Abstract
Background
Asthma is an increasingly common disorder responsible for considerable morbidity and mortality. Although obesity is a risk factor for asthma and weight loss can improve symptoms, many patients do not adhere to low calorie diets and the impact of dietary restriction on the disease process is unknown.
Objective
A study was designed to determine if overweight asthma patients would adhere to an alternate day calorie restriction (ADCR) dietary regimen, and to establish the effects of the diet on their symptoms, pulmonary function and markers of oxidative stress and inflammation.
Methods
Ten subjects with BMI>30 were maintained for 8 weeks on a dietary regimen in which they ate ad libitum every other day, while consuming less than 20% of their normal calorie intake on the intervening days. At baseline, and at designated time points during the 8 week study, asthma control, symptoms and Quality of Life questionnaires (ACQ,ASUI, mini-AQLQ) were assessed and blood was collected for analyses of markers of general health, oxidative stress and inflammation. Peak Expiratory Flow (PEF) was measured daily on awakening. Pre and post bronchodilator spirometry was obtained at baseline and 8 weeks.
Results
Nine of the subjects adhered to the diet and lost an average of 8% of their initial weight during the study. Their asthma related symptoms, control and QOL improved significantly, and PEF increased significantly, within 2 weeks of diet initiation; these changes persisted for the duration of the study. Spirometery was unaffected by ADCR. Levels of serum β-hydroxybutyrate were increased and levels of leptin were decreased on CR days indicating a shift in energy metabolism towards utilization of fatty acids and confirming compliance with the diet. The improved clinical findings were associated with decreased levels of serum cholesterol and triglycerides, striking reductions in markers of oxidative stress (8-isoprostane, nitrotyrosine, protein carbonyls, and 4-hydroxynonenal adducts) and increased levels of the antioxidant uric acid. Indicators of inflammation, including serum tumor necrosis factor-α and brain-derived neurotrophic factor, were also significantly decreased by ADCR.
Conclusions
Compliance with the ADCR diet was high, symptoms and pulmonary function improved, and oxidative stress and inflammation declined in response to the dietary intervention. These findings demonstrate rapid and sustained beneficial effects of ADCR on the underlying disease process in subjects with asthma, suggesting a novel approach for therapeutic intervention in this disorder.
Keywords: AQLQ, isoprostanes, peak expiratory flow, protein carbonyls, nitrotyrosine, BDNF, spirometry, tumor necrosis factor, oxidative stress
INTRODUCTION
The cause(s) and pathogenic mechanisms of asthma are poorly understood, and available treatments can alleviate symptoms but do not reverse the disease process1. The prevalence of asthma in industrialized countries throughout the world has increased significantly during the past 30 years, particularly in children where rates have nearly doubled2. This recent surge of asthma prevalence does not appear to be the result of increases in specific allergens. Instead, increasing evidence points to a link between overeating/obesity and asthma. Weight loss often improves asthma symptoms in obese subjects3, and low calorie diets and exercise programs result in weight loss and can reduce asthma symptoms in overweight children and adults4, 5. However, while obesity is a risk factor for asthma-related symptoms such as wheezing, it may not be a cause of airway hyperresponsiveness5, 6. It is therefore unclear whether weight loss modifies the asthma disease process.
The molecular and cellular mechanisms underlying airway hyper-responsiveness and asthma symptoms are complex and poorly understood. Two general alterations in the lungs are increased oxidative stress and inflammation7–11. The local changes in the lungs are associated with increases in markers of inflammation and oxidative stress in the blood including TNF12, interleukin-613 and lipid peroxidation products14. In addition, circulating levels of brain-derived neurotrophic factor (BDNF) are increased in patients with asthma and other allergic disorders15, 16. Although capable of transiently relieving asthma symptoms, agents such as corticosteroids and β-adrenoreceptor agonists do not block or reverse the underlying disease process and their long-term use poses a considerable risk of morbidity and mortality17, 18.
Caloric restriction (CR) improves numerous health indicators in rodents, monkeys and humans, including those associated with risk of cardiovascular disease, type 2 diabetes and cancers19–21. Similarly to daily CR (on a long-term basis), intermittent CR can extend lifespan and protect multiple organ systems against disease in rodents22–24. However, despite considerable evidence that intermittent CR is beneficial in rodent disease models, the potential application of intermittent CR to human diseases is largely untested25. In light of the poor adherence of subjects to continuous CR diets and adverse consequences associated with gastric bypass surgery and pharmacological interventions26, we designed a pilot study aimed at determining the feasibility and efficacy of an intermittent CR diet in treating overweight patients with moderate asthma.
