Abstract
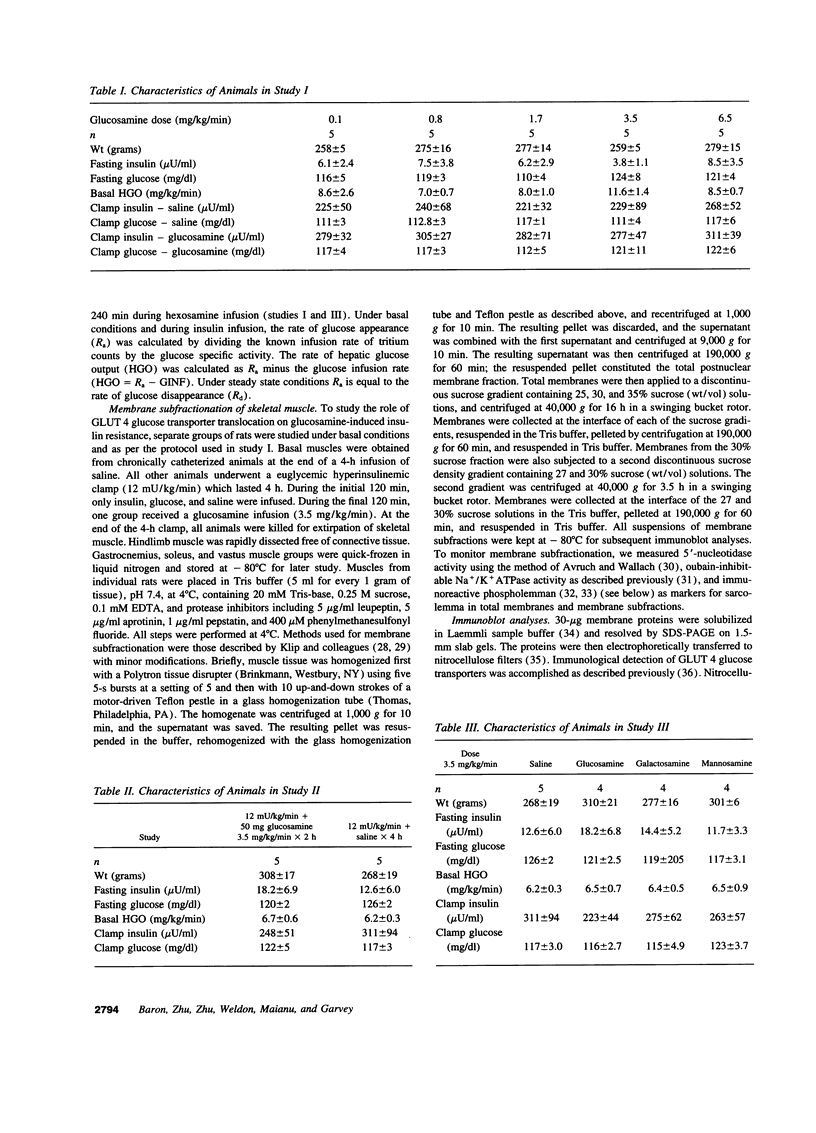
