Abstract
The amplitude of the H-reflex increases chronically after incomplete SCI and is associated with the development of exaggerated hindlimb reflexes. Although the mechanism for this increased H-reflex is not clear, previous studies have shown that pharmacological activation of the 5-HT2 receptors (5-HT2R) can potentiate the monosynaptic reflex. This study tested the hypothesis that increased expression of 5-HT2R on motoneurons is involved in increased H-reflex amplitude after a standardized clinically-relevant contusive SCI. Adult female rats were subjected to contusion, complete surgical transection, or a T8 laminectomy only. At 4wks after surgery, H-reflex recordings from the hindpaw plantar muscles of contused rats showed twice the amplitude of that in laminectomy controls or transected rats. To probe the role of 5-HT2R in this increased amplitude, dose response studies were done with the selective antagonists, mianserin or LY53857, and the 5-HT2R agonist, (±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI). The drugs were intrathecally infused into the lumbar cord while recording the H-reflex. Mianserin did not have any significant effects on the H-reflex after transection, consistent with the loss of distal serotonergic innervation. After contusion, both 5-HT2R antagonists reduced the H-reflex reflex amplitude with a significantly higher ID50 compared to the uninjured controls. The 5-HT2R agonist, DOI, significantly increased reflex amplitude in contused but not control rats. Furthermore, while 5-HT immunoreactivity was similar, contused rats displayed increased 5-HT2AR immunoreactivity in plantar muscle motoneurons compared to uninjured controls. We conclude that increased expression of 5-HT2R is likely to be involved in the enhanced H-reflex that develops after contusive SCI.
Keywords: MASCIS, contusion, transection, spasticity, serotonin, monosynaptic Ia afferent reflex, hyperreflexia, H-reflex pharmacology, lumbar spinal cord, 5-HT2A receptor
Introduction
Segmental hind limb reflexes located in the lumbar spinal cord are heavily influenced by descending and ascending pathways (Carpenter, et al., 1966, Chen, et al., 2001). When these pathways are disrupted after spinal cord injury (SCI), abnormalities of the local lower limb reflexes often develop (Hornby, et al., 2004, Thompson, et al., 1992). One of the most commonly studied local reflexes after SCI is the monosynaptic connection between the primary muscle afferents and the alpha motoneurons, which is often studied in patients and animals using the Hoffman (H)-reflex. The H-reflex is elicited by an electrical stimulation of a peripheral nerve that innervates the muscle from which the reflex is recorded. A single stimulus generates a short-latency M-wave, resulting from direct stimulation of the motor axons innervating the muscle, and a long-latency H-wave, which is a measure of the alpha motoneurons activated by Ia afferents (Gozariu, et al., 1998).
Properties of the H-reflex are known to be affected in both humans and rodents after SCI (Lee, et al., 2005, Schindler-Ivens and Shields, 2000, Thompson, et al., 1998, Valero-Cabre and Navarro, 2001). One of these alterations is elevated reflex amplitude that develops over time after SCI (Lee, et al., 2005, Thompson, et al., 1998). The mechanism for this elevated reflex is still unclear. Our previous study demonstrated that the increased H-reflex amplitude observed at 4wks after different severities of contusive SCI in rats is positively correlated with serotonin immunoreactivity around motoneurons involved in the reflex (Lee, et al., 2005). That is, the greater their apparent chronic serotonergic (re)innervation the more abnormally elevated their reflex activation. Pharmacological activation of 5-HT2 receptors (5-HT2R) has been shown to potentiate the monosynaptic reflex and restore the excitability of the extensor stretch reflex in spinalized rat and cat preparations, respectively (Hasegawa and Ono, 1996, Miller, et al., 1996), in which serotonergic innervation has been completely lost. However, whether alterations in 5-HT2 receptors are involved in the elevated H-reflex after SCI, particularly after clinically-relevant incomplete contusive SCI, has not previously been examined.
We therefore investigated the role of 5-HT2R in the increased H-reflex amplitude observed after a standardized incomplete contusive SCI using a well-characterized rat model of injury (Basso, et al., 1996, Lee, et al., 2005, Pikov and Wrathall, 2001). We employed local intrathecal administration of the 5-HT2R antagonists, mianserin and LY53857, and the agonist, DOI, as well as immunohistochemistry of 5-HT2R expression on plantar motor neurons and found evidence in support of the hypothesis that increased 5-HT2R expression by plantar muscle motoneurons is causally related to the increased H-reflex amplitude of the plantar muscle after contusive SCI.
Materials and Methods
Spinal cord injury and animal care
All animal protocols were in accordance with NIH Guide for the Care and Use of Laboratory Animals and approved by the Georgetown University Animal Care and Use Committee. Adult female Sprague-Dawley rats (200–250g, Zivic Miller, Pittsburgh, PA) were housed 2 or 3 per cage, with food and water provided ad libitum, and kept on a 12 hour light-dark cycle. Animals were generated for two independent sets of experiments, which will be referred to as the mianserin study and the LY53857/DOI study. The details of the drug administration are discussed below. The mianserin study consisted of the following experimental groups: uninjured controls (n=5), 4wk transection SCI (n=6), and 4wk contusion SCI (n=7). The LY53857/DOI study consisted of the following experimental groups: uninjured controls (n=4), and 4wk contusion SCI (n=5). For each study, rats were assigned to the different experimental groups according to a randomized block design.
Rats were anesthetized with chloral hydrate (360mg/kg, i.p., Sigma, St.Louis, MO) and a laminectomy was performed at T8 to remove the vertebral bone overlying the spinal cord, exposing a circle of dura. The spinal column was stabilized by clamping the T7 and T9 vertebra, and the MASCIS weight drop device (Basso, et al., 1996) was used to produce a Mild contusive SCI with a 10g impounder dropped from 12.5mm. This model of SCI has been well-characterized and is widely used in a number of laboratories (for review see Young, 2002). The contusion results in a central hemorrhagic lesion that spares a peripheral rim of white matter at the lesion epicenter and partially spares descending control fibers such as some of the serotonergic fibers from the brainstem to the plantar motor neurons, as illustrated in our initial report examining H-reflex alterations (Lee et al., 2005). For transections, irridectomy scissors were used to completely sever the spinal cord at T8, and gel foam was placed into the lesion site. No serotonergic innervation of the lumbar-sacral spinal cord remains after this complete SCI (Lee et al, 2005). Age-, sex-, and weight-matched controls were only laminectomized. After injury, bladders were manually expressed twice daily until no longer needed and oral antibiotics (sulfamethoxazole and trimethoprim oral suspension, 4mg/1mg, Hi-Tech Pharmacal Co., Inc, Amityville, NY) were given in cases of suspected urinary tract infections.