METHODS
Subjects
This study was approved by an independent Review Board (Cresent City IRB) and analyses of serum samples was approved by the IRB of the National Institute on Aging Intramural Research Program. Participants were recruited through newspaper advertisements in the New Orleans metropolitan area. Inclusion and exclusion criteria were assessed by telephone, an in person interview, and a physician-conducted examination. Participants meeting the following criteria were included in the study: stable body weight with BMI>30 and less than 300 pounds; prior diagnosis of stable moderate persistent asthma as defined by the “Expert Panel Report 2 (NHLBI)27; FEV1 or peak expiratory flow (PEF) >50%; daily symptoms with use of inhaled short-acting beta2-agonist and controller, medication regimen stable for at least 30 days prior to the screening visit; medical history provided by the subject or the subject’s physician did not indicate any potential risk to the subject as the result of the study. The subjects were in general good health based on assessment by the investigators, willing to follow instructions and complete study procedures as required by the protocol. All subjects had demonstrated a >12% post-bronchodilator increase in FEV1 documented in the past two years. Subjects were excluded if they had a history of smoking, were taking systemic corticosteroids within the prior six weeks, were using hypoglycemic agents or insulin at screening or if it was felt such medication might be needed during the study. The dosage of all medications, including over the counter, herbals, and dietary supplements were recorded.
Experimental Design
Ten subjects (8 females and 2 males) with inactive lifestyles and stable moderate persistent asthma with daily symptoms were enrolled in the study as a single cohort. The experimental design involved evaluation of clinical and biochemical variables in subjects at baseline and at designated time points during the course of a 2 month alternate day CR (ADCR) dietary regimen. In this longitudinal design, the baseline value for each subject served as the control value for that subject to which ADCR diet values were compared. After a 14 day pre-diet period during which baseline variables were recorded, all subjects initiated ADCR in which women were instructed to consume 320 calories and men 380 calories of a commercially available canned meal replacement shake (Atkins Advantage or Carb Solutions) provided to the subjects. On the other day subjects ate ad libitum (AL). Diary cards and instructions were given to the subjects during the 14 day baseline period. On the last day of the baseline period subjects returned their diary cards and were given new cards and instructions in how to follow the diet, including the number of calories to be consumed on each CR day. They were told to eat on the AL day whatever they normally ate and to the point of satisfaction but not to intentionally overeat. The subjects were told to continue taking the vitamins and herbal supplements they were taking prior to the study. The principal investigator and ancillary personnel met each week with all the participants for 1 hour in the evening to provide group support. Topics of discussion were limited to subjects’ reaction to the dietary pattern. Subjects were weighed on days 1, 15, 29, 57 using a calibrated balance scale. Blood draws were taken at baseline and on consecutive AL and CR days at the 2, 4 and 8 week time points
Evaluation of Asthma Symptoms and Pulmonary Function
Three different questionnaires were used. The Juniper mini-Asthma Quality of Life Questionnaire (mini-AQLQ) and the Juniper Asthma Control Questionnaire (ACQ) were completed baseline and end of study. The Asthma Symptom Utility Index (ASUI) was completed at baseline and every two weeks. The mini-AQLQ has 4 domains: symptoms, activity limitations, emotional function, and environmental stimuli. The ASUI has five domains, all of which are symptoms: cough, wheeze, dyspnea, sleeplessness, and medication side effects. The ACQ has six domains and spirometry. It measures degree of control of the disease, mainly with questions related to symptoms. Thus, the mini-AQLQ measures perceived QOL improvement and emotional response, whereas the ASUI and ACQ measure primarily symptoms. Scores for the mini-AQLQ and ACQ were analyzed using the package provided by Dr. Juniper. The ASUI was scored according to published methods28. Participants were trained in the use of the peak flow meter (mini-Wright by Ferraris). The best of three Peak Flow (PEF) measurements were recorded on awakening, during a 14 day baseline period and daily during the 58 day study period. Spirometry before and after albuterol was performed during baseline and at 8 weeks by a certified respiratory therapist using the Schilling spirometer (Model: Type SP-1) under the supervision of the pulmonologist. The best of three attempts was recorded before and after albuterol during baseline and at the end of the study.
