Abstract
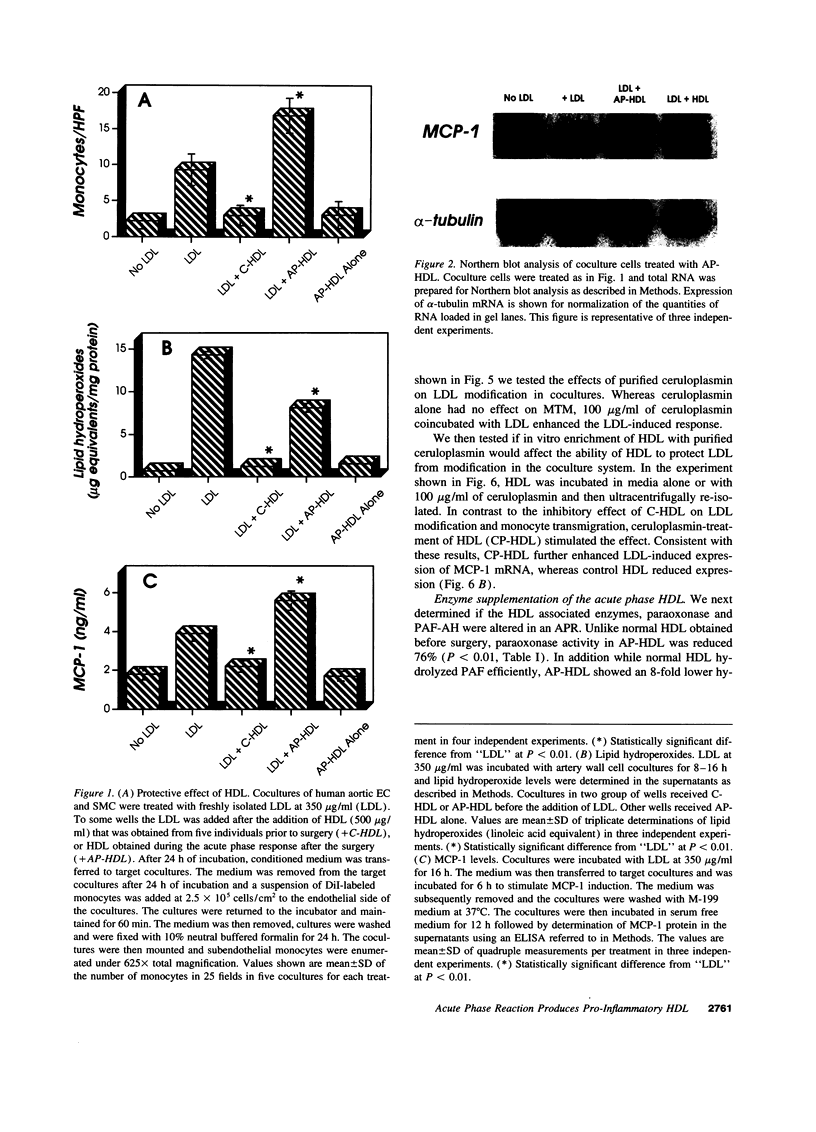
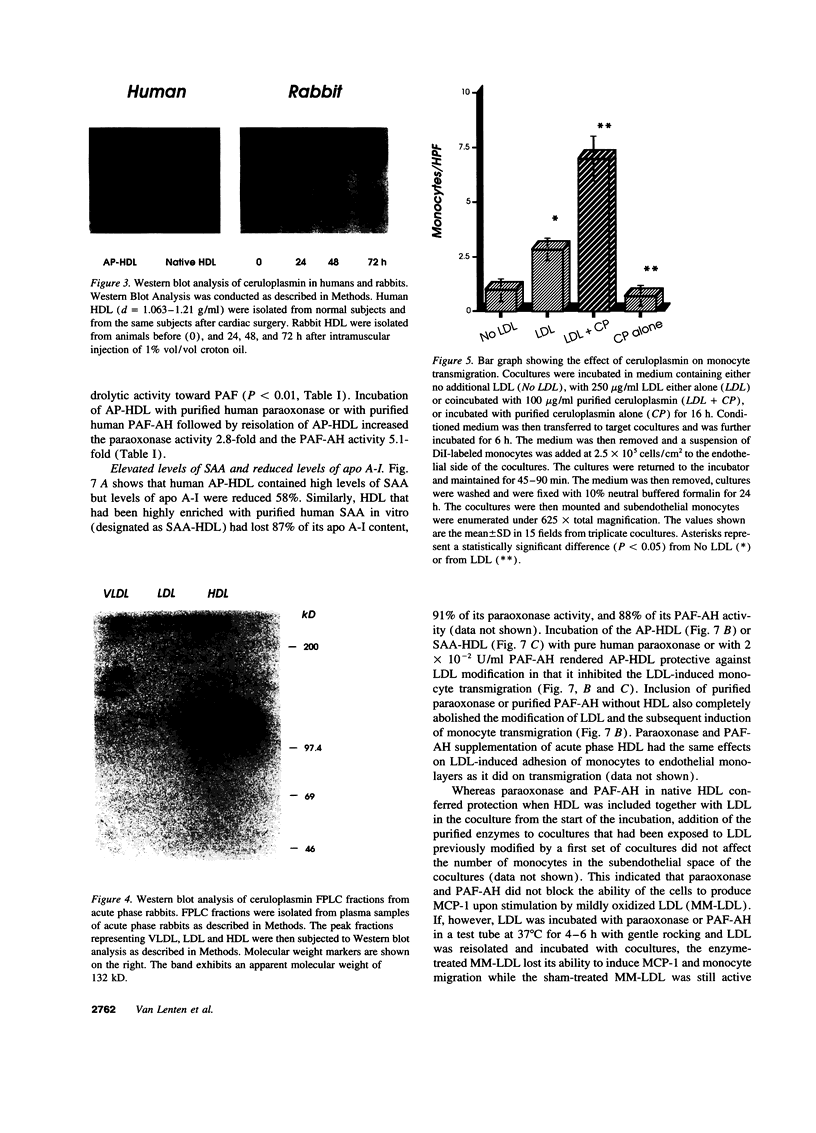
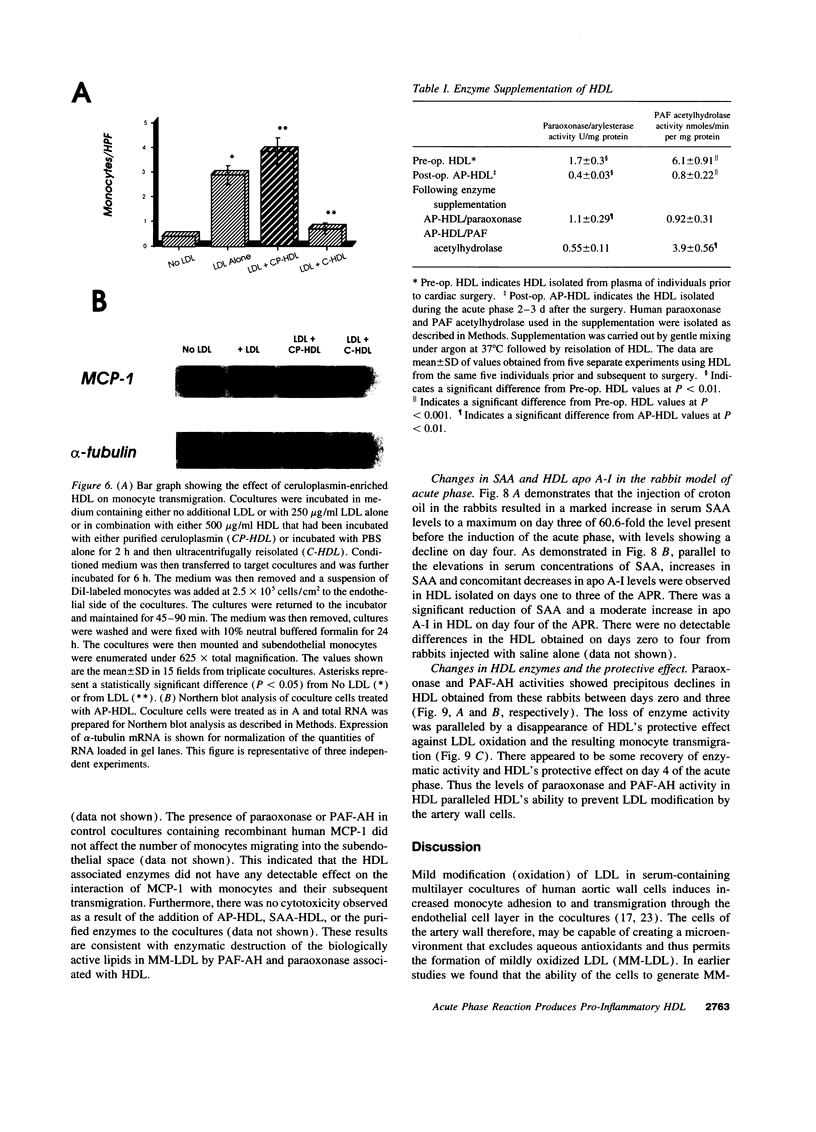
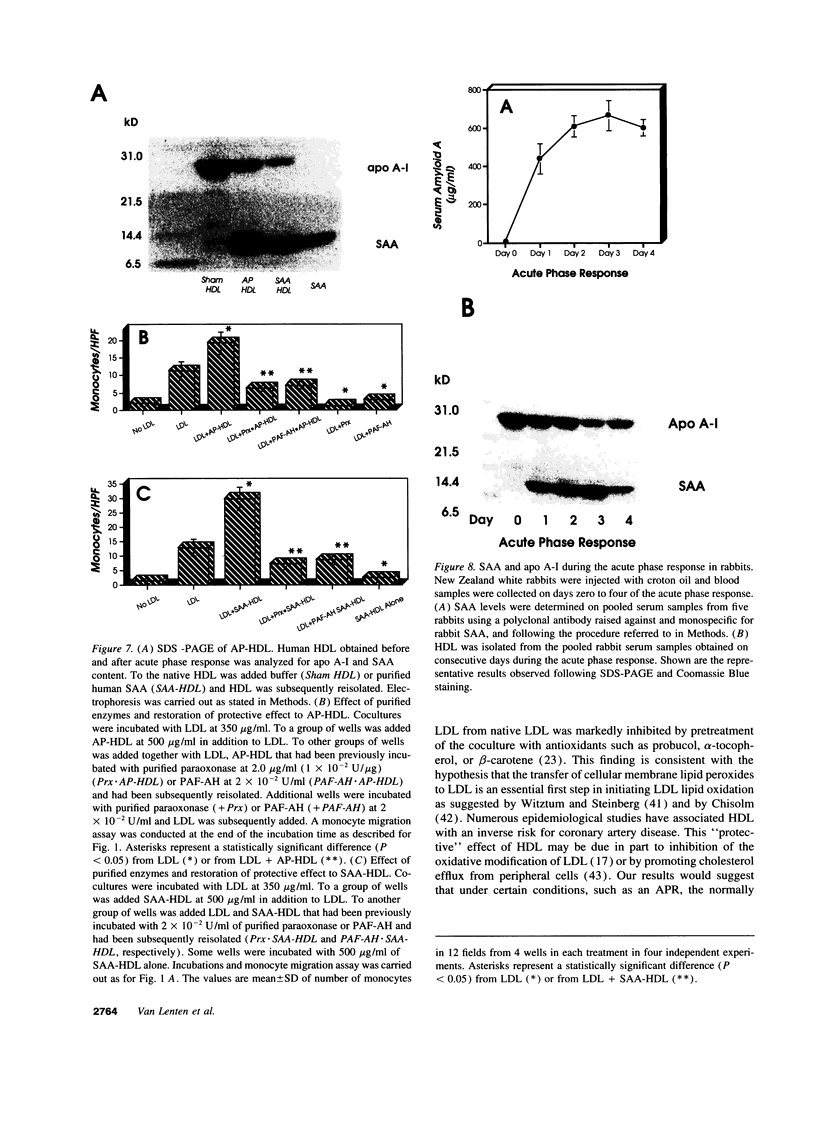
We previously reported that high density lipoprotein (HDL) protects against the oxidative modification of low density lipoprotein (LDL) induced by artery wall cells causing these cells to produce pro-inflammatory molecules. We also reported that enzyme systems associated with HDL were responsible for this anti-inflammatory property of HDL. We now report studies comparing HDL before and during an acute phase response (APR) in both humans and a croton oil rabbit model. In rabbits, from the onset of APR the protective effect of HDL progressively decreased and was completely lost by day three. As serum amyloid A (SAA) levels in acute phase HDL (AP-HDL) increased, apo A-I levels decreased 73%. Concomitantly, paraoxonase (PON) and platelet activating factor acetylhydrolase (PAF-AH) levels in HDL declined 71 and 90%, respectively, from days one to three. After day three, there was some recovery of the protective effect of HDL. AP-HDL from human patients and rabbits but not normal or control HDL (C-HDL) exhibited increases in ceruloplasmin (CP). This increase in CP was not seen in acute phase VLDL or LDL. C-HDL incubated with purified CP and re-isolated (CP-HDL), lost its ability to inhibit LDL oxidation. Northern blot analyses demonstrated enhanced expression of MCP-1 in coculture cells treated with AP-HDL and CP-HDL compared to C-HDL. Enrichment of human AP-HDL with purified PON or PAF-AH rendered AP-HDL protective against LDL modification. We conclude that under basal conditions HDL serves an anti-inflammatory role but during APR displacement and/or exchange of proteins associated with HDL results in a pro-inflammatory molecule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla D. S., Campa A., Monteiro H. P. Low density lipoprotein oxidation by stimulated neutrophils and ferritin. Atherosclerosis. 1992 Dec;97(2-3):149–159. doi: 10.1016/0021-9150(92)90128-4. [DOI] [PubMed] [Google Scholar]
