Abstract
Background
The initial tension applied to an ACL graft at the time of fixation modulates knee motion and the tibiofemoral compressive loads.
Purpose
To establish the relationships between initial graft tension, tibiofemoral compressive force, and the neutral tibiofemoral position in the cadaver knee.
Study Design
Controlled Laboratory Study.
Methods
The tibiofemoral compressive forces and joint positions were determined in the ACL-intact knee at 0°, 20° and 90° knee flexion. The ACL was excised and reconstructed with a patellar tendon graft using graft tensions of 1, 15, 30, 60 and 90 N applied at 0°, 20° and 90° knee flexion. The compressive forces and neutral positions were compared between initial tension conditions and the ACL-intact knee.
Results
Increasing initial graft tension increased the tibiofemoral compressive forces. The forces in the medial compartment were 1.8 times those in the lateral compartment. The compressive forces were dependent on the knee angle at which the tension was applied. The greatest compressive forces occurred when the graft was tensioned with the knee in extension. An increase in initial graft tension caused the tibia to rotate externally compared to the ACL-intact knee. Increases in initial graft tension also caused a significant posterior translation of the tibia relative to the femur.
Conclusions
Different initial graft tension protocols produced predictable changes in the tibiofemoral compressive forces and joint positions.
Clinical Relevance
The tibiofemoral compressive force and neutral joint position were best replicated with a low graft tension (1–15 N) when using a patellar tendon graft.
Keywords: ACL, ligament, reconstruction, tension, biomechanics
INTRODUCTION
Clinical studies suggest that 11 to 100% of patients undergoing ACL reconstruction surgery will develop osteoarthritis (OA) in the reconstructed knee.35, 49, 51 Although the mechanisms behind post-traumatic OA in the ACL injured and reconstructed patient remain unknown, it has been hypothesized that altered joint contact mechanics may be responsible.8, 9, 24, 57 It has been shown that normal knee kinematics are not restored following reconstruction surgery,57 and this in turn may alter the tibiofemoral contact mechanics which places the patient at risk to early OA. 9, 24 Several parameters have been shown to affect knee joint biomechanics following ACL reconstruction, including the tension applied to the graft at the time of fixation (“initial graft tension”). The optimal graft tensioning protocol remains controversial.2, 3, 19
Current clinical protocols for graft tensioning vary. In general, they are based on applying an initial graft tension to restore normal anterior-posterior laxity to the knee.2 It is generally agreed that the initial graft tension should be applied with the knee close to full extension to control the excessive rises in graft tension that occur with full extension of the knee after fixation. 1, 2 A surgical textbook recommends that the theoretical tension should be “sufficient to obliterate the instability (Lachman test)” while avoiding over-tensioning.19 Another approach is to over-tension the graft at the time of fixation to compensate for the changes in knee laxity that may occur during healing.2 A graft tension protocol that restores joint contact and optimizes graft healing may be important to the long-term outcome.
There are two surgical parameters under direct control of the surgeon that will influence knee kinematics, graft loading, and the tibiofemoral compressive forces; 1) the magnitude of the tension applied to the graft just prior to fixation, and 2) the knee flexion angle at which the tension is applied.1, 16, 18, 23, 27, 32, 45, 54
Cadaver studies have been performed to determine which initial graft tension condition best restores normal knee joint laxity.1, 16, 18, 21, 23, 36, 45, 54, 64, 69 Although many of these studies are difficult to compare due to differences in anterior-posterior laxity measurement protocols and variations in intra-operative parameters (e.g. graft type, intra-articular placement) these studies have generally found that initial graft tensions between 0 and 60 N performed between full extension and 30° flexion best matched the anterior-posterior laxity measurements of the ACL-intact knee. In contrast, another study found no significant effect on laxity when comparing an initial graft tension of 22 N versus 68 N, although the angle at initial tension application did affect anterior-posterior laxity (0° versus 30° flexion).27 The focus on anterior-posterior laxity is motivated by the fact that a primary role of the ACL is to oppose anterior translation of the tibia,17 althouth the ACL is also a restraint to axial rotation.25, 44 More recent cadaver studies evaluating the relationship between initial graft tension and axial rotation have determined that the ACL stabilizes the knee against internal-external rotational torques when the knee is positioned at low angles after the tension is applied, 4 that increases in initial graft tension cause external rotation of the tibia,52 and that tibial rotation in the ACL-reconstructed knee may differ from both ACL-deficient and intact knees at low flexion angles under muscle loads.56, 66
It has been demonstrated that increasing initial graft tension increases tensile load in the graft at other angles of flexion.1, 52 The general pattern during active or passive extension is that graft tensile load remains low while the knee is in flexion but rises sharply when the knee is moved into full extension in both the cadaver and living knee.1, 13, 46, 52 This has led in part to the rationale for applying initial graft tension while the leg is close to full extension so that the surgeon can control the maximal graft tensile load.2 Excessive graft tensile loads are of clinical significance not only due to altered joint motion but also because of undesirable graft healing, which has been shown by histological examination in an in vivo dog study comparing 1 N versus 39 N initial graft tension.67
The hallmark of osteoarthritis is loss of articular cartilage. It has been hypothesized that a shift in the contact location or an alteration in the contact stress may be responsible for producing local degenerative changes in articular cartilage.9, 15 Clinical studies suggest that abnormal motion often precedes degenerative changes at the knee, and knees with increased general joint laxity or ACL deficiency have been associated with increased incidence of osteoarthritis.9
While there has understandably been a focus on restoring normal kinematics under anterior-posterior loads with cadaver ACL-reconstruction, the effects of initial graft tension on the joint contact mechanics in the cadaver knee without muscle loading have not been characterized. This is an important relationship because initial graft tension and the knee angle at which the tension is applied will affect the joint contact mechanics (magnitude and location of the joint contact areas) and could potentially lead to the long-term degradation of the articular cartilage.
