Abstract
Protein transduction with cell penetrating peptides over the past several years has been shown to be an effective way of delivering proteins in vitro and now several reports have also shown valuable in vivo applications in correcting disease states. An impressive bioinspired phenomenon of crossing biological barriers came from HIV transactivator Tat protein. Specifically, the protein transduction domain of HIV-Tat has been shown to be a potent pleiotropic peptide in protein delivery. Various approaches such as molecular modeling, arginine guanidinium head group structural strategy, multimerization of PTD sequence and phage display system have been applied for taming of the PTD. This has resulted in identification of PTD variants which are efficient in cell membrane penetration and cytoplasmic delivery. Inspite of these state of the art technologies, the dilemma of low protein transduction efficiency and target specific delivery of PTD fusion proteins remains unsolved. Moreover, some misconceptions about PTD of Tat in the literature require considerations. We have assembled critical information on secretory, plasma membrane penetration and transcellular properties of Tat and PTD using molecular analysis and available experimental evidences.
Keywords: Protein transduction domain (PTD), nuclear export signal (NES) analysis, nuclear/nucleolar HIV-Tat, cytoplasmic protein delivery, lysosomotropic agent, transcellular-PTD
1. Introduction
The past decade has witnessed tremendous advances in the field of protein transduction, aiming to correct defects for proteins involved in a variety of disease processes. At present, it is possible to produce a given protein molecule by recombinant DNA technology for in vivo therapeutic applications. Nevertheless, it still remains a challenge to deliver the recombinant proteins to desired targets in vivo, although small molecules or peptides capable of crossing cellular membranes have been successfully designed to deliver small or moderately large proteins. Despite developments in the area of protein transduction peptides, the classical delivery methods of protein coding genes via adeno-associated virus (AAV) [1, 2], adenovirus (AV) [3, 4], lentivirus [5], herpes virus (HSV) [6, 7] vectors, and plasmid expression vectors [8, 9] remain the preferred choice for expression of proteins.
Because of their natural abilities in delivering the specific genes to permissive cells, viral vector-mediated gene expression is considered the most efficient and reliable approach for expressing functional proteins de novo in mitotically active or postmitotically blocked cell types (HIV viral vectors). Nonetheless, viral vectors invariably are required in large doses to achieve therapeutic expression levels of intended protein (s). Moreover, viral vectors integrate with the host chromatin material. These properties may have consequences from long term effects on host genetic systems, and therefore, safety remains a serious concern for their ultimate clinical application [10-13].
An alternative approach that appears to be the safest is to produce recombinant proteins exogenously and then deliver them systemically or by localized injections into the target organs. The delivery and bioavailability of recombinant proteins into cells or tissues need further improvements, however. Discovery of the HIV-Tat protein transduction domain (PTD) has opened avenues for directing in vitro and in vivo delivery of proteins into cells. Several studies have shown the potential of PTD in drug delivery [14, 15] and transduction of proteins as large as 110 kDa into different cells [16]. In vivo injection of fusion proteins systemically has demonstrated the effectiveness of the PTD in protein delivery [15, 16]. In the present review we discuss the current status of the protein transduction focusing mainly on the PTD domain of HIV Tat.
2. Cell penetrating peptides (CPPs) for protein delivery
Various approaches have been designed to develop CPPs for introducing recombinant proteins into the cells. Penetratin [17], polylysine [18, 19], polyarginine [20], Tat PTD [16, 21], HSV VP22 [22-24], Kaposi FGF [25], Syn B1 [26], FGF-4 [27, 28], nuclear localization signal (NLS) [29], and anthrax toxin derivative 254-amino acids (aa) peptide segment [30], diphtheria toxin ‘R ’ binding domain [31], MPG (HIV gp41/SV40 Tag NLS) [32], pep-1 [33], WR peptide [34], and exotoxin A [35] have all been used successfully in protein transductions. The first cargo transduction was achieved by using a homeodomain of Antennapedia (Antp) [36], which transduced neurons and other cells. Antp-fusion proteins work well for proteins smaller than 100 amino acid residues but toxicity is always a concern with these peptides. The transduction by penetratin-fusion proteins also revealed toxicity in the brain [37]. Recently penetratin peptide has been introduced commercially for delivery of siRNA. It is possible that lower concentrations of this peptide may be required for siRNA delivery, where toxicity can be avoided.
Another important molecule which has shown potential in protein transduction is VP22, a part of the viral tegument in HSV-1 virus that is secreted from infected cells and has been shown to enter cells through its C-terminal region [38]. Though documented evidence exists for cell permeability of VP22 [23, 38-41], many studies could not verify this property and have shown failures in transcellular activity [22, 42, 43]. It seems possible that fusion of VP22 with different proteins will attain a different conformation every time, which obviously will affect the transduction behavior. Further investigations, however, are required to verify the limited protein transduction property of VP22 upon fusion with various proteins. Interestingly, a new fusion peptide ‘MPG ’ has been described for efficient transduction of nucleic acids. This peptide is a bipartite amphipathic peptide obtained by combining the fusion domain of HIV-gp41 protein and the NLS domain of SV40 large T antigen [44, 45], but its potential in protein transduction has not yet been demonstrated. This peptide is being used as a nanoparticle for transduction of siRNA in vitro and is also available commercially. This peptide may have great potential for siRNA delivery in vivo.
The most intensely studied yet less understood peptide in protein transduction is the PTD of HIV Tat. In the first exon of HIV Tat (Fig. 1), a region coding for the basic domain from 48-56 amino acid residues is responsible for nuclear localization and protein transduction [21, 46, 47]. Several studies have reported strong protein transduction property in vivo and in vitro after fusion with various full length or truncated proteins [15]. Surprisingly, the protein transduction property of PTD is currently looked with skepticism due to failures in protein transduction as well as misinterpretation of post cell fixation procedures during immunofluorescence studies. The general consensus is that Tat is secreted from the expressing cells and reenters the cells through its PTD domain. Several misconceptions about Tat and PTD in the literature need to be considered. Perhaps the most significant is that Tat or PTD has a transcellular behavior, i.e., intracellular PTD can spread from producer cells to the non-producer cells by an unknown mechanism. The transcellular behavior of PTD is discussed in more detail in a separate section “transcellular property of PTD”. Taken together, CPPs appears weakly potent in protein transduction and require extensive further modifications to improve their protein transduction property with minimum toxicity.
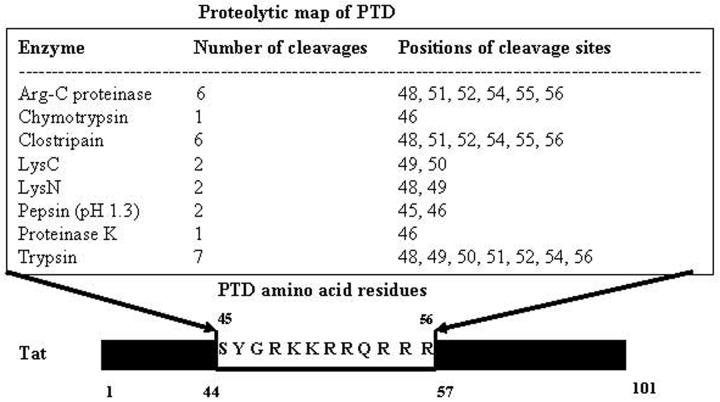
Figure 1.
Proteolytic map of Tat-PTD with amino acid residues from 48-56 is shown. Full length Tat is 101 amino acids and PTD contains mainly charged amino acids lysines and arginines.
3. Potential of PTD-fusion protein transduction in vitro
Several years ago, Frankel and Pabo [48] and Green and Lowenstein [49] demonstrated that extracellular HIV Tat can cross the plasma membrane and enter the cell, reaching the nucleus. The successful entry of extracellular Tat was investigated by others and has become a common test to monitor HIV-LTR promoter activity. Subsequently, more detailed analysis of Tat protein [21, 47, 50, 51] identified a PTD of 9-11 aa residues, a basic domain that can transduce itself, as well as the bigger proteins fused with it, into the cells [15, 16, 46, 52]. In vitro PTD-fusion protein is used by direct addition to the cell culture medium, which resulted in [53-57] efficient transduction of PTD-AT1 receptor domain and PTD-BCL-x in neurons [58, 59]. In another example, PTD-PDX1 fusion protein (PDX1 is a transcription factor that plays a central role in pancreatic development) has shown remarkable biological activity and induction of insulin production in human embryonic stem cells [60]. It suggests that protein transduction through PTD occurs in mitotically active, non-dividing or embryonic cells, which is indeed a useful property to be exploited. Precisely, the principle of Tat entry is not understood. However, it has been demonstrated that the RGD domain present in the second exon of Tat bind integrin receptor on the cell membrane [61]. Alternatively, Tat bind through HSPG followed by LRP receptor mediated endocytosis to enter the cells [62]. In particular, entry of PTD is least understood and the possible mechanism of PTD entry is described in “mechanism of PTD internalization” section below.
Despite successful applications, questions about potency of PTD mediated protein transduction still remain unsolved. Further, Brask et al. [63] have shown PTD and Tat-mediated transduction of non-structural protein (NS1) and nucleoprotein (NP) of influenza virus into neuroblastoma cells and primary neurons. Even after using a high level of recombinant PTD-fusion proteins extracellularly (∼35-60 μg/ml), transduction efficiency was never achieved to the extent reported by others [21]. Further investigations revealed that extracellular application of PTD-fusion proteins just a day after seeding of cells was successful albeit with low transduction efficiency. Surprisingly, with relatively older and confluent cells, no transduction was observed. However, scrape loading followed by chloroquine treatment facilitated the protein transduction in these old cultures [Chauhan et al, unpublished]. In particular, efficiency of denatured PTD-fusion protein has been recommended for successful protein transduction in earlier studies. Indeed, simple but regaining biological activity of denatured protein is questionable. Nonetheless, this seems unlikely when Tat protein expression is examined in vivo. Soluble Tat protein enters the cells more efficiently compared with its denatured counterpart [48, 49].
Furthermore, some studies have also demonstrated failures in PTD-mediated fusion protein transduction in vitro/in vivo as well as an inability to induce an immune response [64-67]. It is not yet understood why PTD-mediated fusion protein transduction failed, but clearly, the overall conformation of the fusion protein must be involved or change in fusion properties after addition to the cultures. It is also possible that PTD might have been rendered ineffective during purification or treatment of cells. In particular, PTD is responsible for nuclear retention of Tat protein due to the presence of strong nuclear localization signal (NLS) (Figs. 1, 2, 3, 4 and 5; Table 1, 2 and 3) and hence will direct the fusion protein to the nucleus, which might be the explanation for low immune response.
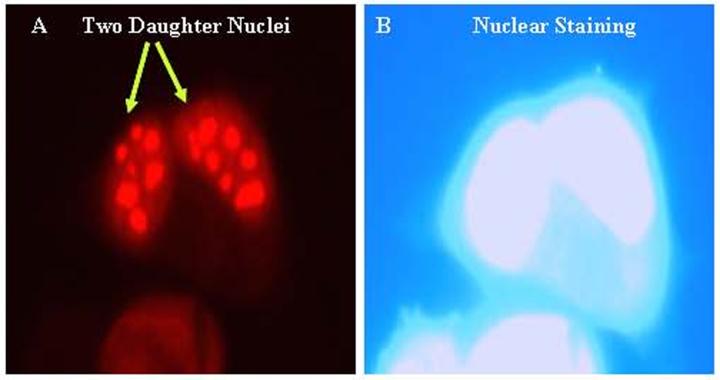
Figure 2.