Behavioral analysis
All rats were tested for behavioral deficits on day 1 after injury and weekly thereafter, using the Combined Behavioral Score (CBS, Gale, et al., 1985) and the Basso, Beattie, and Bresnahan (BBB, Basso, et al., 1995) scale. The CBS provides an overall measure of hindlimb functional deficits, ranging from 100 (most deficits) to 0 (normal hindlimb function). The BBB is a neurological rating scale of the use of hindlimbs in open field locomotion after SCI, ranging from 0 (complete hindlimb paralysis) to 21 (normal hindlimb locomotion). All animals in the study displayed a BBB score of 1 for both hindlimbs at 1 day after SCI, confirming the expected acute behavioral effect of SCI.
Intrathecal infusion of drugs
The drugs used in this study were 1,2,3,4,10,14b-hexahydro-2-methyl-dibenzopyrazinoazepine hydrochloride (mianserin, Sigma-Aldrich, St. Louis, MO), 6-methyl-1-(1-methylethyl)ergoline-8β-carboxylic acid 2-hydroxy-1-methylpropyl ester maleate (LY53857, Sigma-Aldrich), and (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI, Research Biochemicals, Inc., Natick, MA). Mianserin (2.69, 5.38, 10.75, 21.5, 43nmol), LY53857 (0.78, 1.56, 3.13, 6.25, 12.5, 25, 50nmol), and DOI (31, 93nmol) were dissolved in saline, and backfilled in 10μl increments into PE-10 (Becton Dickinson, Sparks, MD) tubing connected to a 1cc syringe (Becton Dickinson) filled with saline. The highest dose was backfilled first, followed by decreasing doses separated by a small air bubble to prevent mixing, finishing with 10 μl of saline. The syringe was then placed on a Harvard ‘22’ syringe pump (Harvard Apparatus, Holliston, MA), and the PE-10 tubing filled with different doses of a single drug was used as an intrathecal catheter.
Intrathecal (i.t.) catheterization via the lumbar subarachnoid space was performed as previously described (Storkson, et al., 1996). Briefly, animals were anesthetized using chloral hydrate (360mg/kg, i.p.) and a 19 gauge needle (Becton Dickinson) containing the drug-filled PE-10 tubing as prepared above was inserted between the L5 and L6 vertebrae. The PE-10 tubing was advanced to the L5-L6 region of the spinal cord while ensuring ease of catheter advancement and easy withdrawal of cerebrospinal fluid into the tubing.
Saline/drugs were infused in 10μl increments at a rate of 1.7μl/min, allowing each dose to diffuse for 10 minutes before recording the H-reflex, as described below. Each animal was infused with saline first, followed by increasing doses of a drug. The H-reflex recording after saline infusion was used to calculate the percent change in the H-reflex at the successive doses of a drug. Saline was not infused between each dose in order to shorten the duration of the recording protocol as much as possible, thereby maximizing the integrity of the reflex. For the mianserin study, mianserin was infused in uninjured control, contusion SCI, and transection SCI rats. For the LY53857/DOI study, LY53857 was infused in an independent set of uninjured control and contusive SCI rats. Four days after measuring the effect of LY53857 on the H-reflex, these animals were re-catheterized for DOI infusions. Behavioral tests (BBB and CBS) one day prior to the DOI infusion were performed to confirm that the initial recording protocol did not have any adverse effects on the animals.
H-reflex recording
Once the animal had been catheterized as described above, additional anesthesia (approximately 50mg/kg every 20–30 minutes) was given throughout the recording procedure to maintain the plane of anesthesia as indicated by the absence of response to forelimb pinch, whisker tremors, and corneal reflex. Chloral hydrate has been shown to produce similar negligible effects on the H-reflex as ketamine (Cliffer, et al., 1998), which is commonly used for H-reflex recordings in rats. Saline (2cc s.c.) was injected approximately 30 minutes before recording to ensure proper hydration of each animal. The animal’s forelimbs and hindlimbs were secured with tape onto a grounded, heated (approximately 37ºC) metal platform. Hindlimbs were only slightly extended away from the trunk (kept consistent between animals), and care was taken not to apply any unnecessary pressure or stretch to any part of the limbs. H-reflex was recorded from the plantar muscles of the left or right hindpaw, chosen randomly, with the active needle electrode (30-gauge) inserted between the fourth and fifth metatarsals, and the reference electrode inserted in the skin of the fifth digit. We elicited the H-reflex by stimulating the tibial nerve at the ankle for 0.1ms at 0.1Hz using a Grass S88 stimulator (Astro-Med, Inc., West Warwick, RI). The cathode needle was inserted subcutaneously at the ankle, just above the heel, and the anode needle was inserted subcutaneously at the plantar surface of the heel. The intensity of the stimulus was adjusted to elicit the maximal M-wave and the maximal H-wave amplitudes. The recorded signal was passed to a differential amplifier (Tektronix AM 502, Richardson, TX) and bandpass filtered at 0.1Hz and 10kHz. The analog signal was then sent to an A/D converter (model PCI-MIO-16E-4; National Instruments, Austin, TX), and the digital waveform (recorded at 30kHz) was stored and displayed online using LabView 7 data acquisition software (National Instruments, Austin, TX). At the end of the infusion of a dose of a drug and the ensuing 10min delay, ten consecutive waveforms were collected. The maximal amplitudes of M- and H-waves as determined by the peak-to-peak values of each waveform were used to calculate the average H/M ratio. The H/M ratio after the initial saline infusion was used to calculate the percent change in the H/M ratio at each ensuing drug dose.
5-HT Immunohistochemistry
After H-reflex recording, rats were deeply re-anesthetized with chloral hydrate (720mg/kg, i.p.) and transcardially perfused with 0.9% saline followed by 0.1M phosphate buffered 4% paraformaldehyde. Spinal cords were removed and post-fixed for 1 hour followed by 10% and 20% sucrose gradients for cryoprotection. Lumbar spinal cords from uninjured control and SCI groups were embedded together in groups of 4 per OCT (Fisher, Pittsburgh, PA) block in order to minimize variability in tissue processing. Ten micrometer coronal serial sections were cut using a Jung Frigocut 2800 E (Leica, Bannockburn, IL) cryostat and thaw-mounted onto Superfrost Plus slides (Fisher, Pittsburgh, PA).