The objective of this study was to investigate these baseline relationships and to determine which initial graft tension condition best restores normal joint contact forces and tibiofemoral position at the time of surgery. The hypotheses were: 1) tibiofemoral compressive forces increase with an increase in the initial graft tension, and these tibiofemoral forces are greater in the medial compartment as compared to the lateral compartment, 2) the tibia rotates externally and translates posterior with an increase in initial graft tension, and 3) the relationships between initial graft tension, tibiofemoral compressive load, and tibiofemoral position are affected by the knee angle at which the graft tension is applied.
MATERIALS & METHODS
Specimens
Twelve fresh frozen human cadaver knees (mean age = 59 (±4.0) years; 4 male/8 female) with no history of knee injury were stored at −20° C following harvest. The knees included all soft tissues and bone from the distal third of the femur through the proximal half of the tibia and fibula. Before testing, the knees were thawed at room temperature and dissected down to the joint capsule. The femur was potted into a PVC pipe using a quick setting epoxy resin (Smooth-Cast 300; Smooth-On, Inc., Easton, PA). A threaded steel rod was inserted into the intramedullary canal of the tibia and secured using the same compound.
Test fixture
A test fixture was designed to rigidly support the femur in the horizontal plane while permitting quasi-static motion (Figure 1). The tibia was supported by a linkage that could be locked at any desired knee flexion angle while the remaining five degrees of motion were left unconstrained. Thus, the natural motion of the tibia was permitted when the knee flexion angle was passively changed.
Figure 1.
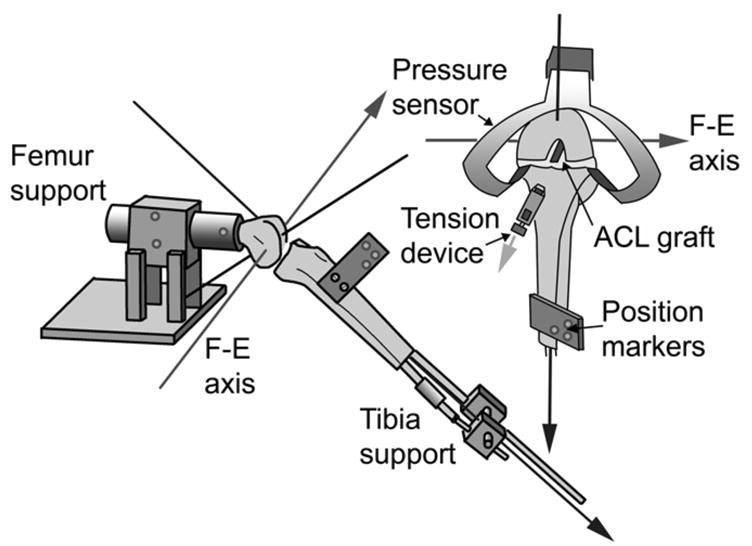
Lateral and anterior view of custom jig for quasi-static knee flexion.
Joint position measurements
The Optotrak Image Analysis System (Northern Digital; Waterloo, Ontario) was used to track the position of the tibia with respect to the femur. Infrared light-emitting diodes were attached to the tibia and femur to define the respective rigid bodies. For the tibia, three sensors were attached to a molded plastic component rigidly secured to the tibia with two screws. For the femur, two sensors were attached to the rigid femur support and one to the femur to ensure that the femur did not move relative to the support (Figure 1). The Optotrak digitizer was then used to digitize bony landmarks to establish an anatomically-based coordinate system commonly used to describe clinical motion.29 The coordinate system was defined for the femur and tibia with the knee at full extension so that the tibial and femoral origins coincided. The epicondyles were digitized and the trans-epicondylar axis running between these two points was assigned as the flexion-extension axis. The axis for internal-external tibial rotation was defined as a line normal to the flexion-extension axis that passed through a point digitized at the center of the distal tibia. The varus-valgus axis was calculated as the line normal to both other axes (Figure 1). The Optotrak Image Analysis System software was used to calculate Euler angles and translation vectors for the rigid body (i.e. tibia) relative to the fixed reference frame (i.e. femur).
Tibiofemoral pressure sensors
The tibiofemoral compressive forces in the medial and lateral compartments of the knee were measured using the K-Scan thin-film pressure sensor (4000/2594T1/1500; Tekscan, Inc., South Boston, MA). The sensor consists of two sensing matrices that could be inserted beneath the menisci, one in the medial and the other in the lateral compartment. The “I-scan” software program (Tekscan, Inc., South Boston, MA) was used to acquire sensor data. A new sensor was used for each knee.
The K-scan output was calibrated using a representative knee and a new sensor. Known compressive loads were applied to the joint by a servo hydraulic material tester (model 8521-S; Instron Corp., Canton, MA). The knee was secured in passive full extension then all ligaments were released. Next, the lateral femoral condyle was removed with a bone saw. One of the sensing matrices was then inserted anteriorly through an incision beneath the medial meniscus and held in place posteriorly with a hemostat clamped to a non-sensing tab. Five preconditioning load cycles to 180N were applied by the material tester following the recommended protocol by Tekscan. After the pre-conditioning cycles were completed, a tare load of 0 N was applied followed by static loads of 50N and 165N. The software converted the raw pressure values into engineering units by calculating the average applied pressure based on the area of loaded sensels and the total force applied to the medial femoral condyle by the material testing system (i.e. 50N and 165N). The calibration was then verified by collecting a range of compressive load measurements from 0-160 N at 20 N increments. Finally, the medial femoral condyle was removed and the lateral condyle was reattached with a screw to allow the same calibration procedure to be repeated for the lateral compartment with the unused sensing matrix.