Assessments of Hunger and Mood
A hunger/ mood/energy scale was created for this study because of anecdotal reporting by previous patients of improved mood and energy levels when on a similar diet, and the lack of a mood/energy level measure in existing asthma or psychological questionnaires. Subjects recorded the level of hunger and mood/energy for each two hour segment daily during baseline and throughout the study. The hunger scale ranged from 1 to 10 with 1 being “not at all hungry, the thought of food is distasteful” and 10 being “extremely hungry, never been hungrier”. The mood/energy scale ranged from 1 to 10 with 1 being “lowest energy level ever” and 10 being “highest energy level ever”
Analyses of Serum Samples
Fasting blood samples were drawn after an overnight fast on consecutive AL and CR days (days 1, 2, 15, 16, 29, 30, 57 and 58). Samples taken on consecutive CR and AL days were analyzed in order to determine whether the variables being measured changed daily in response to the ADCR regimen. Most variables changed progressively with increasing time on the ADCR diet, but did not change acutely between consecutive AL and CR days. Serum lipids, insulin, glucose and C-reactive protein were measured in the clinical laboratory (Qwest Diagnostics, New Orleans, LA) using standard methods in samples drawn on days 1, 15, 29 and 57 (only days 1 and 57 were used for statistical analysis). Serum TNFα, BDNF, protein carbonyls, nitrotyrosine, 8-isoprostane, 4-hydroxynonenal adducts and ceramides were measured in samples drawn on days 1, 2, 15, 16, 29, 30, 57 and 58. TNFα levels were measured using a commercially available ultra-sensitive ELISA kit (Biosource Int., Camarillo, CA). Serum BDNF concentrations were measured using a commercially available ELISA kit (Promega, San Luis Obispo, CA). Levels of protein carbonyls, nitrotyrosine and 8-isoprostane were quantified using methods described previously29, 30. Levels of lysine and histidine adducts of 4-hydroxynonenal, and long-chain ceramides were measured by tandem mass spectrometry methods described previously31. Serum leptin and ghrelin concentrations were quantified using ELISA kits from Linco Research Inc. (St. Charles, MO) and Phoenix Pharmaceuticals (Belmont, CA), respectively. Concentrations of total ketone bodies (acetoacetate and 3-hydroxybutyrate) were measured using a Total Ketone Bodies kit (catalog no. 415-73301 & 411-73401) from Wako Diagnostics USA, Richmond VA, on a Roche Cobas Fara II robotic chemical analyzer according to the manufacturers specifications. The Total Ketone Body calibrator set (catalog no. 412-73791) was used to produce the standard curve and the Total Ketone Body control (catalog no. 418-73891) was used to insure accuracy between assay runs. Uric acid was measured using a Uric Acid kit (catalog no. 237-60) from Diagnostic Chemicals Limited, Oxford, CT, on a Roche Cobas Fara II robotic chemical analyzer according to the manufacturer's specifications.
Statistical Analyses
For those measurements that were normally distributed, paired t-tests and Pearson's correlation coefficients were used for the analyses. Statistical comparisons of variables in serum samples during the course of the study with the baseline values were made using ANOVA and either the Student-Newman-Keuls or Bonferroni post-hoc tests. For non-normal measurements, Wilcoxon signed rank sum test and Spearman's correlation coefficients were used. Two-sided tests were used for all the comparisons and a p value of 0.05 or less was considered statistically significant and a p value of 0.01 or less was considered highly statistically significant. All the analyses were done using SAS version 9.1.
RESULTS
Alternate Day Calorie Restriction Improves Asthma Symptoms and Pulmonary Function
Of 40 responders to the newspaper advertisement, 23 met inclusion and exclusion criteria and fourteen agreed to enroll in the study. Of these, one died of unknown causes during the baseline, one dropped out due to a change in vacation plans during baseline, one decided not to continue during the first study week, and one dropped out the second study week due to work-related travel. Of the remaining 10, nine completed the study; one subject did not complete the study because she volunteered that she was non-compliant with the CR regimen. Subjects lost an average of 8%(8.5 kg.) of their body weight during the course of the study, confirming their adherence to the ADCR regimen (Fig. 1a). The perceived mood and energy of the subjects increased progressively during the first 3 weeks of the ADCR diet and remained significantly elevated for the duration of the study (Fig. 1b). Analysis of the hunger rating scale indicated that the subject's perceived hunger did not increase significantly over baseline values during the course of the study (Fig. 1c). There was a significantly higher level of hunger on CR days compared to the ad libitum days throughout the study. PEF increased by a highly significantly amount from a baseline level of 335 L/min to a level of 382 L/min during the first three weeks of the ADCR period, and remained elevated throughout the 8 week study period (Fig. 1d) (p< 0.009 at 8 weeks). There were no significant differences between FEV1 (forced expiratory flow in 1 sec) values at baseline and at 8 weeks (Table 1). However, the FEV1 after albuterol administration was significantly greater at 8 weeks compared to baseline (Table 1), suggesting that the ADCR diet resulted in improved bronchial responsiveness.
Figure 1.

Asthma subjects lose weight and exhibit improved mood and peak airflow when maintained on and alternate day calorie restriction diet. Body weights (a), mood/energy scores (b), hunger scores (c) and peak expiratory flow (d) were measured at baseline and at the indicated time points during the 2 month ADCR period.
Table 1.
Results of analyses of pulmonary variables
| Variable | Baseline | After 8 weeks | Change | P value |
|---|---|---|---|---|
|
| ||||
| Peak Flow (L/min) | 334.7 ± 26.0 | 379.3 ± 27.9 | 14.4 ± 4.1 (%) | 0.0081 |
| FEV1 (predicted) | 67.4 ± 5.7 | 69.8 ± 5.3 | 5.3 ± 3.7 (%) | 0.2152 |
| FEV1 (predicted after Albuterol) | 71.9 ± 4.8 | 77.5 ± 4.0 | 10.5 ± 5.1 (%) | 0.0156 |
| FEV1 (Albuterol) – FEV1 | 4.4 ± 1.6 | 7.8 ± 1.7 | 3.5 ± 1.3 | 0.0425 |
| Mini-AQLQ | 3.4 ± 0.3 | 5.6 ± 0.3 | 2.1 ± 0.5 | 0.0039 |
| ACQ | 2.4 ± 0.3 | 1.0 ± 0.1 | −1.3 ± 0.7 | 0.0015 |
| ASUI | 0.66 ± 0.20 | 0.91 ± 0.10 | 0.25 ± 0.17 | 0.0022 |
There was also a highly significant improvement in the ASUI scores ( 0.25+-0.17 ( p<0,002) Table1;Fig 2a) which occured within 2 weeks and was maintained throughout the 8 week ADCR diet. The mini-AQLQ scores of the subjects were significantly higher in all four domains (asthma symptoms, activity limitations, emotional function and environmental stimuli) at the end of the study compared to baseline, demonstrating a beneficial effect of the ADCR diet on weight related or on asthma quality of life (Fig. 2b). The overall change in the mini-AQLQ was 2.1 ± 1.4 units (p<0.004) or 61%. Similarly, there were significant positive effects of the ADCR diet on the ACQ score which changed −1.3 ± 0.7 (p<0.0015) or 54%
Figure 2.