Slides containing the rostral Dorsolateral nucleus (rDLn), in which the plantar muscle motoneurons are located as previously described (Crockett, et al., 1987, Lee, et al., 2005), were used for 5-HT immunohistochemistry. Sections were post-fixed for 5 minutes in 10% buffered formalin (Fisher, Pittsburgh, PA), treated with 0.3% hydrogen peroxide for 45 minutes, and incubated overnight at room temperature with serotonin antibody (1:40,000, Immunostar, Hudson, WI) diluted in 5% normal goat serum in high salt buffered solution (500mM NaCl, 9.2mM NaH2PO4, 12.5mM Na2HPO4, 0.3% Triton-X100). Next day, sections were incubated in biotinylated goat anti-rabbit IgG secondary antibody (1:300, Vector, Burlingame, CA) followed by Vector Elite ABC kit for an hour each. Immunoreactivity was visualized with nickel-enhanced DAB (Sigma, St. Louis, MO). Photographs of three consecutive sections of the left and right rDLn (6 total), which contain the plantar muscle motoneurons, included in the field of view were captured with a Zeiss Axiocam camera mounted on a Zeiss Axiophot microscope using Axiovision software. Immunoreactivity was quantified using NIH Scion Image software to count the number of immunopositive pixels set above a threshold to selectively detect serotonin immunoreactivity. The total number of immunoreactive pixels for the six photos were averaged to represent the 5-HT immunoreactivity (5-HT-IR) for each animal.
5-HT2AR Immunohistochemistry
For 5-HT2AR immunohistochemistry, we used spinal cord sections adjacent to those used for 5-HT immunohistochemistry as described above. Sections were post-fixed for 5 minutes in 10% buffered formalin (Fisher, Pittsburgh, PA), treated with 0.3% hydrogen peroxide for 45 minutes, and incubated overnight at 4°C with a rabbit 5-HT2A receptor antibody (1:400, Immunostar, Hudson, WI) diluted in 3% bovine serum albumin (Fisher, Pittsburgh, PA) in PBS-triton (1X PBS, 0.3% Triton-X100). No-primary controls were incubated in 3% bovine serum albumin without 5-HT2A receptor antibody. Next day, sections were incubated in biotinylated goat anti-rabbit IgG secondary antibody (1:200, Vector, Burlingame, CA) followed by Vector Elite ABC kit for 30min each. Immunoreactivity was visualized with nickel-enhanced DAB (Sigma, St. Louis, MO). Slides were dehydrated in increasing ethanol gradients and finally with xylene before coverslipping with Permount (Fisher, Pittsburgh, PA). Photographs were captured with a Zeiss Axiocam camera mounted on a Zeiss Axiophot microscope using Axiovision software. For each animal, photographs of three consecutive sections of rDLn were taken.
Immunoreactivity of each section was quantified using the NIH Scion Image software to measure the mean optical density within a boundary drawn around the rDLn (Fig. 6D) or individual motoneurons (Fig. 6E) located within the field of view. The boundary drawn around the motoneurons was also used to calculate the area of individual motoneurons (Fig. 6F). Motoneurons were identified by the presence of a nucleus as well as a nucleolus under phase contrast microscopy so that the same motoneuron would not be counted twice in adjacent sections. Background mean optical density was also measured for each section by using the white matter near the rDLn that did not contain 5-HT2AR immunoreactivity (5-HT2AR-IR). 5-HT2AR-IR ratio (Fig. 6) was calculated by dividing the mean optical density of the rDLn or individual motoneurons by the mean optical density of the background. 5-HT2AR-IR ratio and motoneuron area for each of the three sections was averaged to represent the final 5-HT2AR-IR ratio per animal.
Figure 6.
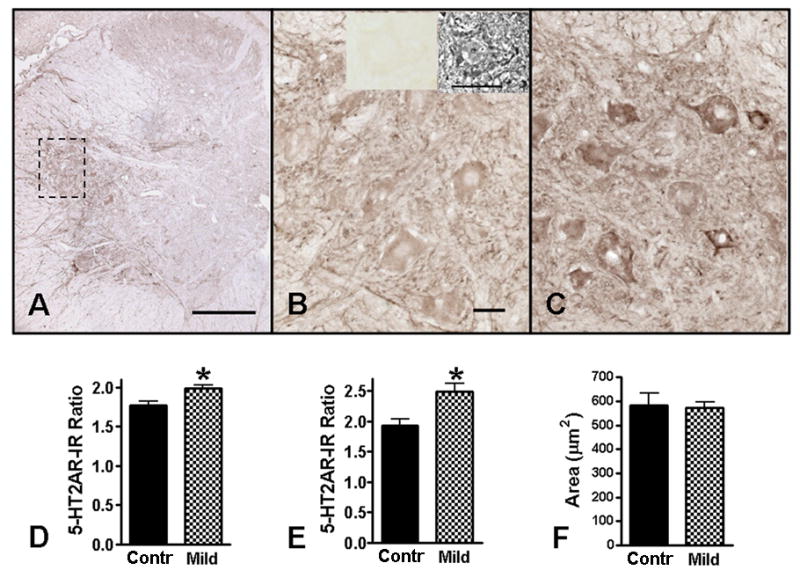
5-HT2A receptor immunoreactivity (5-HT2AR-IR) in the rostral dorsolateral nucleus (rDLn) of the L5-L6 spinal cord. B is a higher magnification photo of the enclosed area of the rDLn in A (uninjured control). The inserts on top of B are phase contrast (right) and brightfield (left) photos of a motoneuron in a section of the same uninjured spinal cord that has been processed without 5-HT2AR antibody (i.e., a no primary control). C is a representative higher magnification photo of the same region in a 4wk Mild SCI animal. In uninjured controls, the 5-HT2AR-IR was strongest around the peripheral rim and motoneuron pools in the ventral horn gray matter (A). At 4wks after Mild SCI (C), the 5-HT2AR-IR was increased compared to uninjured controls (B). Quantification of the optical density of 5-HT2AR-IR indicated a significant increase in the rDLn (D) as well as in the somas of the individual motoneurons located within the rDLn (E) of 4wk Mild SCI animals (n = 5) compared to uninjured controls (n = 4). The average motoneuron area in the uninjured controls and the 4wk Mild SCI animals was not statistically different from each other (F), thus did not contribute to the differences in 5-HT2AR-IR. Scale bar in A = 300μm. Scale bars in B, C = 30μm. *p<0.05 as compared to controls, unpaired t-test.
