Abstract
The fruit shape is important quantitative trait closely related to the fruit quality. However, the genetic model of fruit shapes has not been proposed. Therefore, in the present study, analysis of genetic effects for fruit shape traits (fruit length and fruit perimeter) in sponge gourd was conducted by employing a developmental genetic model including fruit direct effects and maternal effects. Analysis approaches of unconditional and conditional variances were applied to evaluate the genetic behavior of fruit shape traits at economical and physiological maturation times. The results of variance analysis indicated that fruit length and fruit perimeter were simultaneously affected by fruit direct genetic effects and maternal effects. Fruit direct genetic effects were relatively more important for fruit shape traits at whole developmental period. The gene expression was most active at the economical maturation stage (1~12 d after flowering) for two shape traits, and the activation of gene was mostly due to direct dominance effects at physiological maturation stage (13~60 d after flowering). The coefficients due to different genetic effects, as well as the phenotypic correlation coefficients, varied significantly between fruit shape traits themselves at various maturation stages. The results showed that it was relatively easy to improve fruit shape traits for industrial purpose by carefully selecting the parents at economical maturation stage instead of that at physiological maturation stage.
Keywords: Fruit length, Fruit perimeter, Genetic effects, Genetic variance, Luffa cylindrical Roem
INTRODUCTION
The sponge gourd is not well known in the vegetable community, but is very popularly grown in China. The unique nature of the fruits, which are used both for food (fresh fruit) and industrial purposes (the tough fibrous netting that remained from the mature fruit is valued for use in the bath and kitchen or as marine steam engine filters), aroused interest in the plants. The length and perimeter of the fruit are two of the important quantitative traits closely related to the sponge gourd exterior quality. Although it is well known that the fruit shape of the sponge gourd affects the marketability as a vegetable and industrial material, the characteristics of fruit shape have not been reported nor have their modes of inheritance been understood. This information is important in selecting appropriate parents and in developing the most appropriate strategy for breeding sponge gourd as vegetable and for industrial use.
Plant growth under natural open field is affected by environmental conditions, e.g. weather, soil, cultivation and management (Prior et al., 1992; Rouphael and Colla, 2005), however the phenotypic variation for many fruit quality traits of sponge gourd is mainly affected by fruit direct genetic effects, and also might be affected by maternal plant. Therefore, it is necessary to understand the fruit direct genetic effect and maternal plant genetic effect on the performance of fruit shape traits in sponge gourd at different maturation stages. Most agronomic, seed and fruit quality traits are complex and controlled by several genes expressed throughout the developmental stages (Beyer et al., 2002; Wu et al., 2006; Zalapa et al., 2006). Those reports have not however revealed that the net genetic effects of gene expression during a developmental stage. According to the theory of developmental genetics, genes are selectively expressed at different growth stages (Shi et al., 2001; Wu et al., 2005). Those studies ignored the dissimilar gene actions at different stages which is an important factor influencing the development of the quantitative traits. The performance of net genetic effects of fruit shape traits at different maturation stages could be obtained according to the conditional genetic models developed by Zhu (1995). Furthermore, understanding the dynamics of gene sequential expression in different environments is a major goal in developmental quantitative genetics (Fabrizius et al., 1997; Shi et al., 2002), and the results will be helpful for improving the manipulation of quantitative traits.
In this experiment, the genetic mechanism for fruit length and fruit perimeter was investigated for unconditional genetic effects (0→t) and conditional net genetic effects in a specific period (t−1→t), and the total fruit maturation time was divided into two stages: economical maturation stage (days from flowering to harvesting fruit for food) and physiological maturation stage (days from food harvesting to full maturity). The genetic model including fruit direct effects and maternal effects was used to investigate the genetic control of fruit length and fruit perimeter in sponge gourd at different maturation stages (economical maturation stage and physiological maturation stage). The objectives of this experiment were to clarify the developmental behavior of gene expression for the fruit shape traits in sponge gourd at various maturation stages. Correlation coefficients were employed to measure the relationship of accumulated behavior or net genetic effects between traits themselves at different maturation stages. The breeding value of parents was predicted for fruit shape traits improvement in breeding program.