Alternate day calorie restriction results in improved symptoms in subjects with asthma. a. MiniAQLQ scores for four domains (symptoms, activity limitations, emotional function and environmental stimuli) in subjects at baseline and after 8 weeks of ADCR. The differences between the 8 week and baseline values were significantly different for each of the four domains (p<0.004). b. ASUI scores increased rapidly and significantly (p<0.003) within 2 weeks of diet initiation.
Effects of ADCR on Markers of Lipid and Energy Metabolism in Asthma Patients
Body weight reduction in obese subjects is often associated with decreases in risk factors for cardiovascular disease and diabetes. We therefore measured concentrations of lipids (total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides), C-reactive protein (CRP), glucose and insulin in serum samples taken at baseline and after 8 weeks on the ADCR diet. Levels of total cholesterol and triglycerides were significantly lower at 8 weeks compared to baseline, while levels of HDL cholesterol were significantly increased at 8 weeks (Fig. 3a; Table 2). The ADCR diet had no significant effect on serum levels of LDL, glucose, insulin or CRP (Table 2).
Figure 3.
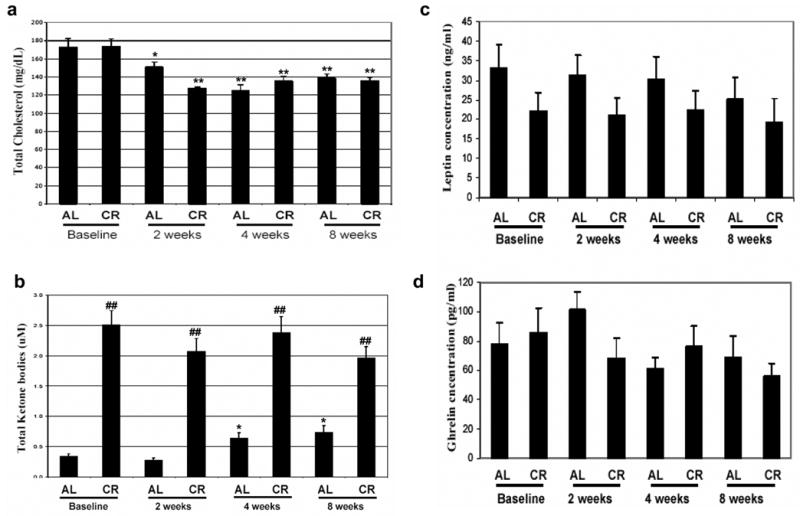
Alternate day calorie restriction results in changes in lipid and energy metabolism indicative of improved health in asthma subjects. Levels of total cholesterol (a), 3-hydroxybutyrate (b), leptin (c) and ghrelin (d) were measured in serum samples from asthma subjects on successive ad libitum (AL) and CR days at baseline and at 2, 4 and 8 weeks of ADCR. *p<0.05, **p<0.01 compared to the baseline value; ##p<0.01 compared the corresponding CR value.
Table 2.