Test protocol
Each knee was thawed to room temperature, potted, and placed in the test fixture (Figure 1). The knee was wrapped in physiologic saline soaked gauze when data were not being obtained to prevent dehydration. The total time from potting to end of the experiment was approximately 4 hours per knee. Transverse incisions were made beneath the menisci anteriorly and posteriorly through which the Tekscan sensor was inserted and held in place with a small hemostat clamped to the non-sensing edge projecting beneath the posterior meniscus. The knee was first preconditioned with 10 flexion-extension cycles and the anatomical coordinate system was established using the digitization procedure described above. Within the test fixture, quasi-static measurements of the tibiofemoral compressive forces and the three-dimensional position of the tibia relative to the femur were recorded as the ACL-intact knee was positioned at 0°, 20°, and 90° of flexion (randomized). The quasi-static approach was used to allow the pressure sensor to reach equilibrium due to the creep response inherent to the transducer.50 The pressure was multiplied by the contact area, which was also measured by the K-Scan, to get the peak force in the respective compartment.
Next, the ACL was excised and surgically reconstructed using a 10-mm wide bone-patellar tendon-bone graft. An endoscopic drill guide system (Arthrex; Naples, FL) was used to standardize anatomic graft tunnel placement. The proximal end of the graft was then fixed within the femoral bone tunnel using a 9x20 mm interference screw (Arthrex; Naples, FL). The distal end of the graft was inserted within the tibial tunnel and secured via #2 FiberWire (Arthrex; Naples, FL) to a tensioning device rigidly mounted on the anterior-medial aspect of the tibia (Figure 1). 22, 23 The knee was then pre-conditioned with 10 flexion-extension cycles, the anatomical coordinate system was reestablished, and the graft was preconditioned by slowly ramping graft tension from 1 to 100N ten times at 20° flexion. The knee was retested as the initial graft tension was sequentially tensioned to 1, 15, 30, 60, and 90 N at each knee flexion angle (randomized). When the desired load was obtained, it was allowed to settle and then the graft was re-tensioned back to the desired initial graft tension; this was repeated until the initial graft tension level stabilized to minimize stress relaxation.
Data/statistical analyses
Repeated measures analysis of variances were performed to evaluate the effects of the initial graft tension condition on the medial and lateral tibiofemoral compressive forces, on the axial rotation position of the tibia, and on the anterior-posterior position of the tibia relative to the femur. Pair-wise comparisons were made between tension levels and the ACL-intact specimen at each knee angle with Dunnett tests.
RESULTS
Tibiofemoral compressive forces
For the ACL-intact knee the mean total tibiofemoral compressive forces were 36 (SD 20) N, 5.4 (SD 3.6) N, and 17 (SD 20) N at 0°, 20° and 90° flexion, respectively. The mean ACL-intact knee medial compartment force for all angles was 18 (SD 16) N and the mean lateral compartment force was 7.5 (SD 7.5) N.
With an increase in initial graft tension there was a significant increase in the total tibiofemoral compressive force when it was applied at all knee flexion angles (p<0.001) (Figure 2). As the initial graft tension was set to 1 N, 15 N, 30 N, 60 N and 90 N, the total tibiofemoral forces increased compared to the ACL-intact knee by a mean of -8.8%, 22%, 60%, 136%, and 210%, respectively, at full extension; 11%, 150%, 340%, 800%, and 1300%, respectively, at 20° flexion; −54%, −12%, 50%, 180%, and 310%, respectively, at 90° flexion. The tibiofemoral compressive forces were also dependent on the knee flexion angle at which the graft tension was applied (p<0.001). The tibiofemoral compressive forces were greatest when the graft was tensioned at 0° of flexion (Figure 2). Medial compartment compressive forces were greater than those of the lateral compartment at all knee flexion angles (p < 0.001) and the difference between the medial and lateral tibiofemoral compressive force increased with increasing initial graft tension (Figure 3).
Figure 2.
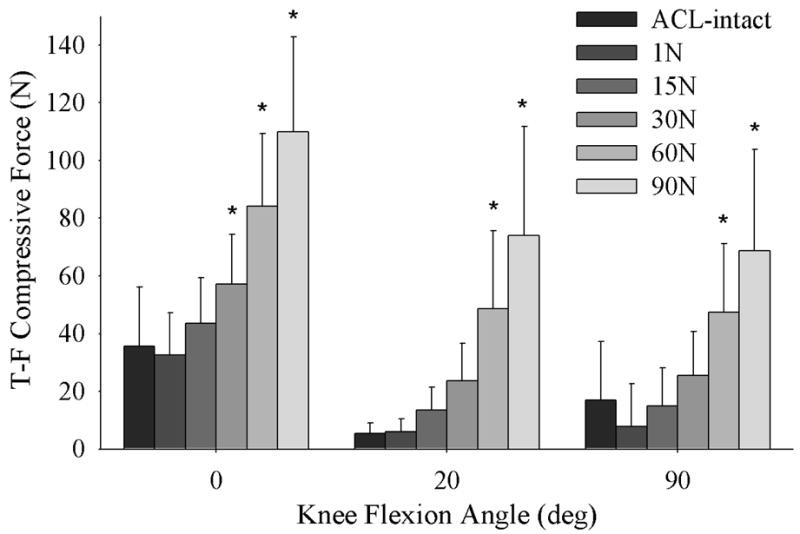
Mean total tibiofemoral (T-F) compressive force (medial + lateral) versus knee flexion angle for the intact and ACL reconstructed knee at 1, 15, 30, 60 and 90 N of initial graft tension. (* = significantly different than that of the ACL intact specimen).
Figure 3.