Nuclear expression of Tat in SVGA (astrocytic) cells. SVGA cells were transiently transfected with 2 exon HIV-Tat (full length 101 amino acids) and immunostained 24 hours post transfection by monoclonal Tat-antibody. (A) Nuclear localization of Tat in two daughter cells. (B) DAPI nuclear staining corresponds to cells in panel (A).
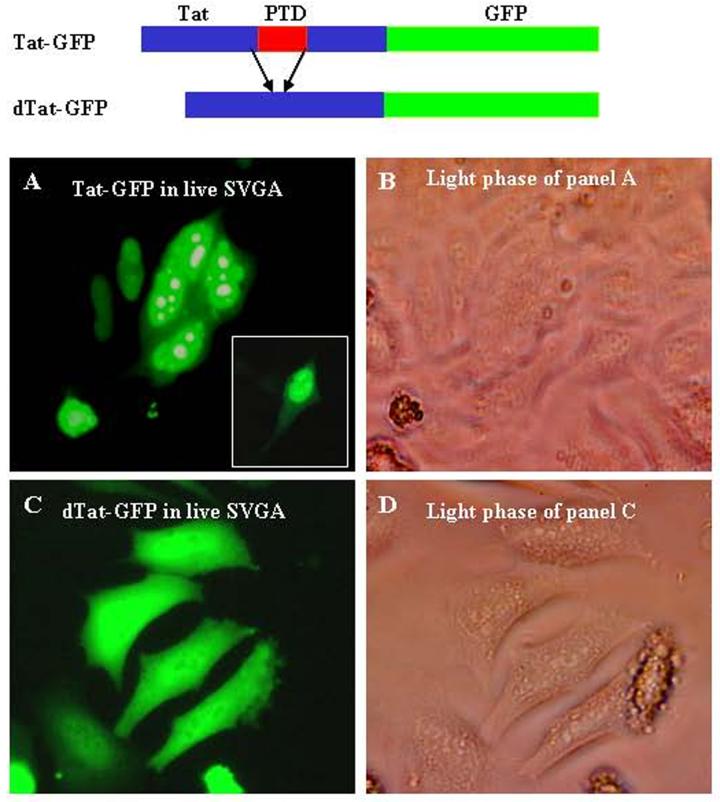
Figure 3.
Wild type Tat-GFP and mutant (Δ) Tat-GFP expression constructs are shown diagrammatically. A, live cell fluorescence in SVGA cells transfected with Tat-GFP; B, light phase of panel A; C, live cell fluorescence in SVGA cells transfected with mutant Tat (48-56)-GFP showing green fluorescence throughout (diffuse) the cells; D, light phase of panel C.
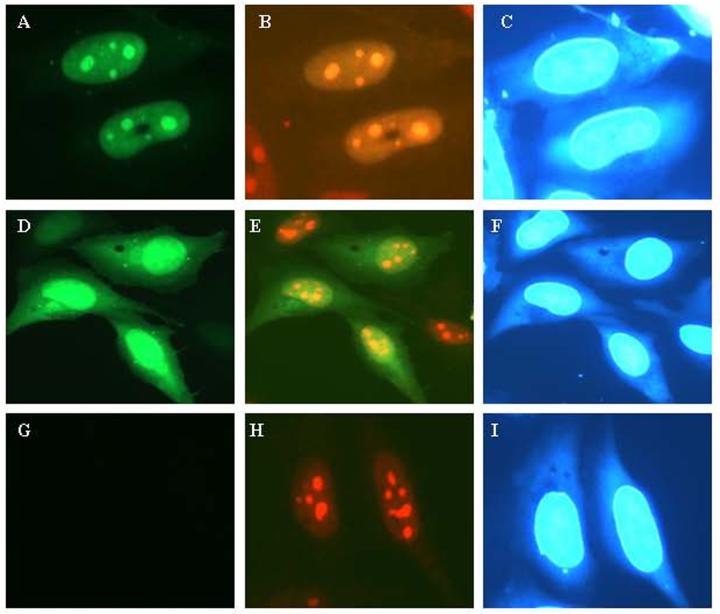
Figure 4.
SVGA cells transiently transfected with Tat-GFP or mutant Tat (dTat)-GFP wherein PTD region (48-56) is deleted and immunostained for nucleoli by using nucleophosmin monoclonal antibody. (A) Tat-GFP (green) expression in the nucleus under green filter; (B) same field as in (A) Tat-GFP (green) and nucleophosmin (red) seen with double filter showing co-localization of Tat and nucleoli; panel (C), DAPI nuclear staining corresponds to panels (A) and (B); panel (D), mutant Tat-GFP shows green fluorescence throughout the cells (diffuse), panel (E) same as (D) showing mutant Tat-GFP (green) and nucleoli (red) under double filter, have lost nucleolar distribution of Tat; panel (F), DAPI nuclear staining corresponds to panel (D) and (E); (G), control cells transfected with vector DNA and seen under green filter and in (H) same as (G) showing nucleolar staining; (I), DAPI nuclear staining for panels (G) and (H).
Figure 5.
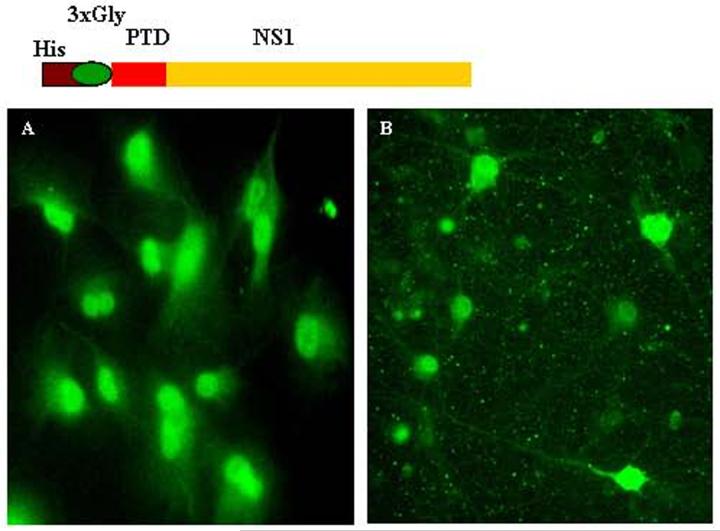
Transduction of PTD-NS1 fusion protein into neuroblastoma cells and primary neurons. His-PTD-NS1 containing a 3-glycine spacer was produced in bacteria. The affinity-purified protein was added to the culture medium with 50 μM chloroquine. After 24 hours, cells were fixed with 2% paraformaldehyde and immunostained using polyclonal rabbit antibody for NS1 protein (gift from Dr. A. Nieto, Spain). (A) PTD-NS1 protein transduction in N1E cells; (B) PTD-NS1 fusion protein transduction in primary hippocampal neurons.
Table 1.
Principal features of Tat PTD
| 1. Strong basic amino acids lysine and arginine |
| 2. Susceptible to furin and other proteases (Fig. 1) |
| 2. Mild protein transduction property |
| 3. Strong nuclear localization property |
| 4. No nuclear export signal |
| 5. No secretory signal |
| 6. No transcellular property |
| 7. No siRNA transduction property |
| 8. PTD delivers fusion proteins into the nucleus except tamed PTDs which deliver into the cytoplasm (19, 80, 81, 84, 168) |
| 9. PTD in combination with transfection reagents enhance transfection efficiency of plasmids and siRNAs |
| 10. Least immunogenic possibly due to NLS and high homology with human proteins. |
Table 2.
Amino acid composition and transduction destinations of the tamed PTDs.
| Type of PTD [references] | Protein sequence | Protein transduction (Nuclear or cytoplasmic) | Homology with human protein data bank (%) |
|---|---|---|---|
| PTD [19] | YGRKKRRQRRR | Nuclear | 85-95 |
| PTD-4 [80] | YARAAARQARA | Cytoplasmic | 75-85 |
| YM-3 [84] | THRLPRRRRRR | Cytoplasmic | 75-85 |
| CTP [81] | GGRRARRRRRR | Cytoplasmic | No significant homology |
Table 3.
Possible effects of PTD fusion on different proteins
| Nature of Protein | Outcome (PTD-fusion protein) |
|---|---|
| Cytoplasmic | aNucleus |
| Nucleo-cytoplasmic | aNucleus |
| Secretory | Nucleus/Extracellular |
| Membrane | Membrane/Nucleus |
= Outcome will depend upon the strength of NES if present on the cytoplasmic protein, conformation of the fusion protein and also upon how strongly the protein binds in the cytoplasm.
To study in greater detail, we analysed the PTD protein sequence to derive a proteolytic map. It is predicted that PTD is prone to degradation by the host proteolytic system (Fig. 1). Also, PTD has been shown to be susceptible to furin [68] and thus one should consider these possibilities while designing potent PTD mutants. Overall, it is concluded that PTD and Tat enter the cells under certain situations, but that in other situations, PTD-fusion proteins may simply stick to the surface of cells probably via heparan sulphate-(HS)-proteoglycans (HSPG) or by some unknown receptors. Further studies are needed on different primary cell cultures to establish the universal protein transduction property of PTD upon fusion with different proteins. In conclusion, we have summarized basic properties of Tat-PTD in Table 1.
4. Potential of PTD-fusion protein transduction in vivo
In 1999 Schwarze et al. [16] showed that PTD-beta-gal fusion protein applied intraperitoneally entered the brain after crossing the blood-brain-barrier (BBB). Subsequently, several other studies have also reported effective biodistribution of therapeutically important proteins in vivo by using PTD [52, 69-75]. In a previous study, however, no PTD mediated transduction in brain was observed, [46] possibly because beta-gal was chemically conjugated to PTD instead of recombinant fusion protein. Similarly, another study also failed to deliver PTD-99Tc to the brain [76]. Since PTD is conjugated to Tc or beta-gal, this might have resulted in structural modifications in the PTD and hence no transduction was observed. Nevertheless, in a different approach, when PTD-beta gal expression plasmid vector was injected directly into liver, enzymatic expression was observed in the liver and heart but not in brain [15]. Mechanistically, intracellular PTD-beta-gal expression is totally different from addition of recombinant protein to cultures and possibly in the former, no secretion of fusion protein occurred owing to the NLS of PTD. Therefore, intracellular expression of PTD-beta-gal or other non-secretory protein might not achieve the same biodistribution as recombinant protein. Indeed, more studies are needed for further verifications.
Of interest, PTD-BCLx fusion protein injected via intraperitoneal injection resulted in protein transduction in neurons in different regions of the brain. Further, PTD-Bcl-xL demonstrated prevention of neuronal death resulting from transient focal ischemia [69]. In another successful in vivo study, Kilic et al. [71] demonstrated that intravenous inoculation of PTD-GDNF fusion protein protected neurons from focal cerebral ischemic injury. Similarly, in a non-neurological model, Hotchkiss et al. [77] showed that Tat-Bcl-xL fusion protein and PTD-BH4 peptide prevented E.coli-induced human lymphocyte apoptosis ex-vivo and markedly decreased lymphocyte apoptosis in an in-vivo mouse model of sepsis.