MATERIALS AND METHODS
Materials
The experiments were conducted in 2004 and 2005. The mating design used by this experiment was a 7×7 diallel cross using sponge gourd cultivars. Their parents were ‘Wuyexiang’ (P1), ‘Lifeng’ (P2), ‘Tianhong’ (P3), ‘Jinke’ (P4), ‘Fengyuan’ (P5), ‘Jiutouniao’ (P6), and ‘Sanjiang 1’ (P7).
Experiments in open field
The seeds of the parents and their 1st generation of hybrid (F1) were obtained by crossing females to males for each cultivar at flowering during the same growing season in the summer of 2004. Seedlings were planted in the open field of experimental farm of Zhejiang University in the spring of 2005. The sponge gourd seeds were sown on Mar. 12 in 2005. Thirty days old seedlings were individually transplanted to a space of 50 cm×180 cm within rows. A randomized complete block design with two replications (each plot with 10 plants) was used. A trellis about 200 cm in height was used to support the climbing vine. Five well-grown plants in each plot of parents and F1 were marked for the determination of fruit shape traits. Fruit length and fruit perimeter were measured using marked plants each with 4 fruits at economical maturation time (12 d after flowering) and physiological maturation time (60 d after flowering), respectively.
Statistical methods
The genetic model including maternal effects as well as additive and dominance effects (Zhu et al., 1993) was employed to study the inheritance of fruit shape traits in sponge gourd. According to the model, unconditional genetic analysis was conducted based on phenotypic value at time t (y (t)), which can be partitioned as:
| y(t)=μ(t)+A(t)+D(t)+M(t)+B(t)+ε(t), |
where µ (t) is population mean, A (t)~N(0, V A) is fruit additive effect. D (t)~N(0, V D) is fruit dominance effect, M (t)~N(0, V M) is fruit maternal effect, B (t)~N(0, V B) is block effect, ε (t)~N(0, V ε) is residual effect.
The phenotypic values at time t conditioned on phenotypic value measured at time (t−1) can be partitioned as (Zhu, 1995):
| y(t|t−1)=µ(t|t−1)+A(t|t−1)+D(t|t−1)+M(t|t−1)+B(t|t−1)+ε(t|t−1), |
where all parameters were defined similarly as the unconditional effects.
Different correlation coefficients between various developmental stages were calculated for phenotypic correlation coefficient (r P), correlation coefficients due to fruit genetic effects [fruit additive correlation coefficient (r A), fruit dominance correlation coefficient (r D)], maternal correlation coefficient (r M) and residual coefficient (r ε).
The genetic effects estimated by using unconditional analysis defined as the total accumulated genetic effects of genes expressed from the initial time (at flowering) to time t (0→t) of maturation stage in sponge gourd fruit, while those measured by using the conditional analysis mean the net genetic effects from genes expressed in the special maturation period from time t−1 to time t. Both unconditional and conditional variances and correlations were estimated by minimum norm quadratic unbiased estimation (MINQUE) method (Zhu, 1992; Zhu and Weir, 1994). The jackknife technique (Miller, 1974; Zhu and Weir, 1994) was applied by sampling means of genetic traits for estimating the standard errors of estimated variances and correlation. A t-test was employed for testing the significance of genetic parameters.
RESULTS
Phenotypic means of generations
The means of parents and F1 generations of fruit length and fruit perimeter showed that there was large variation among materials studied at two maturation stages (Table 1). The phenotypic values of both shape traits differed considerably among the 7 cultivars over two maturation stages. Range at 12 d (12 d after flowering) was smaller than that at 60 d (60 d after flowering), for example, the range for fruit length was from 33.81 to 84.10 cm at 12 d and from 39.27 to 102.36 cm at 60 d, for fruit perimeter from 13.02 to 26.50 cm at 12 d and from 18.11 to 31.79 cm at 60 d. The means of the two shape traits increased relatively rapidly from initial time to the economical maturation time, and maintained the increasing tendency from economical maturation time to the physiological maturation time. These revealed that the variations of the two fruit shape traits in sponge gourd might be influenced by genotypic and maternal effects and might be different at various maturation stages.