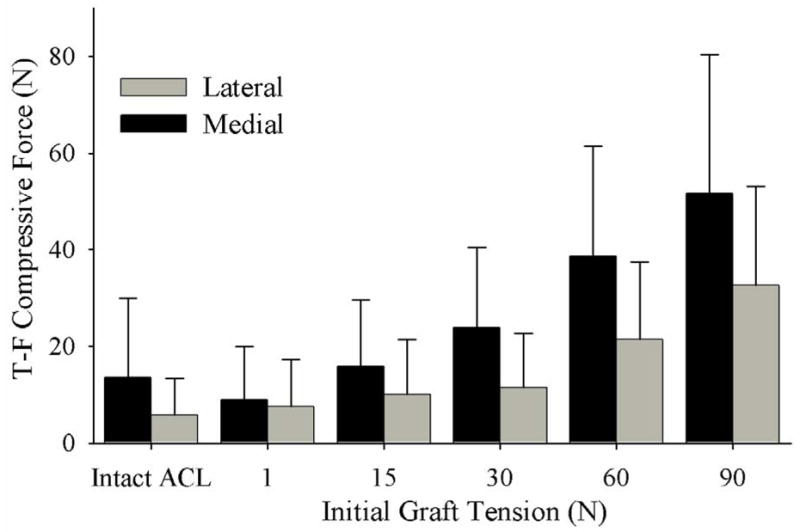
Graft tension versus compressive load (pooled by knee flexion angle) showing that the medial compressive loads are greater than those of the lateral compartment at all initial graft tension levels (p<0.001).
Neutral Tibiofemoral Position
Mean anterior translation of the tibia in the ACL-intact knee compared to full extension was 2.4 (SD 4.6) mm at 20° flexion, and 26 (SD 8.9) mm at 90° flexion. Internal-external tibial rotation in the ACL-intact knee compared to full extension was 1.0 (SD 3.0) degrees internal rotation at 20° flexion and 11 (SD 6.0) degrees internal rotation at 90° flexion.
The tibia rotated externally with an increase in initial graft tension when applied at all knee flexion angles (p=0.001) (Figure 4). With an increase in initial graft tension there was a posterior translation of the tibia relative to the femur (only significant when the graft tension was applied at 90° of flexion; p=0.001) (Figure 5).
Figure 4.
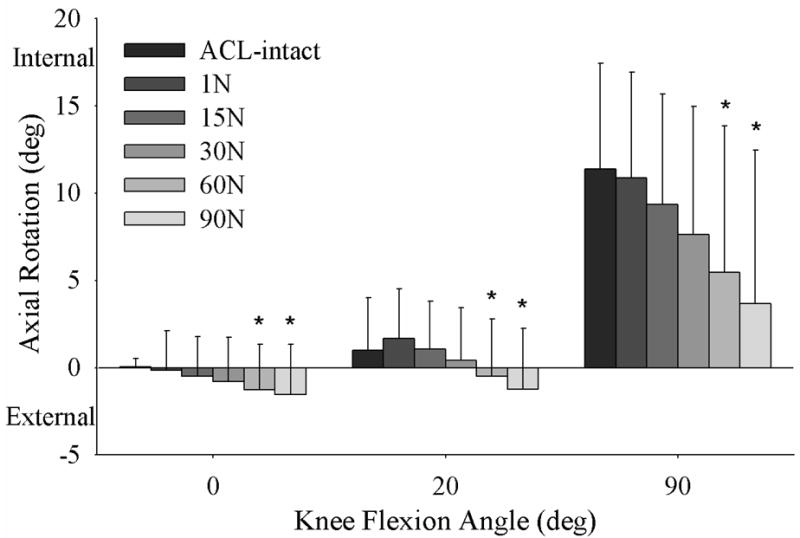
An increase in initial graft tension caused the tibia to rotate externally at all knee flexion angles.
Figure 5.
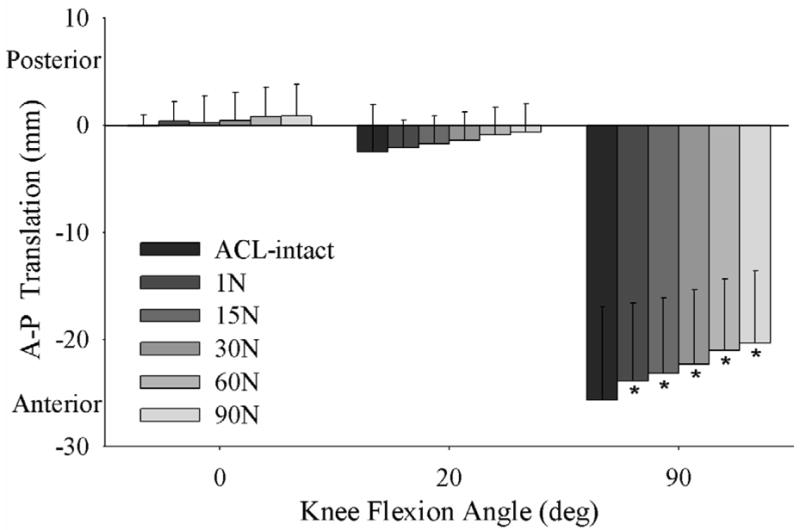
An increase in initial graft tension caused a posterior neutral shift of the tibia relative to the femur at 0°, 20° and 90° flexion.
DISCUSSION
This is the first study to directly measure the compressive forces in the medial and lateral compartments in response to different initial graft tension conditions. In the ACL reconstructed knee, the tibiofemoral compressive forces were dependent on both the initial graft tension level and the knee flexion angle at which it was applied. Increasing initial graft tension increased the total tibiofemoral compressive forces, with the medial compartment forces greater than those of the lateral compartment. Tibiofemoral compressive forces were dependent on the knee flexion angle at which the initial graft tensions were applied. These data provide baseline information quantifying the initial loading conditions of the joint during reconstruction surgery. These data will be particularly useful for ongoing studies evaluating the relationship between tibiofemoral joint compression and the onset of arthritis following ACL reconstruction surgery.