- Al-Timimi D. J., Dormandy T. L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977 Nov 15;168(2):283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach B. J., Kiely J. S., Cornicelli J. A. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem. 1992 Mar;201(2):375–380. doi: 10.1016/0003-2697(92)90354-a. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner J. A., Territo M., Almada L., Carter A., Shafonsky E., Fogelman A. M. Monocyte chemotactic factor produced by large vessel endothelial cells in vitro. Arteriosclerosis. 1986 May-Jun;6(3):254–258. doi: 10.1161/01.atv.6.3.254. [DOI] [PubMed] [Google Scholar]
- Blatter M. C., James R. W., Messmer S., Barja F., Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993 Feb 1;211(3):871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- Bustamante J. B., Mateo M. C., Fernandez J., de Quiros B., Manchado O. O. Zinc, copper and ceruloplasmin in arteriosclerosis. Biomedicine. 1976 Sep 30;25(7):244–245. [PubMed] [Google Scholar]
- Cabana V. G., Gewurz H., Siegel J. N. Interaction of very low density lipoproteins (VLDL) with rabbit C-reactive protein. J Immunol. 1982 May;128(5):2342–2348. [PubMed] [Google Scholar]
- Cabana V. G., Siegel J. N., Sabesin S. M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989 Jan;30(1):39–49. [PubMed] [Google Scholar]
- Cabana V. G., Siegel J. N., Sabesin S. M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989 Jan;30(1):39–49. [PubMed] [Google Scholar]
- Cheung M. C., Wolf A. C., Lum K. D., Tollefson J. H., Albers J. J. Distribution and localization of lecithin:cholesterol acyltransferase and cholesteryl ester transfer activity in A-I-containing lipoproteins. J Lipid Res. 1986 Nov;27(11):1135–1144. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenwald E., Chisolm G. M., Fox P. L. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J Clin Invest. 