Statistical analysis
Statistical differences in the behavioral scores were calculated by two-way repeated measures ANOVA with Bonferonni post-test to compare the uninjured control, contusion, and transection groups at 1day and 4wks after SCI. Statistical differences between the H/M ratios of the different injury groups at each drug dose were also calculated using a two-way ANOVA with Bonferonni post-test. The dose of mianserin and LY53857 required to produce a 50% decrease in the H/M ratio (ID50) was calculated by non-linear regression analysis. Statistical differences in ID50, 5-HT2AR-IR ratio, 5-HT-IR pixels, and motoneuron area between control and contusion groups were calculated using unpaired t-tests. We used GraphPad Prism 4.0 for all statistical analysis and non-linear regression. Error bars in all figures represent standard error of the mean.
Results
Behavioral scores after SCI
Both the Mild and the transection groups showed almost complete hindlimb paralysis at 1 day after injury (BBB 1), but then recovered to different extents by 4wks, when the BBB and CBS scores have reached a plateau (Fig.1). At 4wks after SCI, most transected animals showed slight movements of all three joints of the hindlimb without any plantar placement of the hindpaws, whereas most Mild injured animals showed weight-supported plantar stepping with frequent coordination of the forelimb and hindlimb as defined by the BBB scale.
Figure 1.
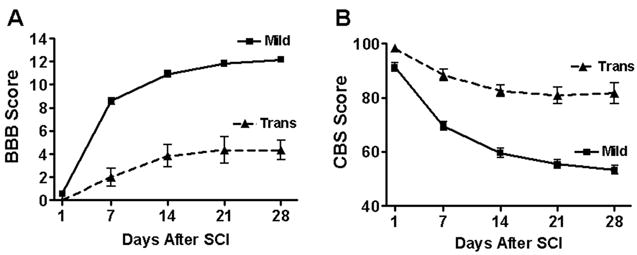
Recovery of hindlimb function after SCI. Hindlimb deficit was greatest at 1 day after SCI on both the BBB (A) and the CBS (B), and recovery reached a plateau by 4 weeks. The Mild group (n = 12) displayed better behavioral scores than transection group (n = 6) at all time points tested, except day 1. Average BBB scores of right and left hindlimb were not different from each other (right hindlimb shown). Uninjured controls (n = 9) did not show any behavioral deficits at any time points tested, as indicated by a BBB score of 21 and CBS score of 0. Data represents animals from both mianserin and LY53857/DOI studies. In some cases, error bars are smaller than symbol.
CBS includes a subset of tests for withdrawal reflexes in response to hindlimb extension, hindpaw pain and pressure (Gale, et al., 1985), and their changes in response to transection and Mild contusion SCI has been previously described (Lee, et al., 2005). Uninjured control animals had normal BBB and CBS scores (21 and 0, respectively) at all time points tested. Figure 1 represents behavioral scores of rats from both the mianserin study and the LY53857/DOI study. The BBB and CBS scores of rats in both studies were not significantly different from each other and thus were combined. The BBB and CBS scores between the LY53857 and DOI infusions (1 day prior to DOI) were not significantly different from pre-LY53857 values.
H-reflex amplitude after SCI
At 4wks after Mild SCI, the H/M ratio of the hindpaw plantar muscles was approximately twice that of uninjured controls. Transection did not result in an increased H/M ratio (Fig. 2). Figure 2 represents H/M ratios from animals in both the mianserin and LY53857/DOI studies, which did not differ in their average H/M ratios (One-way ANOVA, p>0.05) and thus were combined. The M-wave amplitude was similar among the three groups (data not shown).
Figure 2.
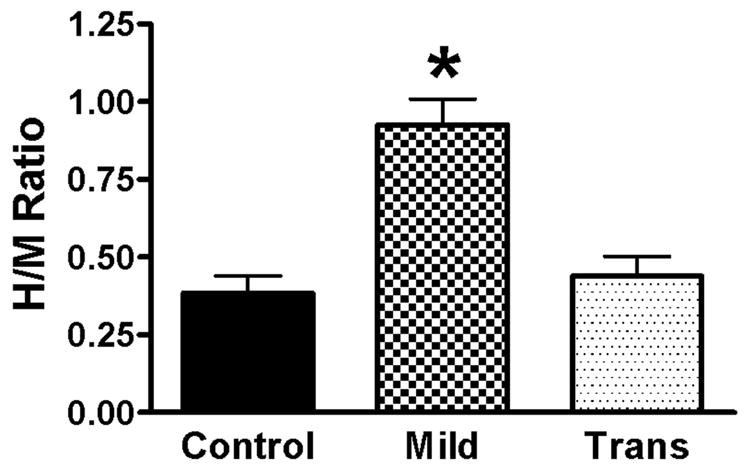
Alteration in H-reflex amplitude after SCI. At 4wks after SCI, the H/M ratio is increased after a Mild (n = 12), but not transection (n = 6), SCI as compared to uninjured controls (n = 9). The graph represents recordings after intrathecal vehicle (saline) infusion. Data is combined from rats in both the mianserin and LY53857/DOI studies. *p<.001 compared to both control and transection groups. One-way ANOVA with Tukey’s post-test.
Effect of mianserin on the H-reflex after SCI
Intrathecal infusion of mianserin resulted in a dose-dependent decrease in the H/M ratio of both uninjured control and Mild SCI animals at 4wks (Fig.3A). However, this decrease in animals with Mild SCI was less sensitive to mianserin as compared to uninjured controls. At 5.38 and 10.75nmol of mianserin, the H/M ratio in animals with Mild SCI was significantly higher than uninjured control animals. This shift in the dose-response curve caused the ID50 for mianserin at 4wks after Mild SCI to be approximately twice that of uninjured controls (Fig.3A’). Increasing doses of mianserin did not have any significant effects on the H/M ratio in animals at 4wks after a complete transection, which resulted in complete depletion of serotonin in the lumbar spinal cord (Fig.5C; Lee, et al., 2005, Fig.5C; Schmidt and Jordan, 2000).
Figure 3.