The results of this study verified the three hypotheses: 1) tibiofemoral compressive forces increased with initial graft tension and those in the medial compartment were greater than the lateral compartment, 2) the neutral joint position was affected by the initial graft tension, and 3) both the tibiofemoral compressive and neutral joint position were dependent on the knee flexion angle at which the tension was applied. We found that the tibiofemoral compressive forces and the position of the tibia relative to the femur were best replicated with a low graft tension when using a patellar tendon graft. An initial graft tension level between 1 and 15 N duplicated the total compressive force at the knee when it was applied at all of the knee flexion angles tested (Figure 2). When applying these tensions with the knee in full extension or at 20° of flexion, the sensitivity of the neutral tibiofemoral position was minimized. In this study, the highest compressive forces were generated when 90 N of tension was applied with the knee at 0° of flexion. This produced a tibiofemoral compressive force 3.1 times that of the ACL-intact knee. It is possible that such high compressive forces could place the knee at risk to OA.
In the intact ACL condition the general trends of anterior translation and internal rotation with passive knee flexion were similar to those previously described by Li et al.42 Our study used a comparable anatomical axis system (i.e. registering the flexion-extension axis of the knee with the trans-epicondylar axis). The anterior tibial translation and internal tibial rotation values at 90° of passive flexion were comparable for the ACL-intact condition in both experiments.42 Li also acquired radiographs of the knee showing posterior translation of anatomical and geometrical flexion-extension axes relative to the tibia with knee flexion, highlighting the importance of having standard joint coordinate systems when comparing studies.
A cadaver study that adjusted graft tension to restore anterior-posterior laxity found that laxity was best restored by grafts tensioned to a mean of 9 +/−14 N (range 0–56) at 20° of flexion.69 Although other ligaments are engaged during anterior-posterior laxity testing, it is a more clinically relevant measure than quasi-static position and this study supports our observation that intact kinematics are best restored with a low (1 – 15N) graft tension.
The results of the study were also consistent with the work of others. Andersen determined that a significant decrease in knee motion occurred when the graft tension was higher than 33N, resulting in lower internal-external and varus-valgus rotations with an applied 6Nm moment, and lower anterior-posterior tibial translation with 140N force compared to the uninjured knee.5 Although we only report on passive kinematics, our data exhibited the same trend of decreased anterior-posterior translation and rotation with increased graft tensions.
In another cadaver study ACL graft tensioning caused posterior translation of the tibia (relative to the femur), which pre-stressed the PCL and compressed the joint even without external forces applied.1 This joint force was not directly observed in the study but was consistent with the increased tibiofemoral force we observed with increasing graft tension (Figure 2) even without muscle loading.
Data relating to the dependence of the tibiofemoral contact point in response to an increase in initial graft tension has not been previously described in the literature. An MRI study demonstrated that internal tibial rotation of 20° occurred during passive flexion both in situ,33 and in vivo in the ACL intact knee.31 The rotations were similar to those measured for the ACL-intact knee in our study (Figure 4), although we only measured rotations to 90° of flexion. An in vivo three-dimensional kinematic study of ACL-deficient knees found that the tibia was rotated internally during the initial swing phase compared to intact-ACL,26 which is consistent with the trend we observed of decreased ACL graft tension associated with relative internal tibial rotation. Another cadaver study showed significantly less internal tibial rotation in ACL reconstructed knees than the intact knees at low flexion angles under muscle loads,66 consistent with the finding that the ACL plays a role in controlling axial rotation and the external tibial rotation with increasing graft tension we observed.4
Several randomized clinical trials have been performed to evaluate outcome in response to different initial graft tensions.47, 60, 65, 68 Yasuda et al randomized patients into three groups in which initial graft tensions of 20, 40, and 80 N were applied to double stranded hamstring grafts connected in series with polyester tape.65 A significant correlation was found between initial graft tension at the time of fixation and anterior-posterior laxity after 2-years, and the high-tension group had significantly less anterior laxity than the low-tension group (i.e. closer to normal). Similar findings were recently reported by Nicholas with patellar tendon grafts. In contrast, Van Kampen and Yoshiya compared initial graft tension levels of 20 and 40 N,60 and 25 and 50 N,68 using patellar tendon grafts. They found no significant difference in anterior-posterior laxity values between treatment groups after two years of healing. The response of articular cartilage to initial graft tension was not assessed in any of these studies, and is important because we have shown that graft tension alters the compressive load at the joint.2
Intraoperative initial graft tension is a variable that we know affects tibiofemoral compressive forces, active and passive knee kinetics, and anterior-posterior joint laxity at the time of surgery. All of these effects are thought to be potential mechanisms for the development and progression of osteoarthritis following ACL-reconstruction by changing normal knee kinematics and joint contact biomechanics. Although cadaver studies are useful in understanding and determining how best to restore normal knee functioning at the time of surgery, the best procedure for tensioning ACL grafts to reduce the incidence of osteoarthritis and other untoward clinical outcomes has not been established. While cadaver specimens allow for a tightly controlled experimental protocol and accurate data collection, the conclusions drawn from any cadaver model may be limited in clinical application. Cadaver specimens provide important information at the time of the ACL reconstruction but do not take into account changes that may occur during graft healing, such as tunnel expansion,34, 37 graft remodeling,20 histologic degeneration and decreased vascularity,67 and substantial changes in the initial set tension despite preconditioning and with flexion-extension in the cadaver.10, 48 Furthermore, assessing the contribution, if any, of modifiable biomechanical factors to initiation and progression of OA in vivo is not possible in the cadaver model. These limitations to clinical applicability can only be overcome with controlled, prospective studies in humans or suitable animal models.
Specimen age is another potential limitation of generalizing cadaver data to younger age groups. The ACL is known to weaken with age (decreased linear stiffness, ultimate energy and energy absorbed), 63 although it is not clear that aging affects knee laxity. The specimens used in this study had a mean age of 59 (±4.0) years.