1994 Apr;93(4):1493–1501. doi: 10.1172/JCI117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenwald E., Fox P. L. Isolation of nonlabile human ceruloplasmin by chromatographic removal of a plasma metalloproteinase. Arch Biochem Biophys. 1994 Mar;309(2):392–395. doi: 10.1006/abbi.1994.1129. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Elahi F., Sykes K., Van Lenten B. J., Territo M. C., Berliner J. A. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988 Sep;29(9):1243–1247. [PubMed] [Google Scholar]
- Furlong C. E., Richter R. J., Chapline C., Crabb J. W. Purification of rabbit and human serum paraoxonase. Biochemistry. 1991 Oct 22;30(42):10133–10140. doi: 10.1021/bi00106a009. [DOI] [PubMed] [Google Scholar]
- Gan K. N., Smolen A., Eckerson H. W., La Du B. N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991 Jan-Feb;19(1):100–106. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick C. C., Castellani L. W., Warden C. H., Puppione D. L., Lusis A. J. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J Biol Chem. 1993 Sep 25;268(27):20676–20682. [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Khouw A. S., Parthasarathy S., Witztum J. L. Radioiodination of low density lipoprotein initiates lipid peroxidation: protection by use of antioxidants. J Lipid Res. 1993 Sep;34(9):1483–1496. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb D. J., Leake D. S. Acidic pH enables caeruloplasmin to catalyse the modification of low-density lipoprotein. FEBS Lett. 1994 Jan 31;338(2):122–126. doi: 10.1016/0014-5793(94)80348-x. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Liuzzo G., Biasucci L. M., Gallimore J. R., Grillo R. L., Rebuzzi A. G., Pepys M. B., Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994 Aug 18;331(7):417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- Lorenzen A., Kennedy S. W. A fluorescence-based protein assay for use with a microplate reader. Anal Biochem. 