Results of analyses of non-pulmonary variables
| Variable | Baseline | After 8 weeks | Change | P value |
|---|---|---|---|---|
|
| ||||
| Weight (kg) | 104.9 ± 6.2 | 96.4 ± 5.5 | −8.5 ± 1.7 | 0.0011 |
| Weight (%) | −8.0 ± 1.4 (%) | 0.0009 | ||
| Total Cholesterol | 204.1 ± 7.9 | 183.6 ± 7.1 | −9.3 ± 4.0 (%) | 0.0480 |
| Triglyceride | 279.3 ± 105.4 | 161.0 ± 40.5 | −118.3 ± 66.8 | 0.0391 |
| HDL | 44.0 ± 5.6 | 48.1 ± 5.9 | 4.1 ± 1.3 | 0.0111 |
| LDL | 116.8 ± 9.5 | 103.4 ± 11.4 | −10.5 ± 8.9 | 0.4295 |
| Trig/HDL | 9.3 ± 4.3 | 4.6 ± 2.0 | −4.6 ± 2.4 | 0.0273 |
| HDLC | 4.9 ± 0.6 | 4.3 ± 0.5 | −0.9 ± 0.3 | 0.0202 |
| Glucose | 75.3 ± 6.9 | 80.4 ± 3.6 | 5.1 ± 4.3 | 0.2679 |
| CRP | 4.6 ± 0.8 | 5.6 ± 1.1 | 1.0 ± 0.9 | 0.2777 |
| Insulin | 23.7 ± 12.4 | 14.9 ± 3.3 | −8.8 ± 9.9 | 0.6797 |
The body weight of subjects on the ADCR diet decreased progressively suggesting that they were compliant with the diet throughout the study. To confirm compliance and to provide insight into the effects of the ADCR diet on energy metabolism we measured concentrations of ketone bodies (acetoacetate and 3-hydroxybutyrate) in serum samples taken on consecutive ad libitum and CR days at baseline and at 2, 4 and 8 weeks. Levels of ketone bodies reliably increase during extended periods of fasting or caloric restriction32. We found that levels of ketone bodies were elevated 4-6 fold on CR days compared to ad libitum days, consistent with adherence of the subjects to the diet (Fig. 3b). There was a significant increase in levels of 3-hydroxybutyrate on ad libitum days at 4 and 8 weeks of the ADCR regimen compared to baseline levels (Fig. 3b). Levels of circulating leptin increase in the fed state and suppress appetite, whereas ghrelin levels increase during fasting and increase appetite33. We found that leptin levels were lower on CR days compared to AL days throughout the study, and there was a progressive decrease in leptin levels on AL days during the 8 week diet period (Fig. 3c). In the case of ghrelin there was a transient increase in levels on the AL day at the 2 week diet point, but ghrelin levels were not significantly affected by diet on either AL or CR days at the 4 and 8 week time points (Fig. 3d). There were no significant differences in ghrelin levels on AL compared to CR days at baseline, 4 and 8 weeks.
ADCR Reduces Markers of Inflammation and Oxidative Stress in Asthma Patients
The concentration of TNFα in serum was unchanged after 2 weeks on the ADCR diet. However, there was a highly significant reduction in serum TNFα levels in the CR day sample at 4 weeks, and in both the ad libitum and CR samples at 8 weeks (Fig. 4a). There was a significant decrease in circulating BDNF levels that occurred within the first 2 weeks of the dietary intervention, decreased further at 4 weeks and remained low at 8 weeks (Fig. 4b). Ceramides are liberated from membrane sphingomyelin in response to inflammatory cytokine receptor activation and oxidative stress and levels of ceramides are elevated in affected tissues and body fluids in several inflammatory and infectious diseases34–36. Levels of ceramides C16:0, C18:0, C22:0 and C24:1 were significantly decreased on both ad libitum and CR days within 2 weeks of ADCR diet initiation and remained at the lower levels for the duration of the 8 week period (Fig. 4c). These reductions in levels of circulating TNFα, BDNF and ceramides in response to the ADCR diet, suggests that this dietary intervention reduces inflammation in asthma patients
Figure 4.

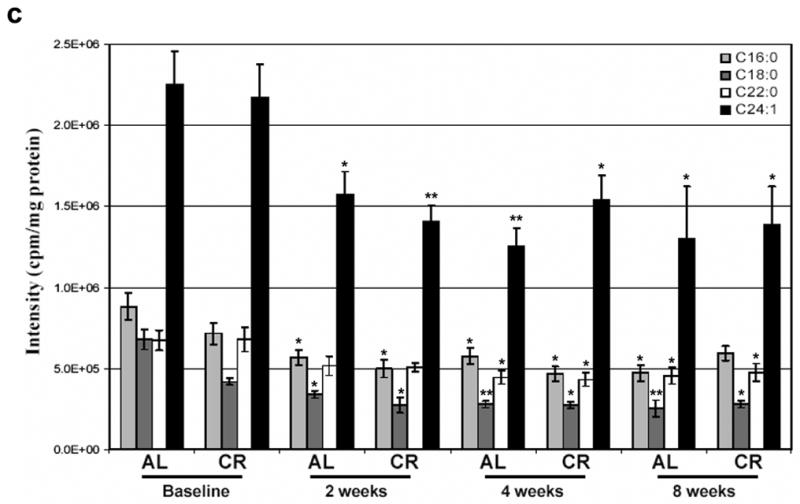
Markers of inflammation are reduced in asthma subjects in response to the ADCR diet. Levels of TNF-α (a), BDNF (b) and ceramides (c) were measured in serum samples from asthma subjects on successive ad libitum (AL) and CR days at baseline and at 2, 4 and 8 weeks of ADCR. *p<0.05, **p<0.01, ***p<0.001 compared to the baseline value.