PTD-fusion protein introduction through intravenous, intraperitoneal or direct injection in the target organs have shown promise for therapeutic and effective vaccination applications. Most importantly, PTD-fusion protein systemic delivery has shown crossing of BBB [16], itself a great achievement to deliver therapeutic molecules effectively. Indeed, a great property to deliver proteins into the brain; however, entry of PTD through BBB remains elusive. In particular, it is difficult to conceive, how PTD-fusion protein will infiltrate between the closely spaced layers of endothelial and astrocytes as well as tight junctions between them. Therefore, it is not an accepted dogma yet and requires further studies.
Intriguingly, in vaccination studies [64], an enhanced immune response to NP of LCMV upon fusion with PTD was shown. Despite increased immune response to NP, the authors ruled out a role for PTD by suggesting that more PTD-NP is deposited on the cell surfaces, and hence NP was readily available to the antigen-presenting cells. In contrast, other studies on pulsing of dendritic cells with PTD-fused antigens revealed robust induction of antigen-specific CTL response and TH1-mediated antitumor immunity in immunized mice [78, 79]. Based on the Tat and PTD properties, it is inferred that fusion protein will enter the cell and then either retained in the endosomes or if released will be trapped in the nucleus and hence not available to mount an immune response. Therefore, it may not be a wise strategy to fuse PTD with the antigen of interest and expect a better immune response especially in DNA vaccinations. Although the tamed PTDs (variants) could show better plasma membrane penetration and immune response to antigen of interest because of the cytoplasmic but not nuclear delivery of the fusion protein.
Further efforts on “taming” the PTD peptide for more effective delivery were achieved by amino acid substitutions (Table 2). In particular, Dowdy ’s group has described PTD peptide variants by molecular modeling approach and a better tamed PTD variant was identified [80]. This variant ‘PTD-4 ’ wherein 3 arginines and two lysines were substituted with alanines, revealed robust transduction in vitro and in vivo than to wild type PTD. Better transduction of tamed PTD-4 was reasoned on obtaining a robust α-helical structure by substituting alanines for basic amino acids in the wild type PTD [80]. Further, another variant of PTD generated by substitutions, known as cytoplasmic transduction peptide (CTP), effectively delivered the cargo into the cytoplasm [81] (Table 2). Interestingly, CTP fusion protein in vivo showed delivery of fusion protein to liver and lymph nodes, but native PTD (as a fusion protein) failed to do so [81, 82]. Moreover, “tamed” PTD i.e., CTP, delivered the fusion protein more efficiently than did PTD. Nevertheless, PTD-based delivery was found to be more efficient for brain, while CTP demonstrated tropism mainly for liver, spleen, and lymph nodes. Paul Wenders group [83] applied arginine guanidinium head group structural strategy for taming the PTD and identified isomers/analogs with better membrane penetration behavior. Further taming of PTD for better cell penetration is possible by phage display system where a library of PTD-peptides fused with indicator protein was screened and the most aggressive cell penetrating mutant ‘YM-3 ’ identified (Table 2) [84].
In brief, PTD-fusion protein does provide in vivo protein transduction application and importantly potential in crossing the BBB. More detailed studies are needed, however, to confirm BBB property and to dissect the distribution pattern of fusion proteins in different organs of the body by using sensitive radioactively labeled PTD-fusion proteins.
5. Mechanism of PTD internalization
It has been observed that histones and cationic polyamines such as polylysine stimulate the uptake of albumin by tumor cells in culture [85, 86]. But, the cellular uptake mechanism(s) of CPPs is currently unknown. CPPs are structurally diverse and highly variable in nature. Nonetheless, their common feature is the high density of basic amino acid residues (Arg and Lys): the presence of basic amino acids in the PTD is considered the hallmark of transduction peptides. There are exceptions, however; for example, a recently described transduction peptide contains 11 amino acid residues [80, 87] having 3 arginines and 6 alanines with 33 times more transduction activity than did conventional PTD. Moreover, the transduction activity, similar to that of other CPPs, was also attributed to be through the α-helical configuration of the peptide [80]. In general, high charge at physiological pH excludes the passive diffusion of CPPs across the lipid bilayer. The guanidine head group of arginine has been predicted to be a critical structural component responsible for the biological activity of CPPs including PTD [83, 88]; further hydrogen bonding between the highly basic arginine guanidine groups and the phospholipids in the membrane lipid bilayer may be involved in protein transduction. Additionally, cationic amphiphilic α-helical peptides (CPPs), which display a hydrophilic and, on the opposing side, a hydrophobic face, are efficient transducers of DNA into cells [89, 90]. With amphipathic peptides and a lipid bilayer, it is known that side chains of cationic peptides bind to anionic lipid bilayers [91].
Cell surface heparan sulphate (HS) proteoglycans (HSPGs) have been shown to play a role in the transduction of Tat proteins [92-95]. HS is present in almost all cells and Tat PTD fusion proteins bind heparin [96, 97], a soluble analogue of heparan sulphate glycosaminoglycans (GAGs) that inhibit uptake of Tat-fusion protein. The role of HS in PTD entry has been described but has not been well characterized. PTD-fusion proteins bind to HSPGs or unknown receptor on the plasma membrane and are then taken up by endocytosis [93, 98-100]. In the endocytosed vesicles HS is degraded by heparinase, which releases the PTD-fusion protein [101]. The influence of HS on PTD-fusion protein has been demonstrated in two ways. In the first, cells treated with heparinase III, which removes the HS, had drastically reduced uptake of PTD [98], although some activity of PTD was still observed and was assigned to chondroitin sulphate, which could not be removed with heparinase III. In the second, HS was added with PTD, which impaired aggregate formation and the uptake of PTD [98].
Cationic peptides such as polyornithine, polylysine, arginine-rich histones, spermine, and DEAE dextran [15, 102] have directed the import of macromolecules such as proteins into eukaryotic cells [86]. This led to the prediction that the minimal mass of a polycationic peptide needed to produce a nominal level of protein import into cells was on the order of ∼500-900 Da, a molecular mass range corresponding to a peptide chain of about 6 or 7 aa residues [103]. Further, internalization and cellular trafficking events are particularly sensitive to valency effects as well [104]. Thus, the efficiency of nuclear import of proteins has been shown to increase with the number of NLS inserted into such conjugates [105]. Furthermore, in many situations PTD fusion peptides are unknowingly present perhaps in multiple copies as a consequence of the quaternary structure of their protein cargo itself or as a result of the strategy involved in linking them to macromolecules [106]. Also it has been observed that particles smaller than 300 nm do not enter the cell through endosomal pathways [107, 108], in contrast to particles of 500-700 nm, which are taken up by endocytosis [109].
In vitro evidences have suggested that Tat/PTD-fusion proteins or Tat peptides enter via an energy-dependent endocytotic (including caveolae) process [110-114], because the membrane inhibitor sodium azide inhibits ATP production and impairs endocytosis [115, 116]. Further, protein transduction was strongly inhibited by energy depletion in cells at low temperature [110, 112]. Also, some studies have pointed to an energy-independent process of uptake of PTD fusion proteins [117-120] which might have resulted from the presence of experimental artifacts during fixation of cells [113, 115, 121]. Moreover, internalized peptides were found to be in the acidic compartment, and the inhibition of endosomal acidification resulted in a marked decrease in peptide internalization [115]. The mechanism of entry by clathrin-coated vesicles has been ruled out, however, and entry is suggested to be mediated by lipid rafts, but not by caveolae-mediated endocytosis [122-124]. Fittipaldi et al [125] have suggested a caveolar endocytic pathway for Tat-fusion proteins entry. Indeed, different internalization pathways such as clathrin mediated and caveolae endocytosis are equally popular in PTD/Tat-fusion proteins entry. However, the particular internalization pathway is dependent on characteristics of the protein fused, conformation attained after fusion with PTD or Tat, cells and experimental conditions.
The receptor-independent endocytosis known as macropinocytosis has been demonstrated for Tat and PTD fusion peptides [126, 127]. It was observed that PTD-fusion protein was localized and sequestered in endosomes. Upon treatment with endosomal releasing polymer, poly-(propylacrylic acid), the fusion protein is released in the cytoplasm [128]. Similar to PTD, CTP (a variant of PTD)-mediated delivery was inhibited by treatment of cells with heparinase III and the entry mechanism has currently been assigned to lipid raft mediated endocytosis [81]. On the other hand, CTP-mediated transduction was not affected by chloroquine, which markedly enhanced PTD transduction, indicating that CTP-based fusion protein is released into the cytoplasm while PTD-fusion protein is retained in endosomes and lysosomes [81]. Interestingly, a recent study has also shown localization of PTD-NP fusion protein in the trans-Golgi network as well, where efficient processing of influenza virus-NP fusion protein for antigen presentation by the proteolytic protease furin in the trans-Golgi occurred [129].
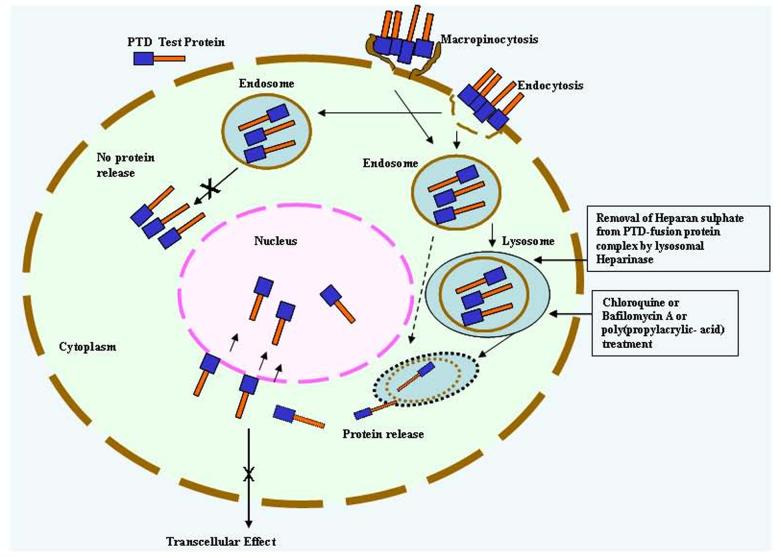
It has been suggested that transduction of proteins in cells is efficient only when denatured recombinant PTD-fusion proteins are used [16, 21, 70, 120, 130-132]. Possibly, denaturation of PTD-fusion proteins attains the optimal conformation for plasma membrane penetration. However, it is not necessary that every protein fused to His-PTD after denaturation will guarantee its entry because of the inherent nature and final conformation attained by the purified fusion protein. Also, for successful transduction, optimization of protein sequences between His-Tag and PTD is required because of the interference by 6 histidine residues. The possible interference by His-Tag can be overcome by inserting a spacer of 1-3 glycine residues [126]. In our studies, we found that when a His-PTD-NS1 recombinant protein was used, no protein transduction was observed. Interestingly, insertion of the 3-glycine spacer between His-tag and PTD resulted in efficient transduction (Fig. 5). This spacer probably permitted the required overall folding needed for fusion protein entry [63]. It is concluded from the above studies that Tat and PTD fusion proteins enter cells via energy dependent endocytosis, which is possibly enhanced upon treatment with lysosomal disrupting agents (Fig. 6).
Figure 6.
