Abstract
Repeated amphetamine (AMPH) administration into the nucleus accumbens does not enhance (sensitize) the locomotor activity produced by a subsequent systemic AMPH challenge. We report here, however, that pretreatment with systemic injections of AMPH does produce a significant enhancement in the locomotor stimulant effects produced by intra-accumbens AMPH given 21 days after the last pretreatment injection of AMPH. These data support the hypothesis that neural adaptations in dopamine (DA) terminal fields are sufficient for the expression of AMPH sensitization, although an action on DA cell bodies may be required for the induction of AMPH sensitization.
Keywords: Sensitization, “Reverse tolerance”, Locomotor activity, Nucleus accumbens, Microinjection, Dopamine
The intermittant administration of amphetamine (AMPH) produces an enduring enhancement in the motor stimulant effects of AMPH, a phenomenon known as behavioral sensitization. Behavioral sensitization to AMPH is thought to be due, at least in part, to enduring changes in mesotelencephalic dopamine (DA) systems, although the locus and nature of the neuronal adaptations responsible for sensitization have not been well characterized (see Robinson and Becker 1986 for review).
A number of researchers have suggested that an action of AMPH in the midbrain, at the level of the DA cell bodies, is necessary for the induction of behavioral sensitization. For example, repeated AMPH injections into the ventral tegmental area, but not into the DA terminal field in the nucleus accumbens, produce an enhanced locomotor response to subsequent peripheral administration of morphine or psychomotor stimulant drugs (Dougherty et al. 1981; Kalivas and Weber 1988; Vezina and Stewart 1990). Also, the local microinjection of a DA D1 receptor antagonist into the ventral tegmental area is sufficient to prevent the development of behavioral sensitization to systemic AMPH treatment (Stewart and Vezina 1989). The idea that an action of AMPH on DA cell bodies is required to induce sensitization is consistent with evidence that the protein synthesis inhibitor, anisomycin, prevents the development of AMPH sensitization (Robinson 1991).
Although the induction of behavioral sensitization may require an action of AMPH at the cell bodies, the expression of sensitization may not. There is considerable evidence showing that the ability of AMPH to enhance locomotor activity is primarily due to its action in DA terminal fields, where it causes DA release. For example, the acute intra-accumbens application of AMPH or DA produces marked locomotor hyperactivity, whereas, administration into the ventral tegmental area does not. Also, a sensitization-related enhancement in DA release is seen in striatal or nucleus accumbens tissue slices, a preparation in which no cell bodies are present (Robinson and Becker 1982; Kolta et al. 1989). We hypothesized, therefore, that the induction of behavioral sensitization may require an action of AMPH in DA somatodendritic regions, but the expression of sensitization is due to changes in the responsivity to AMPH in DA terminal fields. If this hypothesis is correct, animals sensitized by systemic treatment with AMPH should be behaviorally hyper-responsive to a subsequent local AMPH challenge into the nucleus accumbens. The purpose of the experiment reported here was to test this hypothesis.
Female Holtzman rats weighing 200–250 g at the start of the experiment were injected with either saline (n = 10) or escalating doses (1–10 mg/kg) of d-AMPH sulfate (n = 13) twice daily on each consecutive weekday for 6 weeks, but not on weekends (see Robinson and Camp 1987 for a description of the injection regimen). Two weeks after the discontinuation of pretreatment, 23 g guide cannulae were stereotaxically placed 2.6 mm below the surface of the neocortex, bilaterally, above the nucleus accumbens in each hemisphere. One week after surgery (20 days after the last pretreatment injection) animals were placed into automated activity monitors (41 × 24 × 18 cm), which are described in detail elsewhere (Robinson and Camp 1987), and allowed to habituate overnight. The next morning 30 g microinjection cannulae were lowered into the nucleus accumbens in both hemispheres via the guide cannulae, and fixed in place. After 1 min, 0.5 μl 0.9% saline was infused over a period of 1 min using a Harvard syringe pump, and the injection cannulae left in place for an additional minute, before being removed. Locomotor activity (crossovers) was recorded in 5-min intervals over the next hour. Next, the injection cannulae were lowered again and 10μg d-AMPH, in a volume of 0.5 μl, was administered to each animal using the same procedure as described for saline. This dose was based on previous studies showing that it was effective in inducing locomotor activity (Carr and White 1987). The injection cannulae were removed I min later, and locomotor activity was recorded for the next hour. Only animals with cannulae located bilaterally within the nucleus accumbens, verified histologically, were included in the analysis.