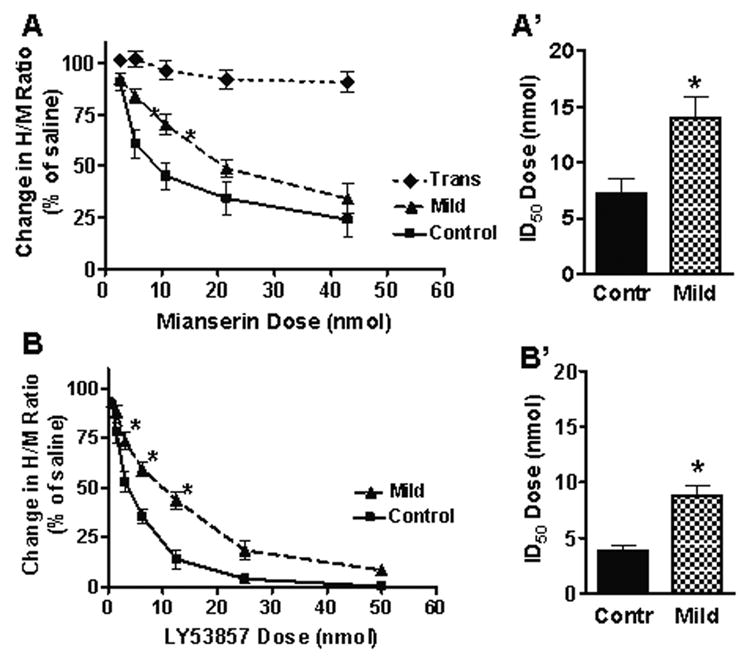
Effect of 5-HT2 receptor antagonists on the H-reflex after SCI. Intrathecal infusion of mianserin (A) and LY53857 (B) in uninjured control animals (n = 5, 4 respectively) resulted in a dose-dependent decrease in the H/M ratio. However, the H/M ratio was less sensitive to mianserin (A’) and LY53857 (B’) at 4wks after Mild SCI (n = 7, 5 respectively), resulting in a significantly greater ID50 as compared to uninjured controls. Since mianserin did not have any significant effect on the H/M ratio at 4wks after transection (n = 6) at any doses tested (A), the study was not repeated using LY53857. *p<0.05 compared to controls. Two-way ANOVA with Bonferonni post-test (A) or unpaired t-test (B).
Figure 5.
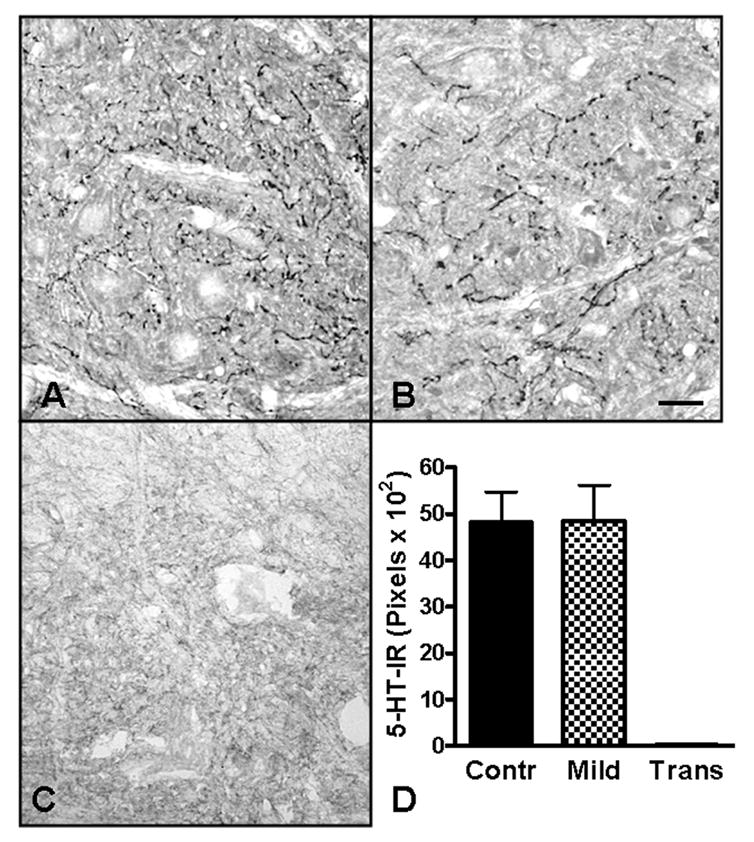
5-HT immunoreactivity (5-HT-IR) in the rostral dorsolateral nucleus (rDLn). 5-HT fibers in the rDLn of uninjured controls (A) and 4wk Mild SCI animals (B) were identified by the dark immunoreactive puncta located throughout the nucleus. 5-HT-IR was not present in the rDLn of transected animals (C). Quantification of the 5-HT-IR pixels showed no significant differences between the uninjured controls and Mild SCI groups (D; unpaired t-test).
Effect of LY53857 and DOI on the H-reflex after SCI
The effect of LY53857 on the H/M ratio after SCI was similar to that of mianserin; it also resulted in a dose-dependent decrease in the H/M ratio of both uninjured control and Mild SCI animals at 4wks that was less pronounced in animals with Mild SCI than uninjured controls (Fig.3B). At 3.14, 6.25, and 12.5nmol of LY58557, the H/M ratio in animals with Mild SCI was significantly higher than uninjured control animals. This shift in the dose-response caused the ID50 for LY53857 at 4wks after Mild SCI to be approximately three times that of uninjured controls (Fig.3B’).
Three days after intrathecal infusion of LY53857, the same animals were re-catheterized and received intrathecal infusion of the 5-HT2 agonist, DOI. At 31 and 93nmol, DOI did not have any significant effects on the H/M ratio of uninjured control animals (Fig.4). However, at 4wks after Mild SCI, there was a significant increase of the H/M ratio at 93nmols, but not 31nmol, of DOI. This response was significantly different from that to the same dose in uninjured controls, or the lower dose of 31nmol in the Mild SCI group.
Figure 4.
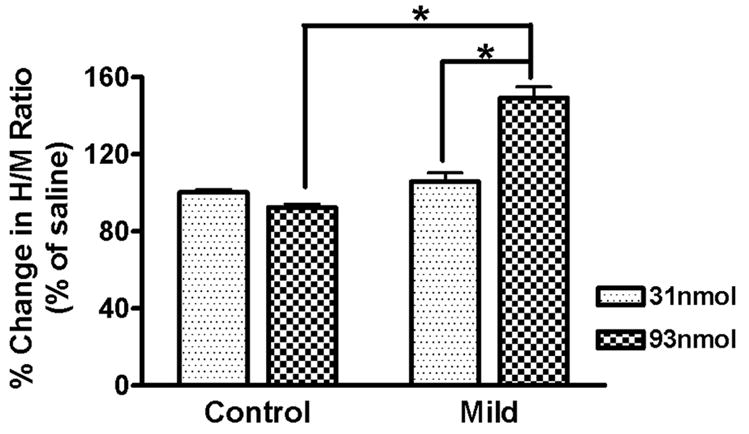
Effect of DOI on the H-reflex after SCI. Intrathecal infusion of DOI in uninjured control animals (n = 4) had no significant effect on the H/M ratio at either dose tested. However, after Mild SCI (n = 5), the higher DOI dose resulted in a significantly potentiated H/M ratio as compared to the lower dose in the Mild or the higher dose in control animals. *p<.001 Two-way ANOVA with Bonferonni post-test.