Table 1.
Phenotypic means of fruit shape traits (fruit length and perimeter) of 7 cultivars and F1 generations at the time of economical (12 d) and physiological maturation (60 d)
| Materials | Fruit length (cm) |
Fruit perimeter (cm) |
||
| 12 d | 60 d | 12 d | 60 d | |
| Parent | ||||
| Wuyexiang (P1) | 39.48 | 54.67 | 17.70 | 24.87 |
| Lifeng (P2) | 84.10 | 102.36 | 13.02 | 18.11 |
| Tianhong (P3) | 42.98 | 52.38 | 20.25 | 23.72 |
| Jinke (P4) | 33.81 | 39.27 | 20.37 | 24.76 |
| Fengyuan (P5) | 33.95 | 41.66 | 26.50 | 31.79 |
| Jiutouniao (P6) | 48.80 | 67.75 | 15.63 | 22.50 |
| Sanjiang 1 (P7) | 64.18 | 73.71 | 16.87 | 20.51 |
| Parent mean | 49.61 | 61.68 | 18.62 | 23.75 |
| F1 generationa | ||||
| F1 (P1·) | 45.41 | 59.56 | 17.02 | 24.00 |
| F1 (P2·) | 63.42 | 81.88 | 16.07 | 22.67 |
| F1 (P3·) | 59.72 | 78.43 | 17.13 | 24.11 |
| F1 (P4·) | 47.74 | 61.56 | 16.70 | 23.63 |
| F1 (P5·) | 59.44 | 78.84 | 19.46 | 26.98 |
| F1 (P6·) | 62.62 | 76.07 | 17.23 | 25.96 |
| F1 (P7·) | 59.87 | 74.09 | 16.70 | 22.75 |
| F1 mean | 56.89 | 72.92 | 17.19 | 24.30 |
F1 (Pi·) represents the mean of all the F1 generations derived from the combination involving female parent i
Performance of F1 generations for fruit shape traits showed that the mean of F1 generations involving a common female parent was different (higher or lower) when compared to that of their common female parent at various maturation stages (Table 1). For example, the positive comparison of fruit length and fruit perimeter at 12 d accounted for about 71.43% and 28.57% of the total comparisons, respectively. Therefore, this suggested that there would be a certain heterosis in F1 seeds for the three seedling traits at various maturation stages.
Variance components
Total phenotypic variance (V P) consists of variance components of fruit genotypic and residual effects (V P=V G+V ε). Among genotypic variance (V G=V A+V D+V M), variance of fruit direct effects (V A+V D) represents the genetic variation contributed by the fruit gene effects and variance of maternal nuclear effects (V M) measures the contribution of maternal plant through effects of maternal nucleolus gene. Residual variance (V ε) covered the remaining random effects, including unexplainable random effects and environment effects around plant.
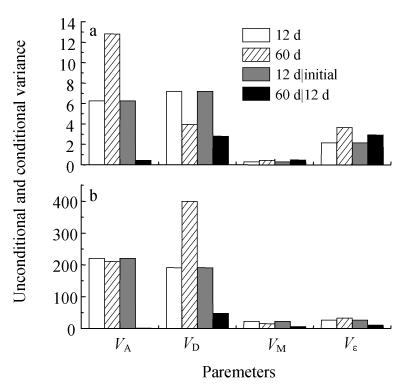
According to the magnitude of each genotypic variance component, the major contribution effects affecting the fruit shape traits of sponge gourd could be found. Estimates of unconditional and conditional variances are presented in Fig.1. The results showed that the performance of fruit length and fruit perimeter at various maturation stages was mainly affected by direct genetic effects of fruit genes [(V A+V D)/(V A+V D+V M)=0.872~0.979]. From the unconditional fruit variance analysis, since the fruit additive effects and fruit dominance effects varied slightly for two shape traits, suggesting that both were important for fruit shape traits in sponge gourd. From the conditional fruit variance analysis, however, the direct dominance variance V D of fruit length and fruit perimeter at the second stages (13~60 d) accounted for about 98.14% and 86.46% of the fruit direct genotypic variance (V A+V D), respectively, indicating that the net expression of genes for fruit shape traits was mainly caused by direct genotypic variance at this stage. It was implied that genetic selection on fruit could be quite effective for improving fruit length and fruit perimeter in specific stages; however, heterosis was important for all various stages. Besides direct genotypic variances, significant unconditional and conditional variances of fruit maternal effects were noticeably detected at all maturation stages for fruit length and fruit perimeter (Fig.1). It was indicated that net genetic effects of maternal plant existed through the whole development period.