Levels of protein carbonyls, a measure of protein oxidation, decreased significantly on both ad libitum and CR days within 2 weeks of diet initiation, continued to decrease through 4 weeks and remained low through 8 weeks (Fig. 5a). Progressive and highly significant decreases in serum levels of nitrotyrosine and 8-isoprostane also occurred during the course of the 8 week ADCR diet period (Fig. 5b, c). Levels of histidine and lysine 4-hydroxynonenal adducts were progressively and significantly decreased during the course of the 8 week ADCR diet period; levels of these adducts were decreased on both ad libitum and CR days (Fig. 5d). The magnitude of the decreases in each marker of oxidative stress were large; at the end of the 8 week study levels of protein carbonyls and 8-isoprostane were less than 20% of baseline levels, levels of nitrotyrosine were less than 10% of baseline values, and levels of 4-hydroxynonenal adducts decreased by approximately 50% (Fig. 5). Finally, we measured levels of uric acid, a major antioxidant scavenger of hydroxyl radical and peroxynitrite37, in serum samples from the subjects. Uric acid levels increased significantly (by approximately 20%) within 2 weeks of ADCR diet initiation and remained elevated through 8 weeks (Fig. 6), consistent with less oxidative stress.
Figure 5.

Markers of oxidative stress are reduced in asthma subjects in response to the ADCR diet. Levels of total protein carbonyls (a), nitrotyrosine (b), 8-isoprostanes (c) and lysine and histidine adducts of 4-hydroxynonenal (d) were measured in serum samples from asthma subjects on successive ad libitum (AL) and CR days at baseline and at 2, 4 and 8 weeks of ADCR. *p<0.05, **p<0.01, ***p<0.001 compared to the baseline value.
Figure 6.
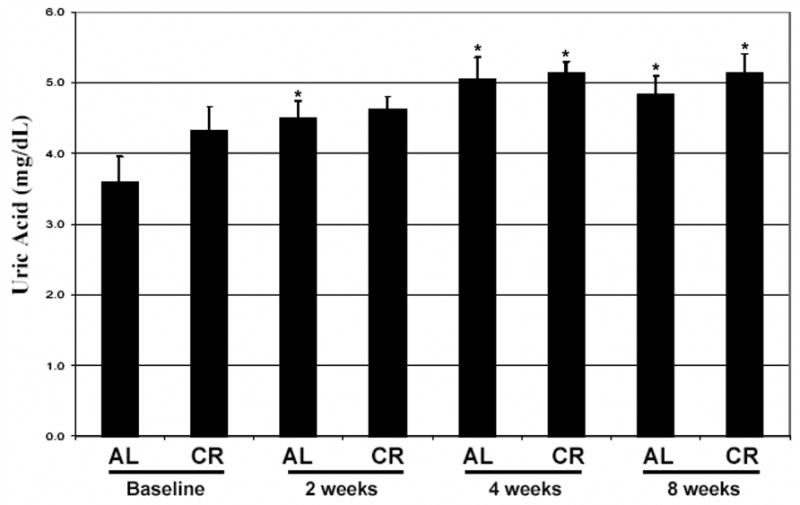
Levels of the antioxidant uric acid are increased in asthma subjects in response to the ADCR diet. Levels of uric acid were measured in serum samples from asthma subjects on successive ad libitum (AL) and CR days at baseline and at 2, 4 and 8 weeks of ADCR. *p<0.05 compared to the baseline value.
DISCUSSION
Nine of the 10 asthma subjects who began the ADCR regimen complied with the diet, as indicated by progressive weight loss, and completed the study. All 9 subjects exhibited improved asthma symptoms, control and quality of life, demonstrating a clinical benefit of the ADCR diet. An improvement of ACQ or mini AQLQ score of 0.5 is considered clinically important and has been repeatedly shown to be useful in research and management of individual asthma patients. In a recent clinical study of 1414 asthma patients newly started on either fluticosone proprionate or montelucast an improvement in ACQ and mini-AQLQ scores of >1 unit or 47% and 25% respectively was observed38. Our ADCR study recorded a 54% improvement in ACQ and 61% improvement in mini- AQLQ in patients already on baseline controller therapy. Although medical and surgical induced weight loss is also associated with similar degrees of quality of life improvement (SF-36) our patients also demonstrated improvement in asthma specific control (ACQ) and symptoms (ACUI ) score. Our study demonstrated a 0.25 improvement in ASUI within 3–4 weeks, when weight loss was only 4%. In studies using the ASUI a change of >0.25–0.3 is associated with a clinically detectable difference in asthma severity classification Although all these scoring systems could be linked simply to weight loss the rapid change associated with change in inflammatory markers are consistent with improvement in asthma burden. The improvement in PEF of 44.6 L/min+-3.8 in our study is consistent with the improvement usually observed after “optimizing” controller medications in mild/moderate asthmatics. Although major weight loss (13%) is known to result in some improvement in pulmonary function, our PEF improvement occurred within 3–4 weeks when weight loss was 4%; PEF thereafter remained constant, whereas continued through 8 weeks, suggesting that the change in pulmonary function in response to the ADCR diet was not due solely to weight loss.
The significant increase in the FEV1 after albuterol in the subjects during the ADCR diet compared to baseline suggests an effect of the ADCR diet on airway smooth muscle responsiveness, consistent with an anti-inflammatory effect. In a study of 58 obese women losing >13% of body weight over six months, there was no change in response to metacholine challenge5, suggesting that the changes were independent of airway reactivity. Although we did not evaluate methacholine responsiveness, the improved airway response to bronchodilators should not be caused by weight loss per se and is consistent with an anti-inflammatory response from the ADCR diet.