5-HT and 5-HT2AR immunoreactivity after SCI
Transected animals showed no 5-HT-IR at the level of the rDLn (Fig.5C). Uninjured controls had similar 5-HT-IR in the rDLn as Mild SCI animals at 4wks after SCI (Fig. 5), as determined by quantitatively analysis of immunoreactive pixels. Qualitatively, the pattern of immunoreactivity appeared somewhat different with 5-HT fibers in the contused animals appearing to be fewer in number but longer in length, perhaps due to sprouting of spared 5-HT fibers.
Uninjured controls and contused animals 4 weeks after injury differed significantly in the quantity of 5-HT2AR immunoreactivity (5-HT2AR-IR) in the rDLn (Fig.6). The Mild SCI animals demonstrated significantly higher receptor immunoreactivity, especially within the cell bodies of rDLn motoneurons.
5-HT2AR -IR in uninjured control rats was strongest around the peripheral rim of the gray matter and pools of motoneurons located in the ventral horn (Fig. 6A). 5-HT2AR -IR was present in fibers, especially those in the white matter that project to and/or from the gray matter, as well as motoneuron somas. 5-HT2AR -IR was also present in small cell somas located in the dorsal horn. 5-HT2AR -IR was very weak around the central canal and absent in the dorsal white matter. At 4wks after Mild SCI, there was a visible increase in the 5-HT2AR -IR of the rDLn (Fig. 7B, C).
Quantification of the optical density of the 5-HT2AR -IR indicated a significant increase in the rDLn (Fig. 6D) as well as individual motoneuron somas (Fig. 6E) located within this nucleus. The average soma area (Fig. 6F) was not different between the control and Mild SCI groups. The average optical density of the white matter used as background was also not significantly different between these two experimental groups.
Discussion
The goal of this study was to better understand if and how the serotonergic system is involved in the increased recruitment of motoneurons in response to afferent input that occurs chronically after an incomplete, clinically-relevant contusive SCI. We first determined that at 4wks after contusive SCI, when an increased amplitude of the H-reflex is apparent (Lee, et al., 2005), there is a reduced sensitivity of the H-reflex to the local infusion of 5-HT2R antagonists, mianserin and LY53857, compared to uninjured controls. Second, local intrathecal infusion of a 5-HT2R agonist, DOI, at a dose of 93nmol increased the H/M ratio in the 4wk contusive SCI group, but had no significant effect on uninjured controls. Third, while there was no difference in 5-HT-IR at 4 weeks after contusion, the 5-HT2AR-IR of the rDLn was higher at 4wks after Mild SCI as compared to uninjured controls. Based on these data, we conclude that up-regulation of 5-HT2R is likely to be involved in the enhanced H-reflex that develops after clinically -relevant incomplete contusive SCI.
The increased expression of 5-HT2R has previously been reported after SCI in a transection and in a hemisection SCI model (Fuller, et al., 2005, Kim, et al., 1999). In the first of these studies, neonatal rats that were subjected to complete transection were examined 8 weeks later for 5-HT2C receptor binding activity in ventral horn tissue rostral and caudal to the injury site. Compared to uninjured controls, a significant 35% increase was seen in the lumbar spinal cord of transected rats (Kim et al., 1999). As expected, no 5-HT-IR was seen distal to the transection site but application of serotonergic agonists demonstrated a super sensitivity of lumbar motor neuron activity associated with treadmill locomotion, consistent with functional stimulation of the increased 5-HT2R. Chronically, after a C2 hemisection, there is up-regulation of ipsilateral 5-HT2AR density in phrenic motor neurons distal to the injury site (Fuller et al., 2005). In this case, the density of 5-HT-IR is not different from that in uninjured controls, similar to what we observed in the rDLn chronically after incomplete contusion injury. After a C2 hemisection, the increased density of 5-HT2AR is believed to contribute to improved respiratory function after injury with chronic intermittent hypoxia after hemisection also leading to increased 5-HT2AR associated with contralateral phrenic motoneurons and long-term strengthening of crossed phrenic pathways. Although the drugs used in this study are not subtype specific, we used the 5-HT2AR antibody because there are no commercially available antibodies that can detect all three 5-HT2R subtypes. However, since all three subtypes have similar molecular structure, pharmacology and signal transduction pathways (Barnes and Sharp, 1999) we used the 5-HT2AR antibody to represent 5-HT2R in general. Furthermore, it is important to note that the increased 5-HT2AR immunoreactivity could have been due to receptor accumulation rather than overexpression.
Our results are consistent with these previous studies in showing evidence for increased expression of 5-HT2R and increased sensitivity to serotonergic drugs by spinal motoneurons distal to SCI. Further, in our SCI model, as in the C2 hemisection model (Fuller et al., 2005), the increased receptor expression is present chronically along with an apparently normal level of serotonergic (re)innervation thus should lead to increased responsiveness of the motoneurons to endogenous serotonergic stimulation. One clinical consequence after SCI may be spasticity, as suggested by the effects of treatment of patients with the 5-HT2R antagonist, cyproheptadine (Barbeau et al., 1982; Nance, 1994; Wainberg et al., 1990)
A theoretically possible alternative explanation for the increased ID50 for serotonin antagonists observed in our experiments is an increased release of 5-HT at serotonergic terminals after SCI without any changes in receptor number. However, this is unlikely for several reasons. If the serotonergic synapse in a normal uninjured spinal cord was flooded with 5-HT such that all of the 5-HT2 receptors were always occupied, then a further increase in 5-HT release after SCI should not result in a change in baseline H-reflex amplitude. Alternatively, if the serotonergic synapse was not flooded with 5-HT in the uninjured spinal cord such that there was a reserve of 5-HT2 receptors that remained unoccupied, then infusion of the 5-HT2 agonist, DOI, should have resulted in a potentiation of the H-reflex in the uninjured animal, which we did not observe. The 5-HT2 receptor reserve indicated by our pharmacological data seems to appear only after contusive SCI when there is evidence for receptor up-regulation, although we cannot rule out the possibility that increased 5-HT release may also occur.