Fig. 1.
Estimates of unconditional and conditional variance of fruit length (a) and fruit perimeter (b) at the time of economical (12 d) and physiological maturation (60 d)
V A: Fruit additive variance; V D: Fruit dominance variance; V M: Fruit maternal variance; V ε: Residual variance
Since development is a dynamic procedure, gene expression should not always be the same for developmental quantitative traits (Zhu, 1995). For instance, new additive effects due to fruit gene expression could affect fruit length and fruit perimeter at the first stage (from initial to 12 d), but with small effect on the fruit shape traits at the second stage (from 13 d to 60 d) (Fig.1). Dynamic consequences of genetic effects could be revealed by the combination of conditional and unconditional methods for different developmental traits at specific periods. For example, the new effect of gene at the second stage (from 13 d to 60 d) was very small but relatively large V A of fruit length was observed by unconditional method, this was due to accumulated results of early stages. Thus, the conditional analysis method could help to clarify the reality of genetic effects of new gene expression that could not be detected by unconditional method.
Since sponge gourd is a dry land plant, higher unconditional and conditional residual variances were observed as compared to those of paddy field plant such as rice (Shi et al., 2002). However, relatively significant small unconditional and conditional residual variances indicated that the genetic effects were the predominant source of variation, with the performances of fruit shape traits at different developmental stages being also influenced by sampling errors or environmental effects.
Analysis of genetic correlations
Genetic variation analysis could only get insight into the gene action of specific stage. It would be useful to examine the correlation between fruit shape traits with themselves (Ye et al., 2003). This would facilitate understanding on the interaction of the gene effects, and whether the genetic association pattern would be altered by various gene expression of each trait at specific time intervals. Since the total genetic effects could be further partitioned into fruit direct additive effect, fruit direct dominance effect and maternal effects, the correlations consisted of components due to fruit direct additive correlation, fruit direct dominance correlation and maternal correlation.
For fruit length, it was relatively large positive correlation that contributed mostly to the performances between the two stages (Table 2). It seemed that r A(12 d & 60 d) was the main contribution. However, actually, the direct additive conditional correlation coefficient between the first and second periods was not significant from zero, indicating that effects of new gene expression caused by additive effect at the first period had no relationship with that at the second period.
Table 2.
Estimates of unconditional and conditional correlation coefficients of fruit shape traits (fruit length and perimeter) at the time of economical (12 d) and physiological maturation (60 d)
| Parameter | Length |
Perimeter |
||
| 12 d & 60 d | 12 d|initial & 60 d|12 d | 12 d & 60 d | 12 d|initial & 60 d|12 d | |
| rP | 0.947** | 0.030 | 0.721** | −0.022 |
| rA | 1.000** | 0.033 | 1.000** | −0.108** |
| rD | 0.947** | 0.046** | 0.544** | −0.007 |
| rM | 0.820** | 0.141** | −0.026** | −0.076** |
| rε | 0.821** | −0.036 | 0.449** | 0.001 |
r P: Phenotype correlation; r A: Embryo additive correlation; r D: Embryo dominance correlation; r M: Embryo additive interaction correlation; r ε: Residual correlation.