Saline infusion and the associated handling produced a very small increase in locomotor activity that subsided within 10 min (Fig. 1). There was, however, no effect of pretreatment condition. In contrast, intra-accumbens AMPH produced a marked increase in locomotor activity that peaked 20 min after the injection and then slowly subsided over the 1-h observation period (Fig. 1). The increase in locomotor activity produced by intra-accumbens AMPH was significantly greater in animals pretreated with AMPH than in saline-pretreated controls (F = 5.79, P < 0.02).
Fig. 1.
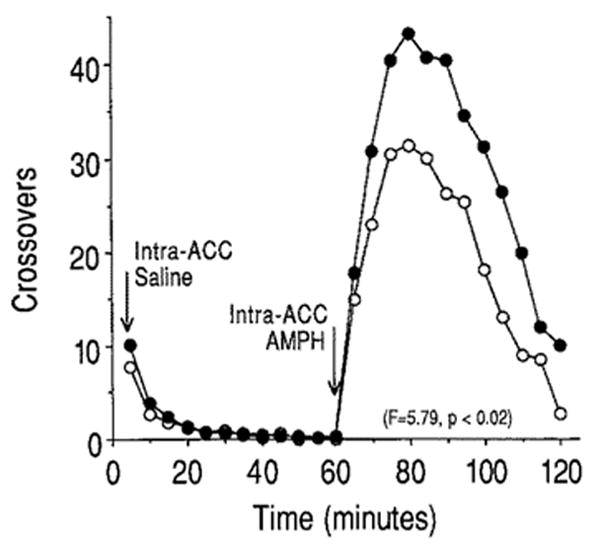
The mean number of crossovers from one side of the test chamber to the other (locomotor activity) recorded for the 1 h following an intra-accumbens injection of saline (0–60 min) and for 1 h following the intra-accumbens injection of 10 μg amphetamine (65–120 min). Animals were tested 3 weeks after discontinuation of pretreatment with either saline (open symbols) or escalating doses (1–10 mg/kg) of amphetamine (closed symbols)
In summary, pretreatment with systemic injections of AMPH produced a significant enhancement in the locomotor stimulant effects of intra-accumbens AMPH given 21 days after the last pretreatment injection of AMPH (also see Rebec and Segal 1979; Kolta et al. 1989). These data support the hypothesis that neural adaptations in DA terminal fields are sufficient for the expression of AMPH sensitization, although an action on DA cell bodies may be required for the induction of AMPH sensitization.
References
- Cart GD, White NM. Effects of systemic and intracranial amphetamine injections on behavior in the open field: a detailed analysis. Pharmacol Biochem Behav. 1987;27:113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Dougherty GG, Ellinwood EH. Chronic d-amphetamine in nucleus accumbens: lack of tolerance or reverse tolerance of locomotor activity. Life Sci. 1981;28:2295–2298. doi: 10.1016/0024-3205(81)90582-8. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kolta MG, Shreve P, Uretsky NJ. Effect of pretreatment with amphetamine on the interaction between amphetamine and dopamine neurons in the nucleus accumbens. Neuropharmacology. 1989;28:9–14. doi: 10.1016/0028-3908(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Segal DS. Enhanced responsiveness to intraventricular infusion of amphetamine following its repeated systemic administration. Psychopharmacology. 1979;62:101–102. doi: 10.1007/BF00426043. [DOI] [PubMed] [Google Scholar]
- Robinson TE. The neurobiology of amphetamine psychosis: evidence from studies with an animal model. In: Nakazawa T, editor. Taniguchi Symposia on Brain Sciences. Vol. 14. 1991. Biological Basis of Schizophrenic Disorders (in press) [Google Scholar]
- Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982;85:253–254. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Microinjections of Sch-23390 into the ventral tegmental area and substantia nigra pars reticulata attenuate the development of sensitization to the locomotor activating effects of systemic amphetamine. Brain Res. 1989;495:401–406. doi: 10.1016/0006-8993(89)90236-9. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]