Schematic diagram showing mechanism of PTD-fusion protein entry into the cell. PTD-fusion protein binds with the heparan sulphate or unknown receptors on the cell surface and enters either via endocytosis or macropinocytosis. Endocytic vesicles fuse with lysosomal vesicles, heparan sulphate from PTD-fusion protein complex is then removed by heparinase present in endosomes, and protein is either released into the cytoplasm or degraded. Chloroquine (lysosomotropic agent) treatment prevents degradation and aids release of fusion protein from lysosomes. The released PTD-fusion protein either stays in the cytoplasm or enters the nucleus, depending on the sequence and nature of the protein under study. Transcellular effect is not possible either for cytoplasmic or nuclear protein, as PTD does not have secretory property.
6. Role of lysosomotropic agents in PTD-mediated protein transduction
Some studies have reported inefficient delivery of PTD-based fusion proteins in vitro [66, 113, 133]. The failure of PTD-mediated protein transduction was explained in two ways. First, nuclear translocation of fusion proteins deposited on the plasma membrane after fixation of cells has been the common explanation, and hence PTD actually does not transduce proteins. Second, deposits of PTD-fusion proteins on the cell surface with no biological activity were demonstrated. These studies may explain, in part, what might be occurring, but the reason for inefficient entry may not be because PTD is incapable of protein transduction, but because of the way in which the fusion protein entered the cell (endocytic) and the overall conformation attained by fusion protein. In particular, the inefficient delivery of fusion proteins in these studies may also be due to the absence of lysosomotropic agents [134].
Many studies on Tat as well as PTD fusion proteins, including our own, have demonstrated that these proteins function only when lysosomotropic agents are used [63, 135], although this may not be the case with every protein. Endocytosed PTD-fusion proteins are retained in endosomes. In order to release and prevent degradation of the fusion proteins in endosomes, lysosomal inhibitors or disruptors such as bafilomycin, chloroquine, endosomal releasing polymer poly(propylacrylic acid), or influenza virus HA2 hemagglutinin subunit are required [127, 136]. Bafilomycin A specifically prevents the acidification of early endosomes, by inhibiting a proton pump known as vacuolar ATPase [137].
Chloroquine is a weak base that inhibits the maturation of the transport vesicles into the late endosomes and neutralizes their pH [138]. Further, Wadia et al. [127] have shown that Tat-Cre fusion protein was trapped in macropinosomes even after 24 hours of treatment and was released only when chloroquine at 100 μM or 200 μM was applied, thus strongly implicating lysosomal inhibitors in efficient targeting of PTD-fusion proteins. We found, however, that the doses of chloroquine used in these studies were toxic within a few hours of treatment. The most effective and safest dose was found to be 50 μM [63, 136]. Similarly, it has been shown that transduction of PTD-Smac (Smac, which suppress the activity of X-linked inhibitor of apoptosis protein) in different cell lines increases significantly upon chloroquine treatment, while transduction of CTP (a variant of PTD which delivers the fusion protein into the cytoplasm) as CTP-Smac fusion protein was insignificantly affected by chloroquine treatment [81].
Moreover, in another approach, endosomal release of transduced protein was enhanced by exposure to fluorescent light, suggesting the importance of photoactivation in protein transduction [139, 140]. It appears that the intracellular route of PTD-fusion protein is dependent on the overall conformation of a mosaic (PTD) fusion protein. It is essential for successful transduction to determine which route (endosomal or non-endosomal) would be followed by PTD-fusion protein in a given study (Table 2 and Fig. 6). It is important to include lysosomotropic agents to facilitate protein transduction; however, exceptions to this rule can not be ruled out.
7. Transcellular property of PTD
Successful protein transductions have resulted in the investigation of the transcellular effect on bystander cells. Limited studies have shown that PTD is conferred with transcellular property [125, 141]. Indeed, this behavior is extremely valuable in delivering therapeutic proteins to surrounding cells. Therefore, it is important to know whether a preformed PTD-fusion protein or a DNA expression vector for the PTD-fusion protein is involved in transcellular transduction. Of note, Tat-PTD is suitable for protein transduction when it is included as a recombinant fusion-protein. Nonetheless, in a DNA expression system, PTD is not directly useful since the expressed PTD-fusion protein will be held in the nucleus until or unless the cargo protein has a strong NES. It has been demonstrated that Tat binds strongly in the nuclei (Fig. 2, 3 and 4) and hence its exit from the nucleus would be difficult unless the cell ruptures [135, 142-148].
The authors own observations revealed that although PTD is capable of entry into different cell systems (Fig. 5), the overall efficiency depends on the type and density of cells as well as the mode of introduction such as scrape loading or loading immediately after attachment of trypsinized cells [63, Chauhan et al., unpublished]. Damage to the cell membranes during trypsinization of cells could explain the enhanced transduction of PTD into the cells. Moreover, failure of the Tat protein to enter the cells when added on the 2nd or 3rdday after seeding of cells could be because the cell membranes are repaired, and the cells have become confluent. Furthermore, the development of tight junctions between cells does not allow PTD-fusion proteins to enter, even though the apical side of cell monolayers is exposed to the culture medium. Importantly, Tat protein does not show full biological activity unless chloroquine is added to the culture medium, which implies that after entry into the cells, Tat stays in the endosomes and is released into the cytoplasm upon chloroquine treatment. The released Tat enters the nucleus and displays its biological activity. Transcellular delivery of PTD remains uncertain and is not well investigated. Nonetheless, it has not been established definitively that PTD has transcellular property. Owing to the strong NLS property of PTD [135, 146] (Figs. 2, 3, 4 and 5), it should not be used for transcellular delivery nor for cytoplasmic targeting of fusion proteins unless prior testing in expression experiments reveals that fusion protein has strong cytoplasmic localization domain(s) that can overcome the PTD ’s NLS strength (Table 3).
Earlier studies with Western blotting, immunoprecipitation, and ELISA showed that the extracellular medium of Tat-expressing Jurkat cells was devoid of Tat [144, 149]. The possible reasons given by these authors to explain the results were that Tat could not be secreted by these cells and that Tat may be degraded by proteases, or that extracellular Tat could bind to the surface of the cells and fail to get internalized. Upon co-culture of Jurkat-Tat cells with Hela-LTRCAT cells, however, transactivation of LTR and expression of CAT enzyme occurred. Surprisingly, anti-Tat antibody did not inhibit the LTR transactivation in these experiments.
Intriguingly, in our investigations on stable SVGA-Tat cells, co-culture with SVGA-LTRCAT or SVGA-LTRGFP did not result in LTR activation [135]. This may be due either to low production of Tat in stable cells or astrocyte-to-astrocyte transcellular Tat blockage. Further, cells transiently transfected with Tat-plasmid upon co-culture with SVGA-LTRGFP revealed sporadic LTR-mediated expression of GFP-positive reporter cells. We also observed that stable HIV-Tat-expressing astrocytic cells (SVGA-Tat) upon co-culture with lymphocytic-LTRGFP cells (D3R5) did not display LTR transactivation. When transiently Tat-transfected SVGA cells were co-cultured with D3R5-LTRGFP cells [150], however, we observed sporadic LTR-mediated GFP expression. Upon treatment with Tat antibody, partial inhibition of LTR activity was observed [135]. These results may be explained, at least in part, by the following: Tat expressed in high concentrations may be secreted from these cells and taken up by the bystander reporter cells but not transcellularly [144] (see supplemental data in ref. 144) or, alternatively, the concurrent mild cell death of the transiently Tat expressing cells might be the potential source of Tat released into the culture medium.
A recent study has clearly shown that upon expression through an adenoviral vector, PTD-fused GFP protein did not demonstrate transcellular behavior [142], which is supported by another study in which PTD-GFP or Tat-GFP expression de novo did not show cell to cell movement [64, 144]. Further, thus far, only few studies are available on PTD transcellular behavior, one with adenoviral vector-mediated expression [151] and the other with PTD-TK expression [152]. In the first study, Xia et al. [151] showed that beta-glucuronidase (GUS) fused with PTD, and expression through adenoviral vector upon direct injection in brain significantly increased distribution of the expressed protein. In contrast, another study [153], however, found no evidence that GUS-Tat crossed the BBB when injected intravenously. The same study also found that overall distribution of GUS alone or GUS-Tat did not differ, but that the clearance rate was slower for GUS-Tat.
Intriguingly, upon NES analysis (www.cbs.dtu.dk/services), we found that wild type glucuronidase enzyme has an NES sequence at positions 514-521 (LELIQLQL) with a signal strength of 0.925, which is quite strong; upon fusion with PTD the overall strength of NES will be decreased. More precisely, the fate of PTD-GUS localization is decided by masking or unmasking of NES (GUS) or NLS (PTD) upon folding of fusion protein. Further, to determine the NLS domain of Tat, we found that upon deletion [Chauhan et al, in communication], PTD-deleted Tat protein is expressed throughout the cell (diffuse) and was not expressed in the nucleolus (Fig. 3 and 4). It is also possible that upon fusion with other proteins, PTD directs the proteins to the nucleus [64, 137, 148], (Fig. 5, Table 2). Nevertheless, when the protein under study has NES(s) that is strong enough to overcome PTD ’s NLS, the PTD-fusion protein will be directed to the cytoplasm rather than to the nucleus. Moreover, when the fusion protein has a secretory signal it will be secreted, and could impart a transcellular property. Overall, the NES and secretory properties of a protein will be weakened upon fusion with PTD (Table 3).
Thymidine-kinase (TK) expressing cancer cells are highly susceptible to cell death upon treatment with ganciclovir (GCV). Upon fusion of PTD with TK, however, TK has been shown to have a mild increase in bystander cell death due to its transcellular effect [152]. In contrast, in another study, intracellular PTD-TK-GFP expression showed no transcellular activity in rat glioma cells. Interestingly, cells expressing PTD-TK-GFP confer the bystander effect on rat glioma and human ovarian carcinoma cells in the presence of GCV, but had only a slight effect on human prostate carcinoma [154, 155]. It is possible that the bystander PTD-TK effect is due to the presence of extracellular PTD-TK concentrated on plasma membranes that has diffused to the cells in the vicinity upon reseeding of second target cells after 4 hours of fusion protein treatment. Moreover, in vivo bystander effect might be possible because of a more vigorous immune response, such as infiltration of macrophages and T-lymphocytes generated by PTD-TK as compared to TK alone [156]. We further analysed this situation and found that TK has a week NES property as judged by NES sequence program analysis (value 0.697) at positions 227-232 (LDLAML). Upon PTD fusion with TK, however, there is either competition between NLS and NES sequences or one of the signals is masked by final conformation attained by the fusion protein, and can only be verified through further testing in cell culture.
Therefore, it is inferred that PTD itself does not possess any transcellular property but, upon fusion with test protein, it would change the efficiency of NES if present. The expected outcome upon fusion of a cytoplasmic protein with PTD will be that it may be retained solely in the nucleus of the cell when expressed either intracellularly or applied as PTD-fusion protein extracellularly (Table 2 and 3). Further evidence is required by investigating fusion of PTD with other proteins to strengthen the generalized transcellular phenomenon. Most important, after PTD-TK treatment, cells should be treated either with trypsin or heparinase III to remove fusion protein that has been concentrated on the surface of the cells in order to avoid undesirable effects on cells in the vicinity [120, 157]. Ideally the best approach would be to express stable PTD-fusion proteins intracellularly to monitor the resultant transcellular effect in vitro which otherwise in vivo would be difficult to differentiate from immune infiltration effect as described above. Overall, it is clear that PTD does not have transcellular property and its fusion with protein of interest will target the cargo to the nucleus and simultaneously will weaken the net nuclear export of the cytoplasmic protein.