Another study limitation is that the joint was passively flexed and extended without any of the effects of musculature or dynamic joint loading. While this is an experimental advantage because it allowed us to isolate the effects of the ligaments and graft, it created a situation different from what the knee would experience during normal gait in which active muscle contractions and the loads applied by bodyweight provides a substantial portion of knee joint stability.7, 53 In vivo studies have shown that the tibial position relative to the femur and anterior-posterior laxity vary with physiological loads.14, 59 Variations in gait have also been observed to affect the rate of progression to OA and affect the outcome of treatment for medial compartment OA.9 While physiological loads and gait biomechanics are important for answering the ultimate question of best surgical methodology for ACL reconstructions, this study contributes to the understanding of the baseline relationships between initial graft tension, tibiofemoral compressive load, and tibiofemoral position. Future experiments will be performed to simulate dynamic loading conditions using this technique.
Tekscan pressure sensors have been used in previous studies to measure contact forces in the knee.6, 28, 30, 41, 55, 61 The system has been previously validated,11, 30, 62 though calibration of these sensors is complex. We calibrated the sensor in a separate knee and followed the recommended protocol of the manufacturer. It is the intent of the calibration procedure to establish the output as a function of the applied load when the sensor is placed within the geometric constraints and stiffness of the contact surfaces. The calibration was performed in a separate knee and not the knees that were used in the experiment to eliminate problems with sensor fatigue and changes due to tissue dehydration that would occur with a post-experiment calibration. The calibration was then applied to all of Tekscan outputs from the 12 knees under study. The 0.1mm thickness of the pressure sensors may also affect the Tekscan readings by changing the relative knee cartilage geometry but we feel the overall effect on the comparative data analyses will be negligible. Although these concerns may raise some questions about the accuracy of the absolute compressive force values, the relative changes would be consistent. Furthermore, each knee served as its own control in the 3-way analysis of variances to reduce errors between specimens.
The menisci are generally considered to only play a part in stabilizing the ACL deficient knee but not the ACL-intact knee.12, 39, 40, 58 We were required to violate a portion of the meniscal attachments to the tibia to insert the pressure sensors. This could have increased tibial translations and rotations, though this is unlikely to be a major concern since the ACL-deficient knee was not tested and the menisci were not completely transected. Additionally, it has been shown that ACL deficiency in the cadaver knee results in insignificant changes in the anterior-posterior reference position,43 and it has been demonstrated that ACL sectioning has no significant effect on rotational stability in the unloaded cadaver knee.38 While the lack of absolute tibia and femur location in our study is not ideal, these other studies support the validity of reestablishing the coordinate system during the protocol and comparing initial joint position of the intact ACL condition to the ACL reconstructed condition. If some additional instability was imparted to the joint coordinate system it would likely increase the kinematic variation and so strengthen our statistical conclusions.
Frictional losses at the graft-tibial tunnel interface could also introduce errors because the load measured extra-articularly may be different than that induced intra-articularly. Some studies have used tibial tunnel sheaths to minimize frictional interactions between the graft and the tunnel.22 Instead we opted to make the tibial tunnel 2mm greater in diameter than the graft because we felt that this would allow ample graft movement in the tunnel while mimicking the procedure as performed in vivo during ACL reconstruction surgery.
In conclusion, we found that a low initial graft tension on the patellar tendon graft (1–15N) best simulated the tibiofemoral compressive forces and neutral position in the quasi-static, passively flexed human cadaver knee. An increase in initial graft tension increased the tibiofemoral compressive force. The compressive forces in the medial compartment were greater than those in the lateral compartment. An increase in initial graft tension also produced a posterior neutral shift of the tibia relative to the femur and external rotation of the tibia.
Acknowledgments
The authors gratefully acknowledge help of Nigel Gomez (Brown Medical School) in the design of the test fixture, and the statistical advice of Gary Badger, M.S. (Department of Biometry of the University of Vermont).
This project was supported by the National Institutes of Health (AR047910 & AR049199) and a research grant from the Arthroscopy Association of North America.
This paper was presented at the AOSSM Specialty day, Chicago Ill, March 25, 2006.
References
- 1.Amis AA. Anterior cruciate ligament replacement: Knee stability and the effects of implants. J Bone Joint Surg. 1989;71B:819–824. doi: 10.1302/0301-620X.71B5.2584254. [DOI] [PubMed] [Google Scholar]
- 2.Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning, and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6:S2–S12. doi: 10.1007/s001670050215. [DOI] [PubMed] [Google Scholar]
- 3.Andersen HN, Amis AA. Review on tension in the natural and reconstructed anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1994;2:192–202. doi: 10.1007/BF01845586. [DOI] [PubMed] [Google Scholar]
- 4.Andersen HN, Dyhre-Poulsen P. The anterior cruciate ligament does play a role in controlling axial rotation in the knee. Knee Surg Sports Traumatol Arthrosc. 1997;5:145–149. doi: 10.1007/s001670050042. [DOI] [PubMed] [Google Scholar]
- 5.Andersen HN, Jorgensen U. The immediate postoperative kinematic state after anterior cruciate ligament reconstruction with increasing peroperative tension. Knee Surg Sports Traumatol Arthrosc. 1998;6:s62–S69. doi: 10.1007/s001670050225. [DOI] [PubMed] [Google Scholar]