The plantar muscles were chosen for this study due to their accessibility and the robustness of the H-reflex that can be elicited from this muscle group as described previously (Cliffer, et al., 1998). Furthermore, the stimulation of the tibial nerve at the ankle allows for the plantar muscles to be isolated as much as possible without stimulating other muscle groups, thereby reducing the possibility of reciprocal inhibition or facilitation from other muscle groups (Crone, et al., 2003, Okuma, et al., 2002, Xia and Rymer, 2005). It would be interesting to see if our results (physiological as well as immunohistochemical) could be observed in more proximal muscle groups, such as the gastrocnemius or tibialis, which play a more prominent role in locomotion.
Since it was not clear from the existing literature as to which of the three 5-HT2R subtypes would mediate the potentiation of the monosynaptic reflex, we chose the non-selective 5-HT2R antagonists, mianserin and LY53857, and the agonist, DOI, due to their relatively high affinities at the 5-HT2A, 5-HT2B, and 5-HT2C subtypes (Barnes and Sharp, 1999). Mianserin also has relatively high affinity for other receptors such as the H1 histamine and the α2 adrenergic receptors (Leonard, 1982). Therefore, we chose a second antagonist, LY53857, which has been characterized as a highly potent 5-HT2R antagonist with minimal affinities for histamine or adrenergic receptors (Cohen, et al., 1983). Both of these antagonists demonstrated significantly higher ID50 for the H/M ratio at 4wks after Mild SCI compared to uninjured controls, which adds confidence to our conclusion that increased 5-HT2R expression in rDLn is involved in increased h-reflex amplitude after incomplete SCI. However, it is important to note that although we used local intrathecal infusion at the level of the rDLn, we cannot rule out the possibility that diffusion of the drugs to other receptor sites might contribute to the results.
The transection group served as a useful control in this study. Since virtually all serotonin in the spinal cord is of supraspinal origin (for review see Schmidt and Jordan, 2000), a mid-thoracic transection leads to a depletion of serotonin in the lower lumbar spinal cord as indicated by the complete absence of 5-HT immunoreactivity in this region (Fig.5C; Hadjiconstantinou, et al., 1984, Lee, et al., 2005). Therefore, the lack of any significant effects of increasing doses of mianserin on the H/M ratio of transected rats indicated that the effect of mianserin observed in contused rats was not due to any non-specific interactions with local lumbar circuitry. Furthermore, it also demonstrated that the decrease in the H/M ratio during mianserin infusion was not due to some detrimental effect of the experimental protocol such as duration under anesthesia. Since the transected animals in this study were not affected by mianserin, and there was no evidence of 5-HT-IR in the distal cord of these rats, studies using LY53857 and DOI were not performed, nor was 5-HT2R expression in rDLn examined in these animals. It should be noted that the transection group in the current study did not show an increased H-reflex amplitude chronically after injury, confirming our previous results (Lee, et al., 2005). For a detailed discussion regarding this unexpected result, the reader is referred to the earlier report.
Partial loss of 5-HT fibers in the lumbar spinal cord after incomplete SCI can be followed by a subsequent normalization due to sprouting of spared fibers (Holmes, et al., 2005, Lee, et al., 2005, Pikov, 2000, Saruhashi, et al., 1996). If this occurs with respect to serotonergic innervation of the plantar motoneurons, the potential effect on the H-reflex needs to be taken into account. In a previous study, we determined both H-reflex amplitudes and 5-HT immunoreactivity around plantar motoneurons at 1, 4 and 8 weeks after Mild contusive SCI, the same model used in the present study. We found increased H-reflex amplitude, as confirmed in the current study, but no evidence of significantly altered 5-HT immunoreactivity around plantar motoneurons at any of the three time points (Lee, et al., 2005). Therefore, the increased H-reflex amplitude after Mild SCI does not seem to be caused by sprouting of spared 5-HT fibers. Rather, these results support the hypothesis that increased 5-HT2R is involved in the increased H-reflex amplitude after contusive SCI.
The role of the serotonergic system in locomotion is well documented mainly due to the ability of serotonin to induce rhythmic activation of the central pattern generator (Cazalets, et al., 1995, Sqalli-Houssaini, et al., 1993). The effects of serotonin or serotonergic agents on spinal cord locomotor output have been studied in various models ranging from in vitro postnatal spinal cord preparations to kinematic analysis in freely moving adult animals (Antri, et al., 2002, Cazalets, et al., 1995). In particular, 5-HT2R agonists have received a lot of attention due to their ability to increase the excitability of motoneurons (Barasi and Roberts, 1974, Elliott and Wallis, 1992, Machacek, et al., 2001, Miller, et al., 1996). The 5-HT2R agonists, quipazine and m-CPP, have been shown to significantly improve treadmill-trained locomotion after complete transection in rats (Antri, et al., 2002, Kim, et al., 2001). In addition, the 5-HT2R also seems to be important for locomotion in intact rats as indicated by complete hindlimb paralysis after a high dose of intrathecally infused cyproheptadine, a 5-HT2R antagonist (Majczynski, et al., 2005). Therefore, based on our results, we speculate that 5-HT2 receptor modulation of the enhanced recruitment of motoneurons in response to afferent input may contribute to locomotor recovery after incomplete SCI.
The results from our study also have important implications in the use of serotonergic anti-depressants in SCI patients. Depression is common among SCI patients (Boekamp, et al., 1996, Dryden, et al., 2005), and selective serotonin re-uptake inhibitors (SSRI) such as fluoxetine (Prozac) and sertraline (Zoloft) are commonly prescribed anti-depressants (Goldberg, 1998). In cases of incomplete SCI where descending 5-HT fibers can be preserved (Lee, et al., 2005), these anti-depressants may cause an increase in the local concentration of serotonin in the lumbar spinal cord. If this increase occurs at a time when increased sensitivity to serotonin has developed due to receptor up-regulation, exaggerated reflexes and spasticity may result in SCI patients as previously noted in a case report (Stolp-Smith and Wainberg, 1999). Interestingly, overdose of SSRI in non-SCI patients has been linked to the development of serotonin syndrome, in which hypertonus of lower limbs is often a symptom (Whipp and Waterfield, 2004). In contrast to these negative effects, administration of SSRI in transected animals transplanted with embryonic serotonergic cells improved locomotor recovery (Feraboli-Lohnherr, et al., 1997). Similar studies in contusive SCI models have not yet been reported, but both beneficial as well as detrimental effects of SSRI may be expected depending on the SCI severity and the time of SSRI administration.