8. Validation of true PTD-mediated protein transduction
PTDs are promising tools for transducing presynthesized proteins across the plasma membrane. Nonetheless, because artifacts result from fixation and endosomal entrapment, true cytosolic distribution or targeting to nucleus is hampered by use of nonvisual methods [134]. There are limited numbers of approaches that can verify the true nature of PTD-fusion protein transduction in vitro. The one most commonly employed is trypsin treatment, which removes the surface-bound fusion proteins and reduces the chances of nonspecific migration of proteins upon fixation of cells during immunostaining. In addition, some studies have shown successful transduction of PTD-fusion proteins into cells without fixation [47, 98, 158-161]. Further, many of these studies have also shown biological effects upon treatment with PTD-fusion proteins, which could be due to the interactions with surface receptors [162] or due to the release of proteins from lysosomes into the cytoplasm and nucleus. It has been demonstrated that PTD entry occurs via endosomal pathways through either caveolae [163, 164] or macropinocytosis where lysosomotropic agents are required [126, 157].
It is emphasized that for targeting PTD-fusion protein to bystander cells, thorough analysis of NES and secretory signal should first be performed to determine the overall score of NES or secretory value after fusion of PTD with the protein of interest. In particular, after verification in cell culture, an additional NES signal sequence, if required, should be used for cytoplasmic delivery. It is also advisable to include the proteolytic sequence between PTD and the protein of interest so that upon endosome-mediated breakdown of fusion protein, the protein of interest is released into the cytoplasm without further influence from PTD. This should be undertaken with caution, to ensure that the proteolytic sequence remains unprocessed in the bacterial system.
Recently, an ubiquitin-specific C-terminal protease method has been described 134]. It was shown that Tat PTD-fusion protein failed to reach the cytosol in many cell types, except dendritic cells, where antigens are taken up efficiently [134]. The assay is based on the processing of PTD-linked proteins by deubiquitin enzymes (DUBs), which are localized in the nucleocytosol. In this method, PTD is fused to an ubiquitin moiety and a protein of interest as a C-terminal extension. This demonstrates that PTD fusion proteins are efficiently taken up by cells but are localized in endocytic vesicles. Nonetheless, dendritic cells were capable of sending the cargo into the cytosol [165]. The best example for verifying PTD behavior is the ultra-sensitive cre-lox reporter assay that allows easy detection of transduced cells [126]. Here, treatment with Tat-cre recombinase fusion protein induced GFP expression via cre-mediated excision of the loxP-stop codon-loxP intervening region in the nuclei of the cells.
9. Cytotoxicity of PTD
There are limited toxicity studies on PTD peptide. Tat peptide of 48-85 aa encompassing a PTD domain did not show neurotoxicity in cultures in vitro [166], but prolonged exposure (24 hours) of Hela cells to Tat peptide containing alpha helical sequence (37-60) resulted in necrosis of 60% of cells [47] while Tat peptide (43-60) revealed 10-15% toxicity. Short exposure of cell cultures with 20-100 μM concentrations of PTD did not exhibit any adverse effects [47, 167], whereas a 500 μM dose of PTD resulted in functional alterations of living fibroblasts even after short time periods [98]. The use of PTD concentration at or less than 100 μM has been suggested and a concentration above 100 μM tends to cause toxicity [122]. The studies that reported toxicity of PTD utilized very high concentrations of peptide or PTD-fusion proteins. Therefore, lower concentrations of peptide and/or PTD-fusion protein should be considered but the relative total amount could vary from one protein molecule to another.
10. Limitations in PTD-mediated protein transduction
Since the inception of protein delivery studies, cell-to-cell movement of transduced fusion protein has not been thoroughly studied. Further, in reality, PTD-based delivery of fusion proteins will invariably result in nuclear targeting [63, 135, 142-148, 168] which may not be required in every case. It is useful, however, in some cases such as expression of single chain antibody fragment, where the product is required in the nucleus to inhibit Tat-mediated HIV-LTR transcription [169, 170]. It is also not possible for all fusion proteins under study to be produced in bacteria because of the requirement for secondary post-translational modifications. Additionally, denaturation of fusion proteins for delivery may impose other limitation, as it will restrict fusion proteins from having optimal biological activity. Nonetheless, very few reports are available on this aspect.
The specific targeting of different cell organelles is possible by engineering a directing signal which otherwise is absent in the native protein sequence. Further, it will be challenging to target every given protein into the cell since it would depend on cell type as well as the nature of protein under investigation. Most of the studies have reported successful transduction of PTD-fusion proteins. One caveat is that in some cases the results may be misinterpreted, because a significant amount of fusion protein simply resides on the cell surface. This is true for Tat-PTD-attached diphtheria toxin A-fragment. The fusion molecule was not found to be cytotoxic on transduction, implying that it did not get transduced at all [171]. Moreover, diphtheria toxin is only 21 kDa in size, far smaller than beta-gal protein which has been shown to be transduced efficiently in neurons and in vivo in the brain [16]. Similarly, His-tag, which is also charged, makes protein entry into the cell difficult. In our experience, when PTD was attached without a spacer to His-tag, PTD-fusion protein failed to transduce neuroblastoma and primary rat neurons. Insertion of a 3-glycine spacer (Fig. 5) led to efficient transduction of fusion protein [63]. Whether a spacer is mandatory for all proteins for transduction is currently unknown. Besides, cluster of more cationic residues contributed by His-tag could make the PTD-fusion protein stickier, thereby, entrapped on the plasma membrane [118], however, needs to be confirmed.
It has been shown that when Tat or its basic domain was fused to GFP and expressed in eukaryotic cells, secretion and uptake were not observed in bystander cells [64, 172, 173]. It seems likely that this is because PTD is an NLS and will direct the cargo to the nucleus. Moreover, if PTD-GFP-positive cells are lysed and the lysate is applied to freshly seeded cells, Tat-GFP fusion protein was not taken up [172]. The failure of Tat-GFP protein uptake may also be due to the change in conformation and improper folding of GFP during protein purification, so that fluorescence is not observed after entry of fusion protein in the cell [142, 174]. Prior denatured PTD-fusion proteins might enter the cells because of the different but transduction-compatible conformation of the protein. Studies have been performed where even small molecules could not be transduced using Tat-PTD [175].
High extracellular concentrations of PTD fusion proteins of the order of 10 μM or above are needed to have observable effects, however [176]. Even if uptake is demonstrated, the protein may not be active inside the cell if it remains within the endocytic compartment. This was observed when Tat-calpastatin fusion protein was taken up by primary cortical neurons which, however, could not inhibit calpain-mediated spectrin breakdown because Tat-calpastatin was retained in the vesicles [177]. This study, did not involve use of any lysosomotropic agents. Similarly, when Tat-GFP was injected intramuscularly, efficient uptake in muscle cells was not observed, and intra-arterial application also failed to show distribution of GFP fluorescence in surrounding vessels [178].
PTD-linked 99Tc was unable to pass through confluent layers of tight junctions formed by epithelial cells. Similarly, PTD-99Tc administered in vivo failed to cross the epithelial lining of the urinary bladder [179]. Importantly, several years ago Melan and Sluder [180] emphasized that misleading apparent localization of soluble proteins can result from their redistribution during the preparation of cells for immunostaining. The use of different fixatives could lead to a redistribution of proteins and thereby an overestimation of transduced cells. The basic fusion protein that binds to the cell membrane could be translocated into the cells upon fixation. Similarly, the endocytosed protein upon fixation could enter the nucleus, implying that membranes get damaged and become permeable upon fixation, especially with methanol and acetone [64, 67, 113, 120, 181].
Although the chance of an immune response to PTD-fusion protein is expected to occur after repeated injections, little information on tamed PTDs is currently available from in vivo studies. Intriguingly, upon our human protein data bank homology search on four of the Tat-PTDs, wild type PTD, PTD-4 and PTD-YM3 revealed 75-95 percent homology while the CTP revealed no significant homology (Table 2). Therefore, the chances of immune response with the first three PTDs are low while with CTP it seems maximum. This must be verified in vivo, however.
11. Challenges and future of PTD
PTD fusion proteins have great potential, especially for in vitro studies. Application of PTD-directed protein delivery in vivo could prove useful under certain situations, where immediate administration of presynthesized proteins is required. But because of limited evidence and lack of a uniform means of producing proteins, and the poor protein transduction property of PTD, its wide scale use is likely to be delayed. Furthermore, because of inherent variations in the properties of different proteins, PTD-fusion proteins may not be useful for delivery in every case. Although all fusion proteins cannot be expressed in bacteria, PTD-fusion proteins can be expressed efficiently as His-tag in eukaryotic cells and subsequently be purified by affinity chromatography if post-translational modifications are required. Since there is precedence for penetration of the BBB by PTD, the major potential of PTD may be the delivery of therapeutic molecules to the central nervous system. Nevertheless, to gain more insight into the BBB penetration and other organ distribution, more detailed investigations with PTD-fusion proteins must be performed by using sensitive and quantitative techniques such as in vivo radio-imaging [182].
Although great efforts have been done to “tame” the PTD by applying various approaches such as molecular modeling and phage display system, however, the basic sequence of this peptide is too short to play around. Indeed, some fruitful results of taming have been obtained wherein new versions of PTDs have shown a stronger protein transduction property and cytoplasmic delivery, which requires further verification, however. In another approach, taming of the PTD for better delivery has been done by multimerization of PTD and attachment with protein of interest [109]. Obviously, efficiency of protein transduction will be improved by multimerization, but potential side effects may arise, especially toxicity. A further improvement in transduction property of PTD is possible with the application of fine knowledge of genomics, proteomics and computational chemistry. Despite possible future improvements in PTD ’s penetration power, however, the most challenging task will be the taming of the PTD for site specific delivery.
Conclusions
PTD of HIV-Tat protein is a nuclear localization signal which is conferred with mild protein transduction property. PTD deliver the cargo to the nucleus but, lack transcellular property. The taming of the PTD has resulted in more potent PTDs for cytoplasmic delivery. Finally, PTD may be envisioned as a universal protein and nucleic acid transducer but obviously not in the present form.
Acknowledgements
The work is supported by NIH grant (RO1 NS050064) to AC. AC also thanks to Drs Krister Kristensson, Department of Neuroscience, Karolinska Institute, Stockholm, Sweden; Suzanne Gartner, Avindra Nath and Pamela Talalay, Department of Neurology, Johns Hopkins University, Baltimore, USA, for discussions and facilities.