Significance at 0.01 level of probability
For fruit perimeter, as compared to fruit length, there existed noticeable negative unconditional correlation in r M(12 d & 60 d) and conditional correlation in r A(12 d|initial & 60 d|12 d) and r M(12 d|initial & 60 d|12 d) (Table 2). Although there existed positive unconditional correlation in r A(12 d & 60 d) with relatively large magnitude, significant negative conditional correlation between two periods was observed. This indicated that extra effects of new gene expression at second development interval would have contrary function on fruit perimeter.
Positive unconditional r P(12 d & 60 d) and r ε(12 d & 60 d) correlation for fruit shape traits were noticeable between two stages; however, no significant conditional r P(12 d|initial & 60 d|12 d) and r ε(12 d|initial & 60 d|12 d) correlation existed in this experiment. Since the unconditional and conditional residual correlations with traits themselves were relatively small but significant, the relationships of two shape traits were also influenced by sampling errors and environment effects.
Parental genetic effects at economical and physiological maturation stage
Potential value of parents in the breeding could be appraised through the prediction of value of additive effect and maternal effect of parents. The predicted fruit additive effects (A) and maternal effects (M) analyzed based on unconditional variance are shown in Table 3 for fruit shape traits at different maturation stages. The results showed that, for fruit length and fruit perimeter, the tendency of predicted additive effects (positive or negative) in each parent at physiological maturation time was consistent with that at economical maturation (Table 3). It was suggested that the expression of additive effects might be suspended at second stage. Compared to the additive effects, however, predicted maternal effects in each parent between two maturation stages were more complex for fruit length and fruit perimeter. For instance, predicted maternal effect of P2 (‘Lifeng’) for length at 12 d was −3.573, but at 60 d was 0.220. This indicated that extra effects of maternal gene expression in P2 (‘Lifeng’) at second maturation stage would have contrary function on fruit length.
Table 3.
Predicted genetic effects of fruit shape traits in sponge gourd for fruit length and fruit perimeter at economical maturation (12 d) and physiological maturation (60 d)
| Parents | Length |
Perimeter |
||||||
| 12 d |
60 d |
12 d |
60 d |
|||||
| A | M | A | M | A | M | A | M | |
| P1 | −3.350+ | −6.827** | −5.355** | −4.389* | 0.475 | −0.505 | 1.355** | −0.797+ |
| P2 | 14.661** | −3.573** | 13.053** | 0.220 | −1.660** | −0.226 | −2.986** | 0.379 |
| P3 | −0.986 | 2.733 | −0.859 | 3.676+ | −0.010 | 0.571 | −0.587 | 0.444 |
| P4 | −7.601** | −1.953 | −7.801** | −4.000** | 0.014 | −0.028 | 0.133 | −0.334 |
| P5 | −3.008+ | −0.218 | −2.401 | 0.419 | 2.262** | 0.702 | 2.763** | 0.767 |
| P6 | −4.172** | 7.848** | −2.992** | 6.169** | 0.128 | −0.754* | 0.004 | 0.423 |
| P7 | 4.456** | 1.990 | 6.354** | −2.094 | −1.119** | 0.241 | −0.682 | −0.881+ |
A: Additive effect; M: Maternal effect
Significance at 0.10 levels of probability
Significance at 0.05 levels of probability
Significance at 0.01 levels of probability
The predicted genetic effects in Table 3 indicated that variations at two maturation stages were significant in most parents. This suggested that most parents had potential value in breeding for different purpose. Since relatively higher positive total genetic effects of fruit and maternal plant existed at different maturation stages, P2 (‘Lifeng’) was better than other parents in increasing fruit length (A+M were 11.088 and 13.273) and P5 (‘Fengyuan’) was better in increasing fruit perimeter (A+M were 2.964 and 3.530). Similar results were observed from other parents. The genetic effects of some parents showed that they were not always suitable for breeding at different maturation stages. For instance, P1 (‘Wuyexiang’) was the best parent in declining fruit length (A+M was −10.177) at 12 d, however, P4 (‘Jinke’) (A+M was −11.801) was the best parent at 60 d.