- 6.Anderson IA, MacDiarmid AA, Harris ML, Gillies RM, Phelps R, Walsh WR. A novel method for measuring medial compartment pressures within the knee joint in vivo. J Biomech. 2003;36:1391–1395. doi: 10.1016/s0021-9290(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 7.Andriacchi TP. Functional analysis of pre and post-knee surgery: Total knee arthroplasty and acl reconstruction. J Biomech Eng. 1993;115:575–581. doi: 10.1115/1.2895543. [DOI] [PubMed] [Google Scholar]
- 8.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after acl injury cause cartilage thinning. Clin Orthop. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 9.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;33:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 10.Arnold MP, Lie DT, Verdonschot N, de Graaf R, Amis AA, Van Kampen A. The remains of anterior cruciate ligament graft tension after cyclic knee motion. Am J Sports Med. 2005;33:536–542. doi: 10.1177/0363546504269938. [DOI] [PubMed] [Google Scholar]
- 11.Bachus KN, Demarco AL, Judd KT, Horwitz DS, Brodke DS. Measuring contact area, force, and pressure for bioengineering applications: Using fuji film and tekscan systems. Med Eng Phys. 2005;19 doi: 10.1016/j.medengphy.2005.07.022. Epub. [DOI] [PubMed] [Google Scholar]
- 12.Bargar WL, Moreland JR, Markolf KL, Shoemaker SC, Amstutz HC, Grant TT. In vivo stability testing of post meniscectomy knees. Clin Orthop. 1980;150:247–252. [PubMed] [Google Scholar]
- 13.Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: A review of previous work. J Biomech. 1998;31:519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 14.Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20:332–337. doi: 10.1016/S0736-0266(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 15.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: A longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 16.Burks RT, Leland R. Determination of graft tension before fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1988;4:260–266. doi: 10.1016/s0749-8063(88)80041-0. [DOI] [PubMed] [Google Scholar]
- 17.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. J Bone Joint Surg. 1980;62A:259–270. [PubMed] [Google Scholar]
- 18.Bylski-Austrow DI, Grood ES, Hefzy MS, Holden JP, Butler DL. Anterior cruciate ligament replacements: A mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522–531. doi: 10.1002/jor.1100080408. [DOI] [PubMed] [Google Scholar]
- 19.Canale: Campbell's Operative Orthopaedics. New York: Mosby, Inc; 2003 . pp. 2264–2265. [Google Scholar]
- 20.Deehan DJ, Cawston TE. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg. 2005;87B:889–895. doi: 10.1302/0301-620X.87B7.16038. [DOI] [PubMed] [Google Scholar]
- 21.Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: A study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22:613–624. doi: 10.1016/j.orthres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Fleming BC, Abate JA, Peura GD, Beynnon BD. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 23.Fleming BC, Beynnon BD, McLeod WD, Howe JG, Pope MH. Effect of tension and placement of a prosthetic anterior cruciate ligament on the anteroposterior laxity of the knee. J Orthop Res. 1992;10:177–186. doi: 10.1002/jor.1100100204. [DOI] [PubMed] [Google Scholar]
- 24.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction, and osteoarthritis. Current Opinion Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabriel MT, Wong EK, Woo SL-Y, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85–89. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 26.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 27.Gertel TH, Lew WD, Lewis JL, Stewart NJ, Hunter RE. Effect of anterior cruciate ligament graft tensioning direction, magnitude, and flexion angle on knee biomechanics. Am J Sports Med. 1993;21:572–579. doi: 10.1177/036354659302100415. [DOI] [PubMed] [Google Scholar]
- 28.Gill TJ, DeFrate LE, Wang C, Carey CT, Zayontz S, Zarins B, Li G. The effect of posterior cruciate ligament reconstruction on patellofemoral contact pressures in the knee joint under simulated muscle loads. Am J Sports Med. 2004;32:109–115. doi: 10.1177/0095399703258794. [DOI] [PubMed] [Google Scholar]
- 29.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three dimensional motions: Application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 30.Harris ML, Morberg P, Bruce WJM, Walsh WR. An improved method for measuring tibiofemoral contact areas in total knee arthroplasty: A comparison of k-scan sensor and fuji film. J Biomech. 1999;32:951–958. doi: 10.1016/s0021-9290(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 31.Hill PF, Vedi V, Williams A, Iwaki H, Pinskerova V, Freeman MAR. Tibiofemoral movement 2: The loaded and unloaded living knee studied by mri. J Bone Joint Surg. 2000;82B:1196–1198. doi: 10.1302/0301-620x.82b8.10716. [DOI] [PubMed] [Google Scholar]
- 32.Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:567–574. doi: 10.1177/03635465010290050801. [DOI] [PubMed] [Google Scholar]
- 33.Iwaki H, Pinskerova V, Freeman MAR. Tibiofemoral movement 1: The shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg. 2000;82B:1189–1195. doi: 10.1302/0301-620x.82b8.10717. [DOI] [PubMed] [Google Scholar]
- 34.Jagodzinski M, Foerstemann T, Mall G, Krettek C, Bosch U, Paessler HH. Analysis if forces of acl reconstructions at the tunnel entrance: Is tunnel enlargement a biomechanical problem. J Biomech. 2005;38:23–31. doi: 10.1016/j.jbiomech.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Johma NM, Borton DC, Clingeleffer AJ, Pinczewski LA. Long term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop. 1999;358:188–193. [PubMed] [Google Scholar]
- 36.Karchin A, Hull ML, Howell SM. Initial tension and anterior load-displacement behavior of high-stiffness anterior cruciate ligament graft constructs. J Bone Joint Surg. 2004;86A:1675–1683. doi: 10.2106/00004623-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 37.L'Insalata JC, Klatt B, Fu FH, Harner CD. Tunnel expansion following anterior cruciate ligament reconstruction: A comparison of hamstring and patellar tendon autografts. Arthroscopy. 1997;5:234–238. doi: 10.1007/s001670050056. [DOI] [PubMed] [Google Scholar]
- 38.Lane JG, Irby SE, Kaufman K, Rangger C, Daniel DM. The anterior cruciate ligament in controlling axial rotation. An evaluation of its effect. Am J Sports Med. 1994;22:289–293. doi: 10.1177/036354659402200222. [DOI] [PubMed] [Google Scholar]