Abbreviations
- CPP
cell penetrating peptide
- CTP
cytoplasmic transduction peptide
- PTD
protein transduction domain
- BBB
blood brain barrier
- NES
nuclear export signal
- NLS
nuclear localization signal
- TK
thymidine kinase
- GUS
beta glucuronidase
- LTR
long terminal repeat
- HSPGs
heparan sulphate proteoglycans
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hennig AK, Lev B, Ogilvie JM, Vogler CA, Galvin N, Bassnet S, Sands MS. Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice. J Neurosci. 2003;23:3302–7. doi: 10.1523/JNEUROSCI.23-08-03302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7:1244–9. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- [3].Akimoto M, Miyatake S, Kogishi J, Hangai M, Okazaki K, Takahashi JC, et al. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–9. [PubMed] [Google Scholar]
- [4].Kugler S, Kilic E, Bahr M. Human synapsin 1 gene promoter confers highly neuron specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003;10:337–47. doi: 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- [5].Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson′s disease. Science. 2000;290:767–73. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- [6].Antonawich FJ, Federoff HJ, Davis JN. BCL-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Exp Neurol. 1999;156:130–7. doi: 10.1006/exnr.1998.7004. [DOI] [PubMed] [Google Scholar]
- [7].Lawrence MS, McLaughlin JR, Sun GH, Ho DY, McIntosh L, Kunis DM, et al. Herpes simplex viral vectors expressing Bcl-2 are neuroprotective when delivered after a stroke. J Cereb Blood Flow Metaba. 1999;17:740–4. doi: 10.1097/00004647-199707000-00003. [DOI] [PubMed] [Google Scholar]
- [8].Hickman MA, Malone RW, Lehmann-Bruinsma K, Sih TR, Knoell D, Szoka FC, et al. Gene expression following direct injection of DNA into liver. Hum Gene Ther. 1994;5:1477–83. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- [9].Hengge UR, Walker PS, Vogel JC. Expression of naked DNA in human, pig, and mouse skin. J Clin Invest. 1996;97:2911–6. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Check E. Gene therapy: shining hopes dented - but not dashed. Nature. 2002;420:735. doi: 10.1038/420735b. [DOI] [PubMed] [Google Scholar]
- [11].Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730–4. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- [12].Cornetta K, Morgan RA, Anderson WF. Safety issues related to retroviral-mediated gene transfer in humans. Hum. Gene Ther. 1991;2:5–14. doi: 10.1089/hum.1991.2.1-5. [DOI] [PubMed] [Google Scholar]
- [13].Marshall E. Viral threat to newborns under radar. Science. 2002;295:1631. doi: 10.1126/science.295.5560.1631a. [DOI] [PubMed] [Google Scholar]
- [14].Gupta B, Torchilin VP. Transactivating transcriptional activator-mediated drug Delivery. Expert Opin Drug Deliv. 2006;3:177–90. doi: 10.1517/17425247.3.2.177. [DOI] [PubMed] [Google Scholar]
- [15].Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse Approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [16].Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- [17].Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;272:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- [18].Shen WC, Ryser HJ. Conjugation of poly-L-lysine to albumin and horseradish peroxidase: a novel method of enhancing the cellular uptake of proteins. Proc Natl Acad Sci U S A. 1978;75:1872–6. doi: 10.1073/pnas.75.4.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ryser HJ, Drummond I, Shen WC. The cellular uptake of horseradish peroxidase and its poly (lysine) conjugate by cultured fibroblasts is qualitatively similar despite a 900-fold difference in rate. J Cell Physiol. 1982;113:167–78. doi: 10.1002/jcp.1041130126. [DOI] [PubMed] [Google Scholar]
- [20].Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res. 2000;56:318–25. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- [21].Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy N, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 1998;4:1449–52. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- [22].Dilber MS, Phelan A, Aints A, Mohamed AJ, Elliott G, Smith CI, O′Hare P. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein. VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- [23].Liu CS, Kong B, Xia HH, Ellem KA, Wei MQ. VP22 enhanced intercellular trafficking of HSV thymidine kinase reduced the level of ganciclovir needed to cause suicide cell death. J Gene Med. 2001;3:145–52. doi: 10.1002/jgm.164. [DOI] [PubMed] [Google Scholar]
- [24].Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Polo JM, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum Gene Ther. 2002;13:553–68. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- [25].Zhao Y, Lou D, Burkett J, Kohler H. Chemical engineering of cell penetrating Antibodies. J Immunol Methods. 2001;254:137–45. doi: 10.1016/s0022-1759(01)00410-0. [DOI] [PubMed] [Google Scholar]
- [26].Kokryakov VN, Harwig SS, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, et al. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–6. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- [27].Chang M, Zhang L, Tam JP, Sanders-Bush E. Dissecting G protein-coupled receptor signaling pathways with membrane-permeable blocking peptides. Endogenous 5-HT (2C) receptors in choroid plexus epithelial cells. J Biol Chem. 2000;275:7021–9. doi: 10.1074/jbc.275.10.7021. [DOI] [PubMed] [Google Scholar]
- [28].Jans DA. Nuclear signaling pathways for polypeptide ligands and their membrane receptors? FASEB J. 1994;8:841–7. doi: 10.1096/fasebj.8.11.8070633. [DOI] [PubMed] [Google Scholar]
- [29].Cutrona G, Carpaneto EM, Ulivi M, Roncella S, Landt O, Ferrarini M, Boffa LC. Effects in live cells of a c-myc anti-gene PNA linked to a nuclear localization Signal. Nat Biotechnol. 2000;18:300–3. doi: 10.1038/73745. [DOI] [PubMed] [Google Scholar]
- [30].Liu XH, Collier RJ, Youle RJ. Inhibition of axotomy-induced neuronal apoptosis by extracellular delivery of a Bcl-XL fusion protein. J Biol Chem. 2001;276:46326–32. doi: 10.1074/jbc.M108930200. [DOI] [PubMed] [Google Scholar]
- [31].Liu XH, Castelli JC, Youle RJ. Receptor-mediated uptake of an extracellular Bcl-x(L) fusion protein inhibits apoptosis. Proc Natl Acad Sci U S A. 1999;96:9563–7. doi: 10.1073/pnas.96.17.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–6. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morris MC, Depollier J, Mery J, Heitz F F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–6. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- [34].Nur-e-Kamal MS, Qureshi MM, Kamal JM, Montague W, Maruta H. Construction of a cell-permeable CDC42 binding fragment of ACK that inhibits v-Ha-Ras transformation. Ann N Y Acad Sci. 1999;886:285–8. doi: 10.1111/j.1749-6632.1999.tb09438.x. [DOI] [PubMed] [Google Scholar]
- [35].Prior TI, FitzGerald DJ, Pastan I. Translocation mediated by domain II of Pseudomonas exotoxin A: transport of barnase into the cytosol. Biochemistry. 1992;31:3555–9. doi: 10.1021/bi00129a001. [DOI] [PubMed] [Google Scholar]
- [36].Perez F, Joliot A, Bloch-Gallego E, Zahraoui A, Triller A, Prochiantz A. Antennapedia homeobox as a signal for the cellular internalization and nuclear addressing of a small exogenous peptide. J Cell Sci. 1992;102:717–22. doi: 10.1242/jcs.102.4.717. [DOI] [PubMed] [Google Scholar]
- [37].Bolton SJ, Jones DN, Darker JG, Eggleston DS, Hunter AJ, Walsh FS. Cellular uptake and spread of the cell-permeable peptide penetratin in adult rat Brain. Eur J Neurosci. 2000;12:2847–55. doi: 10.1046/j.1460-9568.2000.00171.x. [DOI] [PubMed] [Google Scholar]
- [38].Elliott G, O′Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–33. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- [39].Wills KN, Atencio IA, Avanzini JB, Neuteboom S, Phelan A, Philopena J, et al. Intratumoral spread and increased efficacy of a p53-VP22 fusion protein expressed by a recombinant adenovirus. J Virol. 2001;75:8733–41. doi: 10.1128/JVI.75.18.8733-8741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wybranietz WA, Gross CD, Phelan A, O′Hare P, Spiegel M, Graepler F, et al. Enhanced suicide gene effect by adenoviral transduction of a VP22-cytosine deaminase (CD) fusion gene. Gene Ther. 2001;8:1654–64. doi: 10.1038/sj.gt.3301564. [DOI] [PubMed] [Google Scholar]
- [41].Phelan A, Elliott G, O′Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–3. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- [42].Roy V, Qiao J, de Campos-Lima P, Caruso M. Direct evidence for the absence of intercellular trafficking of VP22 fused to GFP or to the herpes simplex virus thymidine kinase. Gene Ther. 2005;12:169–76. doi: 10.1038/sj.gt.3302394. [DOI] [PubMed] [Google Scholar]
- [43].Hakkarainen T, Wahlfors T, Merilainen O, Loimas S, Hemminki A, Wahlfors J. VP22 does not significantly enhance enzyme prodrug cancer gene therapy as a part of a VP22-HSVTk-GFP triple fusion construct. J Gene Med. 2005;7:898–907. doi: 10.1002/jgm.737. [DOI] [PubMed] [Google Scholar]
- [44].Morris MC, Chaloin L, Mery J, Heitz F, Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27:3510–7. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Deshayes S, Plenat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry. 2004;43:7698–706. doi: 10.1021/bi049298m. [DOI] [PubMed] [Google Scholar]
- [46].Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B. Barsoum, Tat-mediated delivery of heterologous proteins into cells. Proc. Natl Acad Sci U S A. 1994;91:664–8. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol Chem. 1997;272:16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- [48].Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1998;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- [49].Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1998;55:1179–88. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- [50].Siomi H, Shida H, Maki M, Hatanaka M M. Effects of a highly basic region of human immunodeficiency virus Tat protein on nucleolar localization. J Virol. 1990;64:1803–7. doi: 10.1128/jvi.64.4.1803-1807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, et al. Complexes of plasmid DNA with basic domain 47-57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–36. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- [52].Dietz GP, Kilic E, Bahr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- [53].Domínguez-Bendala J, Klein D, Ribeiro M, Ricordi C, Inverardi L, Pastori R, Edlund H. TAT-mediated neurogenin 3 protein transduction stimulates pancreatic endocrine differentiation in vitro. Diabetes. 2005;54:720–6. doi: 10.2337/diabetes.54.3.720. [DOI] [PubMed] [Google Scholar]
- [54].Klein D, Ribeiro MM, Mendoza V, Jayaraman S, Kenyon NS, Pileggi A, et al. Delivery of Bcl-XL or its BH4 domain by protein transduction inhibits apoptosis in human islets. Biochem Biophys Res Commun. 2004;323:473–8. doi: 10.1016/j.bbrc.2004.08.116. [DOI] [PubMed] [Google Scholar]
- [55].Wang W, El-Deiry WS. Targeting p53 by PTD-mediated transduction. Trends Biotechnol. 2004;22:431–4. doi: 10.1016/j.tibtech.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [56].Shen H, Mai JC, Qiu L, Cao S, Robbins PD, Cheng T. Evaluation of peptide-mediated transduction in human CD34+ cells. Hum Gene Ther. 2004;15:415–9. doi: 10.1089/104303404322959560. [DOI] [PubMed] [Google Scholar]
- [57].Ozaki D, Sudo K, Asoh S, Yamagata K, Ito H, Ohta S. Transduction of anti-apoptotic proteins into chondrocytes in cartilage slice culture. Biochem Biophys Res Commun. 2004;313:522–7. doi: 10.1016/j.bbrc.2003.11.144. [DOI] [PubMed] [Google Scholar]
- [58].Vazquez J, Sun C, Du J, Fuentes L, Sumners C, Raizada MK. Transduction of a functional domain of the AT1 receptor in neurons by HIV-Tat PTD. Hypertension. 2003;41:751–6. doi: 10.1161/01.HYP.0000047878.13793.41. [DOI] [PubMed] [Google Scholar]