DISCUSSION
Inheritance of fruit quality traits has been reported in previous study (Umaharan et al., 1997; Lavi et al., 1998; Zalapa et al., 2006; Sun et al., 2006), but these efforts do not contain the maternal plant effect. The nutrition for fruit composed of carpodermis, pulp and seed was supported by maternal plant. Therefore, fruit shape traits of sponge gourd might be controlled by maternal gene. Zhu et al.(1993) proposed a genetic model for quantitative traits controlled by direct genes and maternal plant genes. By using this model, the influences of maternal effects on fruit length and fruit perimeter were found. Therefore, the maternal plant genes were also important for the performance of fruit shape traits in sponge gourd as well as fruit direct genes. The performance of quantitative traits of fruit shape would be controlled by the gene expression and regulation during maturation periods. The genetic mechanisms that control the performance of quantitative traits might vary in different developmental stages. Furthermore, correlations due to different genetic effects existed among various developmental stages (Ye et al., 2003). It is helpful to clarify the developmental genetic mechanism of genes and to improve the fruit shape traits in sponge gourd by studying the genetic effects of different genetic effects and the variation of gene expression at different maturation stages. Furthermore, the performance of net genetic effects at different periods and the relationship between various periods could be obtained for developmental behavior of the quantitative traits by using the conditional analysis approach (Zhu, 1995). Therefore, the genetic model and statistical analysis method used in this experiment might be helpful to other horticultural plants for studying quantitative traits of fruit quality.
The present study showed that the genetic effects of fruit direct gene and maternal plant genes affect fruit length and fruit perimeter at various maturation time. Genetic effects due to fruit direct genetic effects were relatively more important for two shape traits throughout the whole developmental period. The results of conditional genetic variances analysis revealed that there was new expression of genes in the two maturation stage for fruit direct genetic and maternal genetic system, especially for economical maturation stage (1~12 d after flowering). The gene expression at this maturation stage was therefore more active. It was implied, by the detection of different genetic variances at various maturation, that genes controlling fruit shape traits might be differently expressed at the various stages of whole maturation period. However, it seemed that the second stage was more active than early stage according to the performance of phenotypic means of parents and F1. Therefore, those results indicated that breeding work only based on the phenotypic data might not represent the reality of fruit gene expression. The correlation coefficients due to different genetic effects, as well as the phenotypic correlation coefficients, varied significantly between fruit shape traits themselves at various maturation stages, and also further confirmed the result of variance analysis.
The result of potential value of parents in the breeding indicated that most parents had potential value for different breeding purpose. Furthermore, since there existed relatively small expression of additive and maternal effects at physiological maturation, the selection of the fruit length and fruit perimeter for industrial purpose might be efficiently obtained based on economical maturation time instead of physiological maturation time. Since the performance of fruit shape traits might be affected by genotype×environment interaction, further evaluation in different environments is required.
CONCLUSION
This investigation has highlighted that fruit length and fruit perimeter are simultaneously affected by fruit direct genetic effects and maternal effects. Fruit direct genetic effects are relatively more important for fruit shape traits throughout the whole developmental period. The gene expression is most active at the economical maturation stage for fruit length and fruit perimeter, and the gene activation is mainly due to direct dominance effects at physiological maturation stage. The coefficients due to different genetic effects, as well as the phenotypic correlation coefficients, varied significantly between fruit shape traits themselves at various maturation stages. Further, the relative small expression of additive and maternal effects at physiological maturation will enable breeders to shorten the breeding time in selecting fruit length and fruit perimeter.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30370911) and the Science and Technology Department of Zhejiang Province (No. 2005C32001), China