Particularly striking were the reductions in levels of TNFα, BDNF and markers of oxidative stress (protein carbonyls, nitrotyrosine and 8-isoprostane) in the serum of the asthma patients during the course of the ADCR diet period. Levels of these markers of inflammation and oxidative stress were decreased on both ad libitum and CR days, indicating a sustained effect of the ADCR diet that did not fluctuate in response to the level of energy intake on the day prior to blood sampling. The decreased levels of TNFα and BDNF suggest that ADCR suppresses inflammation, which may contribute to the beneficial effects of ADCR on asthma symptoms and hyperresponsiveness. Indeed, studies of asthma patients and animal models of asthma have provided evidence that TNFα10, 12 and BDNF16, 39 are important mediators of airway inflammation and associated symptoms. It was previously reported that levels of protein carbonyls, nitrites and nitrates, and lipid peroxidation products were increased in plasma from patients with bronchial asthma compared to control subjects40. The consistent and progressive decrease in levels of oxidative stress in our subjects may therefore be a marker of, or to have contributed to, the improvement in symptoms on the ADCR diet. The striking reduction in markers of oxidative damage which we observed have not been described in daily calorie restriction studies. Other authors have reported modest or non-significant changes in levels of protein carbonyls with various CR regimes41, 42, 43. Similarly, in a previous weight loss study nitrotyrosine levels declined 23% in the Caucasian women and remained unchanged in African American women44, suggesting that different groups of subjects exhibit differential reductions in oxidative stress in response to weight loss.
The mechanism(s) by which ADCR reduces oxidative stress and inflammation in asthmatic subjects remains to be established. However, based upon previous studies of the effects of alternate day fasting on cellular physiology in rodents, two general mechanisms are likely. First, because subjects on ADCR exhibit a reduction in overall energy intake and lose weight, there is likely a reduction in cellular oxygen free radical production24, 41, 42. The latter effect of ADCR would be associated with lower levels of oxidatively modified proteins and lipid peroxidation products in the blood. Second, ADCR may impose a mild beneficial stress, to which cells respond adaptively by up-regulating the expression of antioxidant systems. Such increased cellular stress resistance has been shown occur in rodents on an alternate day energy restriction regimen, resulting in increased disease resistance24. It will be of considerable interest to determine the effects of ADCR on gene expression in tissue involved in the pathogenesis of asthma.
We found that serum leptin levels were lower in subjects on CR compared to AL days throughout the 8 week study period, and that leptin levels on AL days decreased progressively during the 8-week study period. Leptin has been shown to exert pro-inflammatory actions45, and it is therefore possible that the reduction in leptin levels contribute to the anti-inflammatory effects of the ADCR diet. On the other hand, the ADCR diet did not significantly affect circulating levels of this hormone, a result consistent with our evidence that the ADCR does not result in a sustained overactivation of the hunger response.
Humans are unable to consistently comply with a long-term daily caloric reduction of 40% (consuming 60% of maintenance), as has been used in most animals studies to date. The authors of a recent three week trial in which 16 volunteers alternated eating ad lib for 24 hours and nothing the next 24 hours concluded that, due to persistent hunger and irritability, it was unlikely subjects would stay on the regime for extended periods of time46. We designed the ADCR pattern of eating intended as an accommodation to human needs and adaptation to human meal pattern of the alternate day total fasting pattern used in rodent studies. When rats or mice are maintained on an alternate day fasting regimen they maintain body weights 10–25% lower than ad libitum fed control animals, live up to 30% longer and exhibit improvement in a range of health indicators47. A regimen which allows ad libitum feeding on one day and reduced food/caloric intake on the next day (for longer periods of time), whereby a stable weight is maintained, may prolong lifespan and healthspan in humans48. Low levels of oxidative stress may be necessary to reach very old age; at least two studies have shown lower oxidative stress in centenarians than in 70 year olds49, 50.
In our study, the ADCR pattern of eating consisted of repeating cycles of a (approximately) 36 hour period of very low calorie intake and a 12 hour period of AL eating was tolerable and efficacious in treating asthma symptoms, at least in obese subjects. Larger studies that include a control group or a cross-over design with measures of airway reactivity and inflammation will be required to further elucidate the full impact of ADCR diets on obese asthma patients. Further studies to improve asthma outcome are desirable since current therapies do not seem to modify the underlying process or factors that determine disease progression. It will also be important to determine if such diets benefit patients with other disorders that involve inflammation and oxidative stress such as atherosclerotic heart disease51.