- [59].Asoh S, Ohsawa I, Mori T, Katsura K, Hiraide T, Katayama Y, Kimura M, Ozaki D, Yamagata K, Ohta S. Protection against ischemic brain injury by protein Therapeutics. Proc Natl Acad Sci U S A. 2002;99:17107–12. doi: 10.1073/pnas.262460299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kwon YD, Oh SK, Kim HS, Ku SY, Kim SH, Choi YM, Moon SY. Cellular manipulation of human embryonic stem cells by TAT-PDX1 protein transduction. Mol Ther. 2005;12:28–32. doi: 10.1016/j.ymthe.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [61].Vogel BE, Lee SJ, Hildebrand A, Craig W, Pierschbacher MD, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121:461–8. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–7. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- [63].Brask J, Chauhan A, Hill RH, Ljunggren HG, Kristensson K. Effects on synaptic activity in cultured hippocampal neurons by influenza A viral proteins. J Neurovirol. 2005;11:395–402. doi: 10.1080/13550280500186916. [DOI] [PubMed] [Google Scholar]
- [64].Leifert JA, Harkins S, Whitton JL. Full-length proteins attached to the HIV tat protein transduction domain are neither transduced between cells, nor exhibit enhanced immunogenicity. Gene Ther. 2002;9:1422–8. doi: 10.1038/sj.gt.3301819. [DOI] [PubMed] [Google Scholar]
- [65].Andrade DM, Scherer SW, Minassian BA. Protein therapy for Unverricht-Lundborg disease using cystatin-B transduction by Tat-PTD Is it that simple. Epilepsy Research. 2006 doi: 10.1016/j.eplepsyres.2006.07.009. epub. ahead of print. [DOI] [PubMed] [Google Scholar]
- [66].Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat Biotechnol. 2001;19:713–4. doi: 10.1038/90741. [DOI] [PubMed] [Google Scholar]
- [67].Lundberg M, Johansson M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem Biophys Res Commun. 2002;291:367–71. doi: 10.1006/bbrc.2002.6450. [DOI] [PubMed] [Google Scholar]
- [68].Tikhonov I, Ruckwardt TJ, Berg S, Hatfield GS, David C. Pauza, Furin cleavage of the HIV-1 Tat protein. FEBS Lett. 2004;565:89–92. doi: 10.1016/j.febslet.2004.03.079. [DOI] [PubMed] [Google Scholar]
- [69].Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In Vivo Delivery of a Bcl-Xl Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J Neurosci. 2002;22:5423–31. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Elliger SS, Elliger CA, Lang C, Watson GL. Enhanced secretion and uptake of beta-glucuronidase improves adeno-associated viral-mediated gene therapy of mucopolysaccharidosis type VII mice. Mol Ther. 2002;5:617–26. doi: 10.1006/mthe.2002.0594. [DOI] [PubMed] [Google Scholar]
- [71].Kilic U, Kilic E, Dietz GP, Bahr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34:1304–10. doi: 10.1161/01.STR.0000066869.45310.50. [DOI] [PubMed] [Google Scholar]
- [72].Pei DS, Wang XT, Liu Y, Sun YF, Guan QH, Wang W, et al. Neuroprotection against ischaemic brain injury by a GluR6-9c peptide containing the TAT protein transduction sequence. Brain. 2006;129:465–79. doi: 10.1093/brain/awh700. [DOI] [PubMed] [Google Scholar]
- [73].Santra S, Yang H, Stanley JT, Holloway PH, Moudgil BM, Walter G, et al. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem Commun (Camb) 2005;25:3144–6. doi: 10.1039/b503234b. [DOI] [PubMed] [Google Scholar]
- [74].Soane L, Fiskum G. TAT-mediated endocytotic delivery of the loop deletion Bcl-2 protein protects neurons against cell death. J Neurochem. 2005;95:230–43. doi: 10.1111/j.1471-4159.2005.03359.x. [DOI] [PubMed] [Google Scholar]
- [75].Krautwald S, Ziegler E, Tiede K, Pust R, Kunzendorf U. Transduction of the TAT-FLIP fusion protein results in transient resistance to Fas-induced apoptosis in Vivo. J Biol Chem. 2004;279:44005–11. doi: 10.1074/jbc.M401327200. [DOI] [PubMed] [Google Scholar]
- [76].Bullok KE, Dyszlewski M, Prior JL, Pica CM, Sharma V, Piwnica-Worms D. Characterization of novel histidine-tagged Tat-peptide complexes dual-labeled with (99m)Tc-tricarbonyl and fluorescein for scintigraphy and fluorescence microscopy. Bioconjug Chem. 2002;13:1226–37. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- [77].Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, et al. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–7. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- [78].Shibagaki N, Udey MC. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168:2393–401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- [79].Shibagaki N, Udey MC. Dendritic cells transduced with TAT protein transduction domain-containing tyrosinase-related protein 2 vaccinate against murine melanoma. Eur J Immunol. 2003;33:850–60. doi: 10.1002/eji.200323709. [DOI] [PubMed] [Google Scholar]
- [80].Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–7. [PubMed] [Google Scholar]
- [81].Kim D, Jeon C, Kim JH, Kim MS, Yoon CH, Choi IS, et al. Cytoplasmic transduction peptide (CTP): new approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res. 2006;312:1277–88. doi: 10.1016/j.yexcr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- [82].Cai SR, Xu G, Becker-Hapak M, Ma M, Dowdy SF, McLeod HL. The kinetics and tissue distribution of protein transduction in mice. Eur J Pharm Sci. 2006;27:311–9. doi: 10.1016/j.ejps.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [83].Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. Molecular transporters: Synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. Chem Biochem. 2006;7:1497–1515. doi: 10.1002/cbic.200600171. [DOI] [PubMed] [Google Scholar]
- [84].Mukai Y, Sugita T, Yamato T, Yamanada N, Shibata H, Imai S, Abe Y, Nagano K, Nomura T, Tsutsumi Y, Kamada H, Nakagawa S, Tsunoda S. Creation of Novel Protein Transduction Domain (PTD) Mutants by a Phage Display-Based High-Throughput Screening System. Biol Pharm Bull. 2006;29:1570–4. doi: 10.1248/bpb.29.1570. [DOI] [PubMed] [Google Scholar]
- [85].Ryser HJ. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967;32:737–50. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ryser HJ, Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. 1965;150:501–3. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- [87].Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–6. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- [88].Wright LR, Rothbard JB, Wender PA. Guanidinium rich peptide transporters and drug delivery. Curr Protein Pept Sci. 2003;4:105–124. doi: 10.2174/1389203033487252. [DOI] [PubMed] [Google Scholar]
- [89].Murphy JE, Uno T, Hamer JD, Cohen FE, Dwarki V, Zuckermann RN. A combinatorial approach to the discovery of efficient cationic peptoid reagents for gene delivery. Proc Natl Acad Sci U S A. 1998;95:1517–22. doi: 10.1073/pnas.95.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Niidome T, Ohmori N, Ichinose A, Wada A, Mihara H, Hirayama T, Aoyagi H. Binding of cationic alpha-helical peptides to plasmid DNA and their gene transfer abilities into cells. J Biol Chem. 1997;272:15307–12. doi: 10.1074/jbc.272.24.15307. [DOI] [PubMed] [Google Scholar]
- [91].Dathe M, Schumann M, Wieprecht T, Winkler A, Beyermann M, Krause E. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 1996;35:12612–22. doi: 10.1021/bi960835f. [DOI] [PubMed] [Google Scholar]
- [92].Ziegler A, Seelig J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys J. 2004;86:254–63. doi: 10.1016/S0006-3495(04)74101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sandgren S, Cheng F, Belting M. Nuclear targeting of macromolecular polyanions by an HIV-Tat derived peptide. Role for cell-surface proteoglycans. J Biol Chem. 2002;277:38877–83. doi: 10.1074/jbc.M205395200. [DOI] [PubMed] [Google Scholar]
- [94].Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–61. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- [95].Rusnati M, Coltrini D D, Oreste P, Zoppetti G, Albini A, Noonan D, et al. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J Biol Chem. 1997;272:11313–20. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- [96].Hakansson S, Jacobs A, Caffrey M. Heparin binding by the HIV-1 tat protein transduction domain. Protein Sci. 2001;10:2138–9. doi: 10.1110/ps.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yanagishita M, Hascall VC. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992;267:9451–4. [PubMed] [Google Scholar]
- [98].Ziegler A, Nervi P, Durrenberger M, Seelig J. The cationic cell-penetrating peptide CPP (TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry. 2005;44:138–48. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]
- [99].Marty C, Meylan C, Schott H, Ballmer-Hofer K, Schwendener RA. Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes. Cell Mol Life Sci. 2004;61:1785–94. doi: 10.1007/s00018-004-4166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–14. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- [101].Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–44. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ryser HJ. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968;159:390–6. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- [103].Hancock R, Ryser HJ. Histones in prophase and their possible role in nuclear membrane breakdown. Nature. 1967;213:701–2. doi: 10.1038/213701a0. [DOI] [PubMed] [Google Scholar]
- [104].Gariepy J, Kawamura K. Vectorial delivery of macromolecules into cells using peptide-based vehicles. Trends Biotechnol. 2001;19:21–8. doi: 10.1016/s0167-7799(00)01520-1. [DOI] [PubMed] [Google Scholar]
- [105].Dworetzky SI, Lanford RE, Feldherr CM. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1998;107:1279–87. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kawamura KS, Sung M, Bolewska-Pedyczak E, Gariepy J. Probing the impact of valency on the routing of arginine-rich peptides into eukaryotic cells. Biochemistry. 2006;45:1116–27. doi: 10.1021/bi051338e. [DOI] [PubMed] [Google Scholar]
- [107].Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U S A. 2001;98:8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D′Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–7. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278:11411–8. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- [110].Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat Protein. EMBO J. 1991;10:1733–9. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Awedikian R, Francois A, Guilbaud M, Moullier P, Salvetti A. Intracellular route and biological activity of exogenously delivered Rep proteins from the adeno-associated virus type 2. Virology. 2005;335:252–63. doi: 10.1016/j.virol.2005.02.024. [DOI] [PubMed] [Google Scholar]
- [112].Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–6. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- [113].Lundberg M, Wikstrom S, Johansson M. Cell surface adherence and endocytosis of protein transduction domains. Mol Ther. 2003;8:143–50. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- [114].Futaki S. Oligoarginine vectors for intracellular delivery: Design and cellular-uptake mechanisms. Biopolymers. 2006;84:241–249. doi: 10.1002/bip.20421. [DOI] [PubMed] [Google Scholar]
- [115].Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- [116].Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48-60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003;307:100–7. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- [117].Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem. 2002;269:494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- [118].Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–40. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- [119].Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- [120].Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- [121].Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277:2437–43. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- [122].Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SM, Newham P, Lindsay MA. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol. 2005;45:1093–102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15:2347–60. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Foerg C, Ziegler U, Fernandez-Carneado J, Giralt E, Rennert R, Beck-Sickinger AG, Merkle HP. Decoding the entry of two novel cell-penetrating peptides in HeLa cells: lipid raft-mediated endocytosis and endosomal escape. Biochemistry. 2005;44:72–81. doi: 10.1021/bi048330+. [DOI] [PubMed] [Google Scholar]