1993 Oct;214(1):346–348. doi: 10.1006/abio.1993.1504. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Abbott C. A., Durrington P. N. Is paraoxonase related to atherosclerosis. Chem Biol Interact. 1993 Jun;87(1-3):161–171. doi: 10.1016/0009-2797(93)90038-z. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Durrington P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991 Jul 29;286(1-2):152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Harty D., Bhatnagar D., Winocour P. H., Arrol S., Ishola M., Durrington P. N. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991 Feb;86(2-3):193–199. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. Redox cycling of iron and lipid peroxidation. Lipids. 1992 Mar;27(3):219–226. doi: 10.1007/BF02536182. [DOI] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Stevenson L. W., Drinkwater D. C., Laks H., Fogelman A. M. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J Clin Invest. 1988 Dec;82(6):1853–1863. doi: 10.1172/JCI113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Takata K., Horiuchi S., Morino Y., Matsuda I. Protective effect of lipoproteins containing apoprotein A-I on Cu2+-catalyzed oxidation of human low density lipoprotein. FEBS Lett. 1989 Nov 6;257(2):435–438. doi: 10.1016/0014-5793(89)81590-x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J., Fong L. G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990 May 22;1044(2):275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Rienhoff H. Y., Jr, Huang J. H., Li X. X., Liao W. S. Molecular and cellular biology of serum amyloid A. Mol Biol Med. 1990 Jun;7(3):287–298. [PubMed] [Google Scholar]
- Samokyszyn V. M., Miller D. M., Reif D. W., Aust S. D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem. 1989 Jan 5;264(1):21–26. [PubMed] [Google Scholar]
