sensitization, and others may be more persistent neuroadaptations related to the expression of sensitization. Furthermore, the neuroadaptations primarily responsible for the expression of behavioral sensitization could change over time. For example, early during withdrawal behavioral sensitization could be expressed because of the action of relatively normal synaptic concentrations of DA acting on supersensitive DA receptors, or DA receptors coupled to upregulated signal transduction mechanisms (Henry and White, 1991; Kalivas and Duffy, 1993). After longer periods of withdrawal behavioral sensitization could be expressed primarily because of changes in the synaptic concentration of DA, in the absence of supersensitive DA receptor systems. Although the most persistent neural correlate of sensitization reported to date is an enhancement in the ability of cocaine or amphetamine to elevate the extracellular concentration of DA, it is becoming increasingly obvious that this alone cannot account for what is a complex behavioral phenomenon.
Indeed, amphetamine-stimulated DA release alone cannot even account for the time course of the enhanced behavioral response within a test session (also see Kuczenski and Segal, 1989). For example, in animals tested after 28 days of withdrawal, the enhanced behavioral response to an amphetamine challenge was evident only during the first hour after amphetamine administration (Fig. 2). There were no sensitization-related group differences in motor activity after this time. In contrast, amphetamine-stimulated DA release in both the nucleus accumbens and caudate nucleus was significantly enhanced over the entire 3 h test session in animals tested after 28 days of withdrawal, relative to control. In this situation the behavioral outcome is clearly not a simple function of enhanced amphetamine-stimulated DA release. Other factors must be involved. For example, amphetamine produces a rapid (within 30 min) desensitization of striatal DA-stimulated adenylate cyclase (Barnett and Kuczenski, 1986; Roberts-Lewis et al., 1986; Roseboom and Gnegy, 1989, and amphetamine sensitization is associated with a decrease in the threshold dose required to produce densensitization of DA-stimulated adenylate cyclase activity (Barnett et al., 1987), so this could lead to a rapid diminution in DA-mediated behavioral responses, despite continued elevation of synaptic DA.
In addition to changes in amphetamine-stimulated DA release, there was also a small time-dependent increase in basal DA metabolism in the nucleus accumbens, as indicated by a significant increase in the concentration of HVA in dialysate in animals tested after 28 days of withdrawal. Similar findings have been reported by Robinson et al., (1988) and Vezina (1993). It is not clear what change in DA dynamics is responsible for this small increase in HVA, and there are many possibilities, including a change in DA synthesis or in the activity of the enzymes responsible for the degradation of DA. Another interesting possibility is that there is a small increase in the basal rate of DA release, and the increase in HVA reflects the metabolism of synaptic DA. This idea is inconsistent with many reports that sensitization to amphetamine or cocaine is not accompanied by any change in the basal extracellular concentration of DA. On the other hand, it has been reported in electrophysiological studies that the basal rate of discharge of DA cells is increased in animals sensitized to amphetamine or cocaine (White and Wolf, 1991). White and Wolf (1991) suggested that the dialysis technique may not be sufficiently sensitive to detect an increase in DA release produced by the very small (25-33%) increase in DA impulse flow seen in sensitized animals. However, it is possible the sensitization-related increase in basal DA metabolism reported here, and elsewhere (Camp and Robinson, 1988; Robinson and Camp, 1987; Robinson et al., 1988, Vezina, 1993, does reflect a small increase in DA impulse flow. With dialysis, it may be easier to detect a small increase in basal DA release by a change in DA metabolism, because the diffusion of DA metabolites out of the synapse would not be restricted by the active reuptake process that impedes the free diffusion of DA (Wightman and Zimmerman, 1990).
One other finding deserves mention. Rossetti et al. (1992) reported that amphetamine withdrawal is accompanied by a decrease in the extracellular concentration of DA in the nucleus accumbens for about 5 days, but in the present study there was no effect of amphetamine withdrawal on the concentration of DA in accumbens dialysate, although withdrawal symptoms are known to persist for up to 7 days following discontinuation of the amphetamine pretreatment regimen used here (Munn and Wise, 1992; Paulson et al., 1991; Robinson and Camp, 1987). This latter observation is consistent with a number of reports that amphetamine withdrawal is not accompanied by a decrease in DA in accumbens dialysate (Crippens et al., 1993; Segal and Kuczenski, 1992; Wolf et al., 1993). On the other hand, the concentration of DA in dialysate obtained from the dorsolateral caudate nucleus was significantly decreased after 3 days of withdrawal. Perhaps this is related to the depression in nocturnal motor activity associated with amphetamine withdrawal (Paulson et al., 1991).