- 39.Levy IM, Torzilli PA, Gould JD. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg. 1989;71A:401–406. [PubMed] [Google Scholar]
- 40.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg. 1982A;64:883–888. [PubMed] [Google Scholar]
- 41.Li G, DeFrate LE, Zayontz S, Park SE, Gill TJ. The effect of tibiofemoral joint kinematics on patellofemoral contact pressures under simulated muscle loads. J Orthop Res. 2004;22:801–806. doi: 10.1016/j.orthres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Li G, Zayontz S, DeFrate LE, Most E, Suggs JF, Rubash HE. Kinematics of the knee at high flexion angles: An in vitro investigation. J Orthop Res. 2004;22:90–95. doi: 10.1016/S0736-0266(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 43.Ma CB, Janaushek MA, Vogrin TM, Rudy TW, Harner CD, Woo SL-Y. Significance of changes in the reference position for measurements of tibial translation and diagnosis of cruciate ligament deficiency. J Orthop Res. 2000;18:176–182. doi: 10.1002/jor.1100180203. [DOI] [PubMed] [Google Scholar]
- 44.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. J Bone Joint Surg. 1990;72A:557–567. [PubMed] [Google Scholar]
- 45.Markolf KL, Hame S, Hunter DM, Oakes DA, Zoric B, Gause G, Finerman GAM. Effects of femoral tunnel placement on knee laxity and forces in an anterior cruciate ligament graft. J Orthop Res. 2002;20:1016–1024. doi: 10.1016/S0736-0266(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 46.Muneta T, Yamamoto H, Sakai H, Ishibashi T, Furuya K. Relationship between changes in length and force in in vitro reconstructed anterior cruciate ligament. Am J Sports Med. 1993;21:299–304. doi: 10.1177/036354659302100222. [DOI] [PubMed] [Google Scholar]
- 47.Nicholas SJ, D'Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP. A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:1881–1886. doi: 10.1177/0363546504265924. [DOI] [PubMed] [Google Scholar]
- 48.Nurmi JT, Kannus P, Sievanen H, Jarvela T, Jarvinen M, Jarvinen TL. Interference screw fixation of soft tissue grafts in anterior cruciate ligament reconstruction: Part 2: Effect of preconditioning on graft tension during and after screw insertion. Am J Sports Med. 2004;32:418–424. doi: 10.1177/0363546503261703. [DOI] [PubMed] [Google Scholar]
- 49.O'Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament - a follow-up report. J Bone Joint Surg. 2001;83A:1329–1332. doi: 10.2106/00004623-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Otto JK, Brown TD, Callaghan JJ. Static and dynamic response of a multiplexed-array piezoresistive contact sensor. Experimental Mechanics. 1999;39:317–323. [Google Scholar]
- 51.Patel JV, Church JS, Hall AJ. Central third bone-patellar tendon-bone anterior cruciate ligament reconstruction: A 5-year follow-up. Arthroscopy. 2000;16:67–70. doi: 10.1016/s0749-8063(00)90130-0. [DOI] [PubMed] [Google Scholar]
- 52.Ringer GW, Wayne JS, Zuelzer WA. Tensioning of the acl does not restore intact knee kinematics following release in vivo. Trans Orthop Res Soc. 1997;22:98. [Google Scholar]
- 53.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 54.Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: An in vitro study. J Bone Joint Surg. 2003;85A:1018–1029. doi: 10.2106/00004623-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Stukenborg-Colsman C, Ostermeier S, Hurschler C, Wirth CJ. Tibiofemoral contact stress after total knee arthroplasty: Comparison of fixed and mobile-bearing inlay designs. Acta Orthop Scand. 2002;73:638–646. doi: 10.1080/000164702321039598. [DOI] [PubMed] [Google Scholar]
- 56.Tashman S, Anderst W, Kolowich P, Havstad S, Arnoczky S. Kinematics of the acl-deficient canine knee during gait: Serial changes over two years. J Orthop Res. 2004;22:931–941. doi: 10.1016/j.orthres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 58.Thompson WO, Fu FH. The meniscus in the cruciate-deficient knee. Clin Sports Med. 1993;12:771–796. [PubMed] [Google Scholar]
- 59.Uh BS, Beynnon BD, Churchill DL, Haugh LD, Risberg MA, Fleming BC. A new device to measure knee laxity during weightbearing and non- weightbearing conditions. J Orthop Res. 2001;19:1185–1191. doi: 10.1016/S0736-0266(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 60.van Kampen A, Wymenga AB, van der Heide HJL. The effect of different graft tensioning in anterior cruciate ligament reconstruction: A prospective randomized study. Arthroscopy. 1998;14:845–850. doi: 10.1016/s0749-8063(98)70022-2. [DOI] [PubMed] [Google Scholar]
- 61.Wallace AL, Harris ML, Walsh WR, Bruce WJM. Intraoperative assessment of tibiofemoral contact stresses in total knee arthroplasty. J Arthroplasty. 1998;13:923–927. doi: 10.1016/s0883-5403(98)90200-5. [DOI] [PubMed] [Google Scholar]
- 62.Wilson DR, Eichler MJ, Hayes WC. Accuracy of the iscan pressure measurement system. Trans Orthop Res Soc. 1998;23:393. [Google Scholar]
- 63.Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: The effect of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 64.Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL-Y. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda K, Tsujino J, Tanabe Y, Kaneda K. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction - autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25:99–106. doi: 10.1177/036354659702500120. [DOI] [PubMed] [Google Scholar]
- 66.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33:240–246. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 67.Yoshiya S, Andrish JT, Manley MT, Bauer TW. Graft tension in anterior cruciate ligament reconstruction. An in vivo study in dogs. Am J Sports Med. 1987;15:464–469. doi: 10.1177/036354658701500506. [DOI] [PubMed] [Google Scholar]
- 68.Yoshiya S, Kurosaka M, Ouchi K, Kuroda R, Mizuno K. Graft tension and knee stability after anterior cruciate ligament reconstruction. Clin Orthop. 2002;394:154–160. doi: 10.1097/00003086-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Zavras TD, Race A, Amis AA. The effect of femoral attachment location on anterior cruciate ligament reconstruction: Graft tension patterns and restoration of normal anterior-posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc. 2005;13:92–100. doi: 10.1007/s00167-004-0541-5. [DOI] [PubMed] [Google Scholar]