- [125].Fittipaldi A, Giacca M. Transcellular protein transduction using the Tat protein of HIV-1. Adv Drug Deliv Rev. 2005;57:597–608. doi: 10.1016/j.addr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- [126].Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–53. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- [127].Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–5. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- [128].Albarran B, To R, Stayton PS. A TAT-streptavidin fusion protein directs uptake of biotinylated cargo into mammalian cells. Protein Eng Des Sel. 2005;18:147–52. doi: 10.1093/protein/gzi014. [DOI] [PubMed] [Google Scholar]
- [129].Bettosini F, Fiorillo MT, Magnacca A, Leone L, Torrisi MR, Sorrentino R. The C-terminus of the nucleoprotein of influenza A virus delivers antigens transduced by Tat to the trans-golgi network and promotes an efficient presentation through HLA class I. J Virol. 2005;79:15537–46. doi: 10.1128/JVI.79.24.15537-15546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–56. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- [131].Vocero-Akbani A, Lissy NA, Dowdy SF. Transduction of full-length Tat fusion proteins directly into mammalian cells: analysis of T cell receptor activation-induced cell death. Methods Enzymol. 2000;322:508–21. doi: 10.1016/s0076-6879(00)22046-6. [DOI] [PubMed] [Google Scholar]
- [132].Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278:50188–94. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- [133].Leifert JA, Whitton JL. Translocatory proteins” and “protein transduction domains”: a critical analysis of their biological effects and the underlying mechanisms. Mol Ther. 2003;8:13–20. doi: 10.1016/s1525-0016(03)00151-5. [DOI] [PubMed] [Google Scholar]
- [134].Loison F, Nizard P, Sourisseau T, Le Goff P, Debure L, Le Drean Y, Michel D. A ubiquitin-based assay for the cytosolic uptake of protein transduction Domains. Mol Ther. 2005;11:205–14. doi: 10.1016/j.ymthe.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [135].Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–9. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- [136].Michiue H, Tomizawa K, Wei FY, Matsushita M, Lu YF, Ichikawa T, et al. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J Biol Chem. 2005;280:8285–9. doi: 10.1074/jbc.M412430200. [DOI] [PubMed] [Google Scholar]
- [137].Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–6. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Maxfield FR. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982;95:676–81. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, et al. Photo-acceleration of protein release from endosome in the protein transduction System. FEBS Lett. 2004;572:221–6. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- [140].Normand N, van Leeuwen H, O′Hare P. Particle formation by a conserved domain of the herpes simplex virus protein VP22 facilitating protein and nucleic acid delivery. J Biol Chem. 2001;276:15042–50. doi: 10.1074/jbc.M010294200. [DOI] [PubMed] [Google Scholar]
- [141].Tasciotti E, Zoppe M, Giacca M. Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 Tat. Cancer Gene Ther. 2003;10:64–74. doi: 10.1038/sj.cgt.7700526. [DOI] [PubMed] [Google Scholar]
- [142].Cashman SM, Morris DJ, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8:130–42. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- [143].Marasco WA, Szilvay AM, Kalland KH, Helland DG, Reyes HM, Walter RJ. Spatial association of HIV-1 tat protein and the nucleolar transport protein B23 in stably transfected Jurkat T-cells. Arch Virol. 1994;139:133–54. doi: 10.1007/BF01309460. [DOI] [PubMed] [Google Scholar]
- [144].Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–82. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [145].Stauber RH, Pavlakis GN. Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology. 1998;252:126–36. doi: 10.1006/viro.1998.9400. [DOI] [PubMed] [Google Scholar]
- [146].Siomi H, Shida H, Maki M, Hatanaka M. Effects of a highly basic region of human immunodeficiency virus Tat protein on nucleolar localization. J Virol. 1990;64:1803–7. doi: 10.1128/jvi.64.4.1803-1807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Michienzi A, Li S, Zaia JA, Rossi JJ. A nucleolar TAR decoy inhibitor of HIV-1 replication. Proc Natl Acad Sci U S A. 2002;99:14047–52. doi: 10.1073/pnas.212229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Efthymiadis A, Briggs LJ, Jans DA. The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties. J Biol Chem. 1998;273:1623–8. doi: 10.1074/jbc.273.3.1623. [DOI] [PubMed] [Google Scholar]
- [149].Helland DE, Welles JL, Caputo A, Haseltine WA. Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J Virol. 1991;65:4547–9. doi: 10.1128/jvi.65.8.4547-4549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Dorsky DI, Harrington RD. An indicator cell assay for T-cell tropic, macrophage-tropic, and primary isolates of HIV-1 based on green fluorescent protein. J Acquir Immune Defic Syndr. 1999;22:213–20. doi: 10.1097/00126334-199911010-00001. [DOI] [PubMed] [Google Scholar]
- [151].Xia H, Mao Q, Davidson BL. The HIV Tat protein transduction domain improves the biodistribution of beta-glucuronidase expressed from recombinant viral vectors. Nat Biotechnol. 2001;19:640–4. doi: 10.1038/90242. [DOI] [PubMed] [Google Scholar]
- [152].Tasciotti E, Giacca M. Fusion of the human immunodeficiency virus type 1 tat protein transduction domain to thymidine kinase increases bystander effect and induces enhanced tumor killing in vivo. Hum Gene Ther. 2005;16:1389–403. doi: 10.1089/hum.2005.16.1389. [DOI] [PubMed] [Google Scholar]
- [153].Orii KO, Grubb JH, Vogler C, Levy B, Tan Y, Markova K, et al. Defining the pathway for Tat-mediated delivery of beta-glucuronidase in cultured cells and MPS VII mice. Mol Ther. 2005;12:345–52. doi: 10.1016/j.ymthe.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Merilainen O, Hakkarainen T, Wahlfors T, Pellinen R, Wahlfors J. HIV-1 TAT protein transduction domain mediates enhancement of enzyme prodrug cancer gene therapy in vitro: a study with TAT-TK-GFP triple fusion construct. Int J Oncol. 2005;27:203–8. [PubMed] [Google Scholar]
- [155].Cao L, Si J, Wang W, Zhao X, Yuan X, Zhu H, Wu X, Zhu J, Shen G. Intracellular localization and sustained prodrug cell killing activity of TAT-HSVTK fusion protein in hepatocelullar carcinoma cells. Mol Cells. 2006;21:104–11. [PubMed] [Google Scholar]
- [156].Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D, Klatzmann D. A “distant” bystander effect of suicide gene therapy: regression of nontransduced tumors together with a distant transduced tumor. Hum Gene Ther. 1997;8:1807–14. doi: 10.1089/hum.1997.8.15-1807. [DOI] [PubMed] [Google Scholar]
- [157].Green I, Christison R, Voyce CJ, Bundell KR, Lindsay MA. Protein transduction domains: are they delivering? Trends Pharmacol Sci. 2003;24:213–5. doi: 10.1016/S0165-6147(03)00076-2. [DOI] [PubMed] [Google Scholar]
- [158].Futaki S, Ohashi W, Suzuki T, Niwa M, Tanaka S, Ueda K, et al. Stearylated arginine-rich peptides: a new class of transfection systems. Bioconjug Chem. 2001;12:1005–11. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- [159].Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A. 1991;188:1864–8. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nori A, Jensen KD, Tijerina M, Kopeckova P, Kopecek J. Tat-conjugated synthetic macromolecules facilitate cytoplasmic drug delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14:44–50. doi: 10.1021/bc0255900. [DOI] [PubMed] [Google Scholar]
- [161].Waizenegger T, Fischer R, Brock R. Intracellular concentration measurements in adherent cells: a comparison of import efficiencies of cell-permeable peptides. Biol Chem. 2002;383:291–9. doi: 10.1515/BC.2002.031. [DOI] [PubMed] [Google Scholar]
- [162].Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276:34880–7. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- [163].Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, Beltram F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol Ther. 2003;8:284–94. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- [164].Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, Giacca M. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J Biol Chem. 2003;278:34141–9. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- [165].Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- [166].Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–80. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta. 2001;1515:101–9. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- [168].Hu M, Chen P, Wang J, Scollard DA, Vallis KA, Reilly RM. (123)I-labeled HIV-tat peptide radioimmunoconjugates are imported into the nucleus of human breast cancer cells and functionally interact in vitro and in vivo with the cyclin-depdendent kinase inhibitor, p21 (WAF-1/Cip-1) Eur J Nucl Med, Mol Imaging. 2006 doi: 10.1007/s00259-006-0189-0. [E Epub a head of print] [DOI] [PubMed] [Google Scholar]
- [169].Theisen DM, Pongratz C, Wiegmann K, Rivero F, Krut O, Kronke M. Targeting of HIV-1 Tat traffic and function by transduction-competent single chain Antibodies. Vaccine. 2006;24:3127–36. doi: 10.1016/j.vaccine.2006.01.055. [DOI] [PubMed] [Google Scholar]
- [170].Mi MY, Zhang J, He Y. Inhibition of HIV derived lentiviral production by TAR RNA binding domain of TAT protein. Retrovirology. 2005;2:71. doi: 10.1186/1742-4690-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Falnes PO, Wesche J, Olsnes S. Ability of the Tat basic domain and VP22 to mediate cell binding, but not membrane translocation of the diphtheria toxin A-fragment. Biochemistry. 2001;40:4349–58. doi: 10.1021/bi002443l. [DOI] [PubMed] [Google Scholar]
- [172].Yang Y, Ma J, Song Z, Wu M. HIV-1 TAT-mediated protein transduction and subcellular localization using novel expression vectors. FEBS Lett. 2002;532:36–44. doi: 10.1016/s0014-5793(02)03624-4. [DOI] [PubMed] [Google Scholar]
- [173].Ford KG, Souberbielle BE, Darling D, Farzaneh F. Protein transduction: an alternative to genetic intervention? Gene Ther. 2001;8:1–4. doi: 10.1038/sj.gt.3301383. [DOI] [PubMed] [Google Scholar]
- [174].Sacchetti A, Cappetti V, Marra P, Dell′Arciprete R, El Sewedy T, Crescenzi C, Alberti S. Green fluorescent protein variants fold differentially in prokaryotic and eukaryotic cells. J Cell Biochem (Suppl) 2001;36:117–28. doi: 10.1002/jcb.1091. [DOI] [PubMed] [Google Scholar]
- [175].Liu J, Zhang Q, Remsen EE, Wooley KL. Nanostructured materials designed for cell binding and transduction. Biomacromolecules. 2001;2:362–8. doi: 10.1021/bm015515c. [DOI] [PubMed] [Google Scholar]
- [176].Lindsay MA. Peptide-mediated cell delivery: application in protein target Validation. Curr Opin Pharmacol. 2002;2:587–94. doi: 10.1016/s1471-4892(02)00199-6. [DOI] [PubMed] [Google Scholar]
- [177].Sengoku T, Bondada V, Hassane D, Dubal S, Geddes JW. Tat-calpastatin fusion proteins transduce primary rat cortical neurons but do not inhibit cellular calpain activity. Exp Neurol. 2003;188:161–70. doi: 10.1016/j.expneurol.2004.03.018. [DOI] [PubMed] [Google Scholar]
- [178].Caron NJ, Torrente Y, Camirand G, Bujold M, Chapdelaine P, Leriche K, et al. Intracellular delivery of a Tat-eGFP fusion protein into muscle cells. Mol Ther. 2001;3:310–8. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- [179].Violini S, Sharma V, Prior JL, Dyszlewski M, Piwnica-Worms D. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well-differentiated epithelial cells: lack of correlation with heparan sulfate. Biochemistry. 2002;41:12652–61. doi: 10.1021/bi026097e. [DOI] [PubMed] [Google Scholar]
- [180].Melan MA, Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy, J. 1992;101:731–43. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- [181].Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J Control Release. 2005;109:77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- [182].Kameyama S, Horie M, Kikuchi T, Omura T, Takeuchi T, Nakase I, Sugiura Y, Futaki S. Effects of cell-permeating Peptide binding on the distribution of (125)I-labeled fab fragment in rats. Bioconjug Chem. 2006;17:597–602. doi: 10.1021/bc050258k. [DOI] [PubMed] [Google Scholar]