In summary, following the discontinuation of repeated treatment with escalating doses of amphetamine behavioral sensitization “emerged” in a time-dependent fashion. Animals were not hypersensitive to the locomotor activating effects of an amphetamine challenge given during the first week of withdrawal, but were hypersensitive after 28 days of Withdrawal. Sensitization of amphetamine-stimulated DA release “emerged” in a similar time-dependent fashion. These data provide further support for the notion that behavioral sensitization may be due, at least in part, to persistent drug-induced adaptations in DA systems, including an increased ability of drugs to enhance synaptic DA. However, the relatively poor relationship between the time course of the sensitized behavioral response and amphetamine-stimulated DA release (during the dialysis test session) suggests that a simple increase in synaptic DA alone cannot account for the behavioral outcome.
ACKNOWLEDGMENTS
This research was supported by grant 04294 from the National Institute on Drug Abuse. We thank Buda Martonyi and Gerry Borgula for technical assistance and Dianne M. Camp for comments on an earlier draft of the manuscript, as well as advice and assistance throughout the study.
Footnotes
The word release is used here as shorthand for the phrase, “amphetamine-induced increase in the concentration of DA in dialysate (or superfusate)”, and is not used to imply anything about the specific mechanism by which this occurs.
REFERENCES
- Akiyama K, Hamamura T, Ujike H, Kanzaki A, Otsuki S. Methamphetamine psychosis as a model of relapse of schizophrenia—a behavioral and biochemical study in the animal model. In: Nakazawa T, editor. Taniguchi Symposia on Brain Sciences, vol. 14, Biological Basis of Schizophrenia. Japan Scientific Societies Press; Tokyo: 1991. pp. 169–184. [Google Scholar]
- Antelman S. Time-dependent sensitization as the cornerstone for a new approach to pharmacotherapy: Drugs as foreign/stressful stimuli. Drug Dev. Res. 1988;14:l–30. [Google Scholar]
- Antelman SM, Chiodo LA. Dopamine autoreceptor subsensitivity: A mechanism common to the treatment of depression and the induction of amphetamine psychosis. Biol. Psychiatry. 1981;16:717–727. [PubMed] [Google Scholar]
- Barnett JV, Kuczenski R. Desensitization of rat striatal dopamine-stimulated adenylate cyclase after acute amphetamine administration. J. Pharmacol. Exp. Ther. 1986;237:820–825. [PubMed] [Google Scholar]
- Barnett JV, Segal DS, Kuczenski R. Repeated amphetamine pretreatment alters the responsiveness of striatal dopamine-stimulated adenylate cyclase to amphetamine-induced desensitization. J. Pharmacol. Exp. Ther. 1987;242:40–47. [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav. Brain Res. 1988;30:69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Castaneda E, Becker JB, Robinson TE. The long-term effects of repeated amphetamine treatment in vivo on amphetamine, KCl and electrical stimulation evoked striatal dopamine release in vitro. Life Sci. 1988;42:2447–2456. doi: 10.1016/0024-3205(88)90343-8. [DOI] [PubMed] [Google Scholar]
- Crippens D, Camp DM, Robinson TE. Basal extracellular dopamine in the nucleus accumbens during amphetamine withdrawal: A “no net flux” microdialysis study. Neurosci. Lett. 1993;164:145–148. doi: 10.1016/0304-3940(93)90878-o. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J. Pharmacol. Exp. Ther. 1991;258:882–890. [PubMed] [Google Scholar]
- Hirabayashi M, Alam MR. Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol Biochem. Behav. 1981;15:925–932. doi: 10.1016/0091-3057(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. Pegamon Press; New York: 1990. pp. 522–573. [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine: I. Dopamine axon terminals. J. Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kazahaya Y, Akimoto K, Otsuki S. Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biol. Psychiatry. 1989;25:903–912. doi: 10.1016/0006-3223(89)90270-9. [DOI] [PubMed] [Google Scholar]
- Kolta MG, Shreve P, De Souza V, Uretsky NJ. Time course of the development of the enhanced behavioral and biochemical responses to amphetamine after pretreatment with amphetamine. Neuropharmacology. 1985;24:823–829. doi: 10.1016/0028-3908(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Kolta MG, Shreve P, Uretsky NJ. Effect of pretreatment with amphetamine on the interaction between amphetamine and dopamine neurons in the nucleus accumbens. Neuropharmacology. 1989;28:9–14. doi: 10.1016/0028-3908(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Kramer J, Fischman V, Littlefield D. Amphetamine abuse. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J. Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane DK, Martonyi BJ, Robinson TE. An inexpensive automated system for the measurement of rotational behavior in small animals. Behav. Res. Meth. Inst. Computers. 1992;243414:419. [Google Scholar]