In summary, our results suggest that increased 5-HT2 receptors are involved in the increased recruitment of motoneurons in response to afferent input after incomplete SCI. Future studies will need to address whether this phenomenon is associated with locomotor recovery and/or development of spasticity after SCI.
Acknowledgments
We would like to thank Dr. Richard Gillis for his assistance in planning these studies. This work was supported by NIH RO1NS035647 and T32NS41218.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Barasi S, Roberts MH. The modification of lumbar motoneurone excitability by stimulation of a putative 5-hydroxytryptamine pathway. Br J Pharmacol. 1974;52:339–348. doi: 10.1111/j.1476-5381.1974.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Boekamp JR, Overholser JC, Schubert DS. Depression following a spinal cord injury. Int J Psychiatry Med. 1996;26:329–349. doi: 10.2190/CMU6-24AH-E4JG-8KBN. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Engberg I, Lundberg A. Primary afferent depolarization evoked from the brain stem and the cerebellum. Arch Ital Biol. 1966;104:73–85. [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Feng-Chen KC, Chen L, Stark DM, Wolpaw JR. Short-Term and medium-term effects of spinal cord tract transections on soleus H-reflex in freely moving rats. J Neurotrauma. 2001;18:313–327. doi: 10.1089/08977150151070973. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Tonra JR, Carson SR, Radley HE, Cavnor C, Lindsay RM, Bodine SC, DiStefano PS. Consistent repeated M- and H-Wave recording in the hind limb of rats. Muscle Nerve. 1998;21:1405–1413. doi: 10.1002/(sici)1097-4598(199811)21:11<1405::aid-mus7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cohen ML, Fuller RW, Kurz KD. LY53857, a selective and potent serotonergic (5-HT2) receptor antagonist, does not lower blood pressure in the spontaneously hypertensive rat. J Pharmacol Exp Ther. 1983;227:327–332. [PubMed] [Google Scholar]
- Crockett DP, Harris SL, Egger MD. Plantar motoneuron columns in the rat. J Comp Neurol. 1987;265:109–118. doi: 10.1002/cne.902650108. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Dryden DM, Saunders LD, Rowe BH, May LA, Yiannakoulias N, Svenson LW, Schopflocher DP, Voaklander DC. Depression following Traumatic Spinal Cord Injury. Neuroepidemiology. 2005;25:55–61. doi: 10.1159/000086284. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Orsal D, Yakovleff A, Gimenez y Ribotta M, Privat A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: specific role of serotonergic neurons. Exp Brain Res. 1997;113:443–454. doi: 10.1007/pl00005597. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ. Selective serotonin reuptake inhibitors: infrequent medical adverse effects. Arch Fam Med. 1998;7:78–84. doi: 10.1001/archfami.7.1.78. [DOI] [PubMed] [Google Scholar]
- Gozariu M, Roth V, Keime F, Le Bars D, Willer JC. An electrophysiological investigation into the monosynaptic H-reflex in the rat. Brain Res. 1998;782:343–347. doi: 10.1016/s0006-8993(97)01402-9. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Panula P, Lackovic Z, Neff NH. Spinal cord serotonin: a biochemical and immunohistochemical study following transection. Brain Res. 1984;322:245–254. doi: 10.1016/0006-8993(84)90114-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Ono H. Effects of 8-OH-DPAT, a 5-HT1A receptor agonist, and DOI, a 5-HT2A/2C agonist, on monosynaptic transmission in spinalized rats. Brain Res. 1996;738:158–161. doi: 10.1016/0006-8993(96)00991-2. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Van Meter MJ, Beattie MS, Bresnahan JC. Serotonergic fiber sprouting to external anal sphincter motoneurons after spinal cord contusion. Exp Neurol. 2005;193:29–42. doi: 10.1016/j.expneurol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Tysseling-Mattiace VM, Benz EN, Schmit BD. Contribution of muscle afferents to prolonged flexion withdrawal reflexes in human spinal cord injury. J Neurophysiol. 2004;92:3375–3384. doi: 10.1152/jn.00152.2004. [DOI] [PubMed] [Google Scholar]
- Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, Simansky KJ. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J Neurosci. 1999;19:6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Murray M, Simansky KJ. The serotonergic 5-HT(2C) agonist m-chlorophenylpiperazine increases weight-supported locomotion without development of tolerance in rats with spinal transections. Exp Neurol. 2001;169:496–500. doi: 10.1006/exnr.2001.7660. [DOI] [PubMed] [Google Scholar]
- Lee JK, Emch GS, Johnson CS, Wrathall JR. Effect of spinal cord injury severity on alterations of the H-reflex. Exp Neurol. 2005;196:430–440. doi: 10.1016/j.expneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Leonard BE. On the mode of action of mianserin. Adv Biochem Psychopharmacol. 1982;31:301–319. [PubMed] [Google Scholar]
- Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT(2) receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537:201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majczynski H, Cabaj A, Gorska T. Intrathecal application of cyproheptadine impairs locomotion in intact rats. Neurosci Lett. 2005;381:16–20. doi: 10.1016/j.neulet.2005.01.075. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Mizuno Y, Lee RG. Reciprocal Ia inhibition in patients with asymmetric spinal spasticity. Clin Neurophysiol. 2002;113:292–297. doi: 10.1016/s1388-2457(02)00004-4. [DOI] [PubMed] [Google Scholar]
- Pikov V. Recovery of detrusor-external urethral sphincter coordination after incomplete spinal cord injury. Georgetown University; DC: 2000. [Google Scholar]
- Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR, Clarac F. Oscillatory properties of the central pattern generator for locomotion in neonatal rats. J Neurophysiol. 1993;70:803–813. doi: 10.1152/jn.1993.70.2.803. [DOI] [PubMed] [Google Scholar]
- Stolp-Smith KA, Wainberg MC. Antidepressant exacerbation of spasticity. Arch Phys Med Rehabil. 1999;80:339–342. doi: 10.1016/s0003-9993(99)90148-x. [DOI] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Parmer R, Reier PJ. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J Neurotrauma. 1998;15:495–508. doi: 10.1089/neu.1998.15.495. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Navarro X. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res. 2001;919:302–312. doi: 10.1016/s0006-8993(01)03052-9. [DOI] [PubMed] [Google Scholar]
- Whipp MJ, Waterfield KE. Serotonin syndrome in the differential diagnosis of spinal cord compression. Palliat Med. 2004;18:69–70. doi: 10.1191/0269216304pm847cr. [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]