References
- 1.Beyer M, Hahn R, Peschel S, Harz M, Knoche M. Analysing fruit shape in sweet cherry (Prunus avium L.) Scientia Horticulturae. 2002;96(1-4):139–150. doi: 10.1016/S0304-4238(02)00123-1. [DOI] [Google Scholar]
- 2.Fabrizius MA, Cooper M, Basford KE. Genetic analysis of variation for grain yield and protein concentration in two wheat crosses. Australian Journal of Agricultural Research. 1997;48(5):605–614. doi: 10.1071/A96152. [DOI] [Google Scholar]
- 3.Lavi U, Tomer E, Gazit S, Hillel J. Components of the genetic variance and genetic correlations between traits in Mango. Scientia Horticulturae. 1998;75(1-2):11–25. doi: 10.1016/S0304-4238(98)00112-5. [DOI] [Google Scholar]
- 4.Miller RG. The jackknife—a review. Biomertika. 1974;61(1):1–15. doi: 10.1093/biomet/61.1.1. [DOI] [Google Scholar]
- 5.Prior LD, Grieve AM, Cullis BR. Sodium chloride and soil texture interactions in irrigated field grown sultana grapevines. I. Yield and fruit quality. Australian Journal of Agricultural Research. 1992;43(5):1051–1066. doi: 10.1071/AR9921051. [DOI] [Google Scholar]
- 6.Rouphael Y, Colla G. Growth, yield, fruit quality and nutrient uptake of hydroponically cultivated zucchini squash as affected by irrigation systems and growing seasons. Scientia Horticulturae. 2005;105(2):177–195. doi: 10.1016/j.scienta.2005.01.025. [DOI] [Google Scholar]
- 7.Shi CH, Wu JG, Fan LJ, Zhu J, Wu P. Developmental genetic analysis of brown rice weight under different environmental conditions in indica rice. Acta Botanica Sinica. 2001;43(6):603–609. [Google Scholar]
- 8.Shi CH, Wu JG, Wu P. Developmental behavior of gene expression for brown rice thickness under different environments. Genesis. 2002;33(4):185–190. doi: 10.1002/gene.10116. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Lower RL, Staub JE. Analysis of generation means and components of variance for parthenocarpy in cucumber (Cucumis sativus L.) Plant Breeding. 2006;125(3):277–280. doi: 10.1111/j.1439-0523.2006.01224.x. [DOI] [Google Scholar]
- 10.Umaharan P, Ariyanayagam RP, Haque SQ. Genetic analysis of pod quality characteristics in vegetable cowpea (Vigna unguiculata L. Walp.) Scientia Horticulturae. 1997;70(4):281–292. doi: 10.1016/S0304-4238(97)00053-8. [DOI] [Google Scholar]
- 11.Wu JG, Shi CH, Zhang HZ. Genetic analysis of embryo, cytoplasmic, and maternal effects and their environment interactions for protein content in Brassica napus L. Australian Journal of Agricultural Research. 2005;56(1):69–73. doi: 10.1071/AR04089. [DOI] [Google Scholar]
- 12.Wu JG, Shi CH, Zhang HZ. Partitioning genetic effects due to embryo, cytoplasm and maternal parent for oil content in rapeseed (Brassica napus L.) Genetics and Molecular Biology. 2006;29(3):533–538. [Google Scholar]
- 13.Ye ZH, Lu ZZ, Zhu J. Genetic analysis for developmental behavior of some seed quality traits in Upland cotton (Gossypum hirsutum L.) Euphytica. 2003;129(2):183–191. doi: 10.1023/A:1021974901501. [DOI] [Google Scholar]
- 14.Zalapa JE, Staub JE, McCreight JD. Generation means analysis of plant architectural traits and fruit yield in melon. Plant Breeding. 2006;125(5):482–487. doi: 10.1111/j.1439-0523.2006.01273.x. [DOI] [Google Scholar]
- 15.Zhu J. Mixed model approaches for estimating genetic variances and covariances. Chinese Journal of Biomathematics. 1992;7(1):1–11. (in Chinese) [Google Scholar]
- 16.Zhu J. Analysis of conditional genetic effects and variance components in developmental genetics. Genetics. 1995;141(4):1633–1639. doi: 10.1093/genetics/141.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Weir BS. Analysis of cytoplasmic and maternal effects. I. A genetic model for diploid plant seeds and animals. Theoretical Applied Genetics. 1994;89(2):153–159. doi: 10.1007/BF00225135. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Ji DF, Xu FH. A genetic approach for analyzing intra-cultivar heterosis in crops. Acta Genetica Sinica. 1993;20(3):262–271. (in Chinese) [Google Scholar]