- Munn EM, Wise RA. The effects of escalating doses of d-amphetamine (AMPH) on lateral hypothalamic intracranial self-stimulation (ICSS) SOC. Neurosci. Abstr. 1992;18:364. [Google Scholar]
- Nauta WJH. Reciprocal links of the corpus striatum with the cerebral cortex and limbic system: A common substrate for movement and thought? In: Mueller M, editor. Neurology and Psychiatry: A Meeting of Minds. Karger; Basel: 1989. pp. 43–63. [Google Scholar]
- Nelson LR, Ellison G. Enhanced sterotypes after repeated injections but not continuous amphetamines. Neuropharmacology. 1978;17:1081–1084. doi: 10.1016/0028-3908(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Patrick SL, Thompson TL, Walker JM, Patrick RL. Concomitant sensitization of amphetamine-induced behavioral stimulation and in vivo dopamine release from the rat caudate nucleus. Brain Res. 1991;538:343–346. doi: 10.1016/0006-8993(91)90453-3. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav. Neurosci. 1994;108:624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. The time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Raven Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Post R. Intermittent versus continuous stimulation: Effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Contel NR. Human and animal studies of cocaine: Implications for development of behavioral pathology. In: Creese I, editor. Stimulants: Neurochemical, Behavioral and Clinical Perspectives. Raven Press; New York: 1983. pp. 169–203. [Google Scholar]
- Roberts-Lewis JM, Roseboom PH, Iwaniec LM, Gnegy ME. Differential down-regulation of D1-stimulated adenylate cyclase activity in rat forebrain after in vivo amphetamine treatments. J. Neurosci. 1986;6:2245–2251. doi: 10.1523/JNEUROSCI.06-08-02245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: Characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology. 1984;84:466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE. The neurobiology of amphetamine psychosis: Evidence from studies with an animal model. In: Nakazawa T, editor. Taniguchi Symposia on Brain Sciences, Vol. 14, Biological Basis of Schizophrenia. Japan Scientific Societies Press; Tokyo: 1991. pp. 185–201. [Google Scholar]
- Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur. J. Pharmacol. 1982;85:253–254. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol. Biochem. Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. The feasibility of repeated microdialysis for within-subjects design experiments: Studies on the mesostriatal dopamine system. In: Robinson TE, Justice JB Jr., editors. Microdialysis in the Neurosciences. Elsevier; Amsterdam: 1991. pp. 189–234. [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: Sex differences. Brain Res. 1982;253:231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by past experience with (+)-amphetamine: A microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Gnegy ME. Acute in vivo amphetamine produces a homologous desensitization of dopamine receptor-coupled adenylate cyclase activities and decreases agonist binding to the D1 site. Mol. Pharmacol. 1989;34:148–156. [PubMed] [Google Scholar]
- Rossetti ZL, Hmaiden Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: A common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur. J. Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Segal DS. Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv. Biochem. Psychopharmacol. 1975;13:247–262. [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- Segal DS, Geyer MA, Schuckit MA. Stimulant-induced psychosis: An evaluation of animal models. Essays Neurochem. Neuropharmacol. 1981;5:95–129. [PubMed] [Google Scholar]
- Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: An in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- Wages SA, Church WH, Justice JB. Sampling considerations for on-line microbore liquid chromatography of brain dialysate. Anal. Chem. 1986;58:1649–1656. doi: 10.1021/ac00121a012. [DOI] [PubMed] [Google Scholar]
- White FJ, Wolf ME. Psychomotor stimulants. In: Pratt J, editor. The Biological Bases of Drug Tolerance and Dependence. Academic Press; New York: 1991. pp. 153–197. [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res. Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wilcox RA, Robinson TE, Becker JB. Enduring enhancement in amphetamine-stimulated striatal dopamine release in vitro produced by prior exposure to amphetamine or stress in vivo. Eur. J. Pharmacol. 1986;124:375–376. doi: 10.1016/0014-2999(86)90244-x. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J. Pharmacol. Exp. Ther. 1993;264:249–255. [PubMed] [Google Scholar]
- Yamada S, Kojima H, Yokoo H, Tsutsumi T, Takamuki K, Anraku S, Nishi S, Inanaga K. Enhancement of dopamine release from striatal slices of rats that were subchronically treated with methamphetamine. Biol. Psychiatry. 1988;24:399–408. doi: 10.1016/0006-3223(88)90176-x. [DOI] [PubMed] [Google Scholar]