Acknowledgments
This research was supported, in part, by the National Institute on Aging Intramural Research Program, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 3.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2005;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Stenius-Aamiala B, Poussa T, Kvarnstrom J, Gronlund EL, Yikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomized controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 6.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 8.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 9.Reynaert NL, Ckless K, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide and redox signaling in allergic airway inflammation. Antioxid Redox Signal. 2005;7:129–143. doi: 10.1089/ars.2005.7.129. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Cytokine-directed therapies for the treatment of chronic airway diseases. Cytokine Growth Factor Rev. 2003;14:511–522. doi: 10.1016/s1359-6101(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 11.Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24:271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]
- 12.Halasz A, Cserhati E, Magyar R, Kovacs M, Cseh K. Role of TNF-alpha and its 55 and 75 kDa receptors in bronchial hyperreactivity. Respir Med. 2002;96:262–267. doi: 10.1053/rmed.2001.1256. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama A, Kohno N, Fujino S, et al. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995;151:1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Bansal S, Nagpal RK. Lipid peroxidation in bronchial asthma. Indian J Pediatr. 2003;70:715–717. doi: 10.1007/BF02724313. [DOI] [PubMed] [Google Scholar]
- 15.Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158:2002–2005. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- 16.Noga O, Hanf G, Schaper C, O’Connor A, Kunkel G. The influence of inhalative corticosteroids on circulating nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 in allergic asthmatics. Clin Exp Allergy. 2001;31:1906–1912. doi: 10.1046/j.1365-2222.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- 17.Hvizdos KM, Jarvis B. Budesonide inhalation suspension: a review of its use in infants, children and adults with inflammatory respiratory disorders. Drugs. 2000;60:1141–1178. doi: 10.2165/00003495-200060050-00010. [DOI] [PubMed] [Google Scholar]
- 18.Rossi GA, Cerasoli F, Cazzola M. Safety of inhaled corticosteroids: Room for improvement. Pulm Pharmacol Ther. 2005 Dec 13; doi: 10.1016/j.pupt.2005.10.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 20.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 21.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodrick CL, Ingram DK, Reynols MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- 23.Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP. The need for controlled studies of the effects of meal frequency on health. Lancet. 2005;365:1978–1980. doi: 10.1016/S0140-6736(05)66667-6. [DOI] [PubMed] [Google Scholar]
- 26.Scheen AJ. Results of obesity treatment. Ann Endocrinol (Paris) 2002;63:163–170. [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program (NAEPP) Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 1997. publication No. 97–4051. [Google Scholar]
- 28.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Hyun D, Jenner P, Halliwell B. Effect of overexpression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative damage and antioxidant defences: relevance to Down's syndrome and familial amyotrophic lateral sclerosis. J Neurochem. 2001;76:957–965. doi: 10.1046/j.1471-4159.2001.00107.x. [DOI] [PubMed] [Google Scholar]
- 30.Hyun DH, Gray DA, Halliwell B, Jenner P. Interference with ubiquitination causes oxidative damage and increased protein nitration: implications for neurodegenerative diseases. J Neurochem. 2004;90:422–430. doi: 10.1111/j.1471-4159.2004.02493.x. [DOI] [PubMed] [Google Scholar]
- 31.Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopp RH, Magee MS, Raisys V, Benedetti T, Bonet B. Hypocaloric diets and ketogenesis in the management of obese gestational diabetic women. J Am Coll Nutr. 1991;10:649–667. doi: 10.1080/07315724.1991.10718184. [DOI] [PubMed] [Google Scholar]
- 33.Sharma V, McNeill JH. The emerging roles of leptin and ghrelin in cardiovascular physiology and pathophysiology. Curr Vasc Pharmacol. 2005;3:169–180. doi: 10.2174/1570161053586868. [DOI] [PubMed] [Google Scholar]
- 34.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 35.Pettus BJ, Chalfant CE, Hannun YA. Sphingolipids in inflammation: roles and implications. Curr Mol Med. 2004;4:405–418. doi: 10.2174/1566524043360573. [DOI] [PubMed] [Google Scholar]
- 36.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor RD, Gilmore AS, Manjunath R, Stanford RH, Legorreta AP, Jhingran PM. Comparing outcomes in patients with persistent asthma: a registry of two therapeutic alternatives. Curr Med Res Opin. 2006;22:453–461. doi: 10.1185/030079906X89793. [DOI] [PubMed] [Google Scholar]
- 39.Lommatzsch M, Schloetcke K, Klotz J, et al. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am J Respir Crit Care Med. 2005;171:115–120. doi: 10.1164/rccm.200406-758OC. [DOI] [PubMed] [Google Scholar]
- 40.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 41.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 43.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000;14:1825–36. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- 44.Fenster CP, Darley-Usmar VM, Landar AL, et al. Weight loss and race modulate nitric oxide metabolism in overweight women. Free Radic Biol Med. 2004;37:695–702. doi: 10.1016/j.freeradbiomed.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Matarese G, Moshchos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 46.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 47.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 48.Johnson JB, Laub DR, John S. The effect on health of alternate day calorie restriction: Eating less and more than needed on alternate days prolongs life. Med Hypotheses. 2006;67(2):209–11. doi: 10.1016/j.mehy.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Wilcox BJ, Wilcox CD. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10:165–71. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 50.Paolisso G, Tagliamonte MR, Rizzo MR, Manzella D, Gambardella A, Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. J Am Geriatr Soc. 1998;46:833–8. doi: 10.1111/j.1532-5415.1998.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 51.Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]