- Sato M., Schilsky M. L., Stockert R. J., Morell A. G., Sternlieb I. Detection of multiple forms of human ceruloplasmin. A novel Mr 200,000 form. J Biol Chem. 1990 Feb 15;265(5):2533–2537. [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Prescott S. M. Platelet-activating factor acetylhydrolase from human plasma. Methods Enzymol. 1990;187:344–357. doi: 10.1016/0076-6879(90)87041-z. [DOI] [PubMed] [Google Scholar]
- Stafforini D. M., Rollins E. N., Prescott S. M., McIntyre T. M. The platelet-activating factor acetylhydrolase from human erythrocytes. Purification and properties. J Biol Chem. 1993 Feb 25;268(6):3857–3865. [PubMed] [Google Scholar]
- Steinbrecher U. P., Pritchard P. H. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res. 1989 Mar;30(3):305–315. [PubMed] [Google Scholar]
- Strachan A. F., Shephard E. G., Bellstedt D. U., Coetzee G. A., van der Westhuyzen D. R., de Beer F. C. Human serum amyloid A protein. Behaviour in aqueous and urea-containing solutions and antibody production. Biochem J. 1989 Oct 15;263(2):365–370. doi: 10.1042/bj2630365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. D., Navab M., Hama S. Y., Sevanian A., Prescott S. M., Stafforini D. M., McIntyre T. M., Du B. N., Fogelman A. M., Berliner J. A. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995 Feb;95(2):774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi K., Gonnerman W. A., Debeer F. C., Debeer M. C., Steel D. M., Sipe J. D., Whitehead A. S. Major acute-phase reactant synthesis during chronic inflammation in amyloid-susceptible and -resistant mouse strains. Inflammation. 1991 Feb;15(1):1–14. doi: 10.1007/BF00917905. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Scheffer M., Havekes L., Kempen H. J. Role of endothelial cells and their products in the modification of low-density lipoproteins. Biochim Biophys Acta. 1986 Aug 14;878(1):49–64. doi: 10.1016/0005-2760(86)90343-7. [DOI] [PubMed] [Google Scholar]
- van Lenten B. J., Fogelman A. M. Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-alpha. J Immunol. 1992 Jan 1;148(1):112–116. [PubMed] [Google Scholar]


