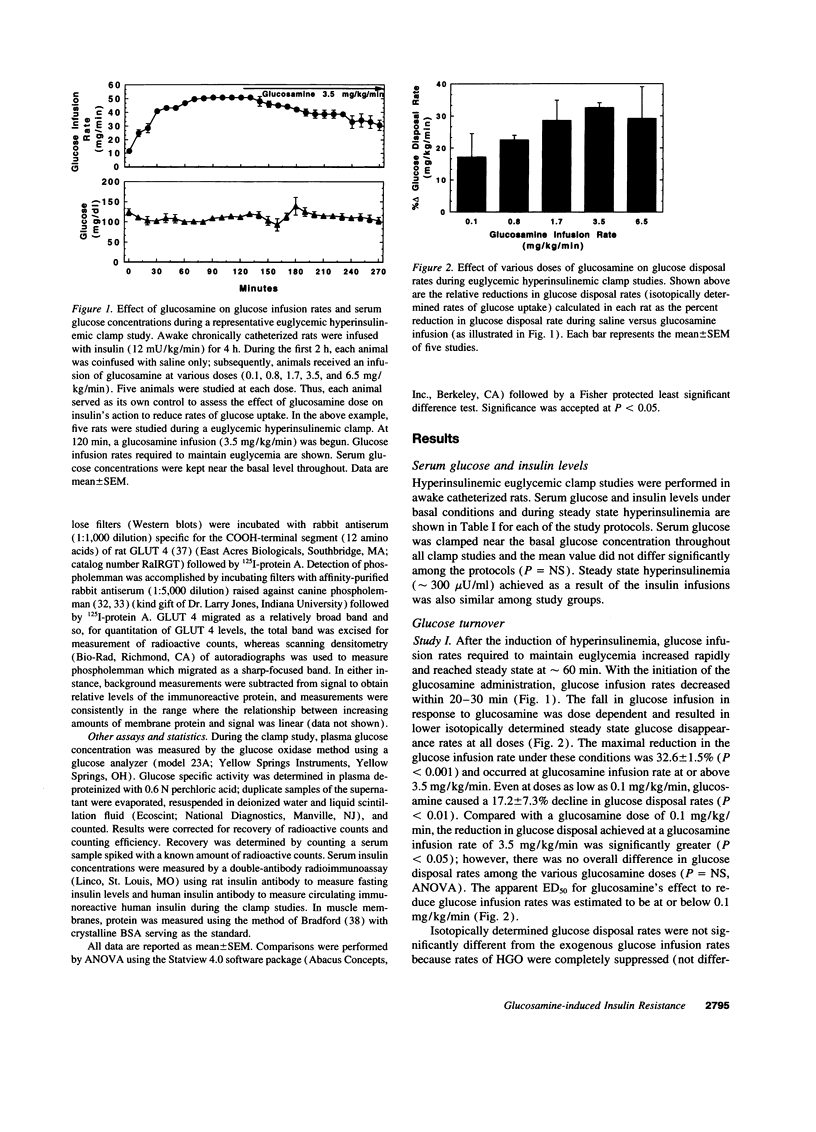
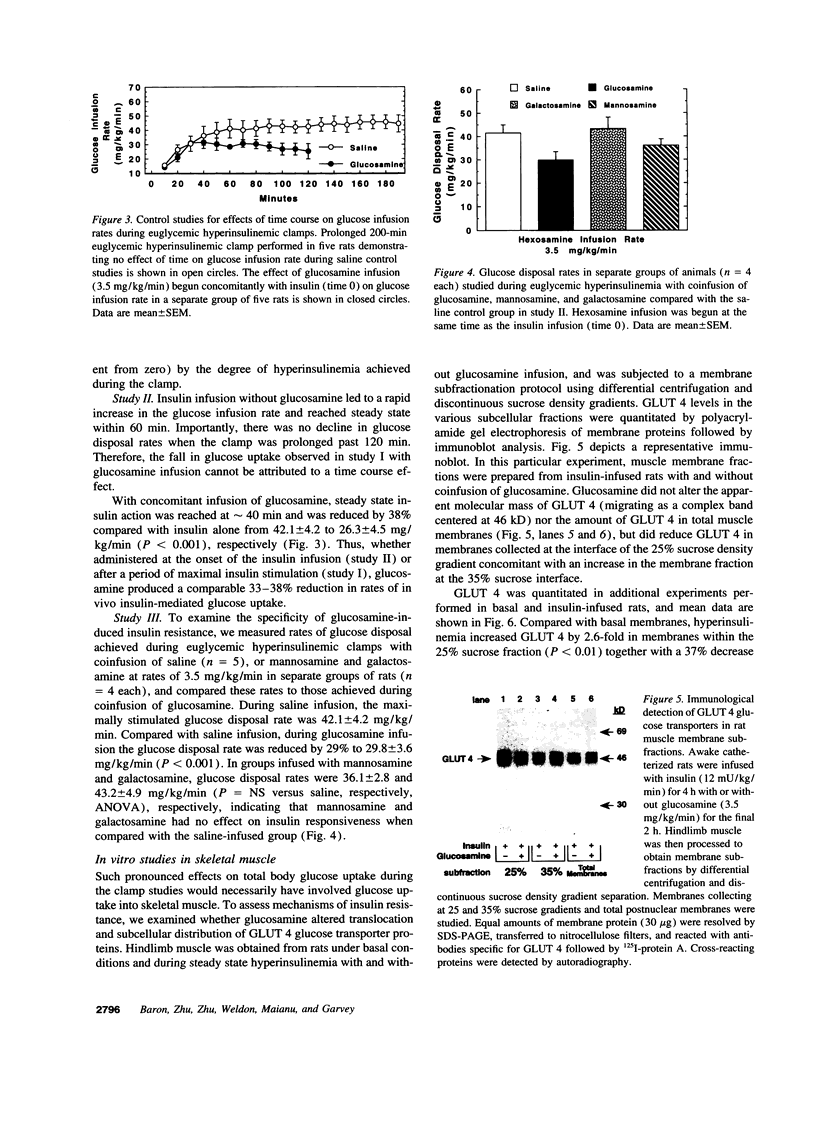
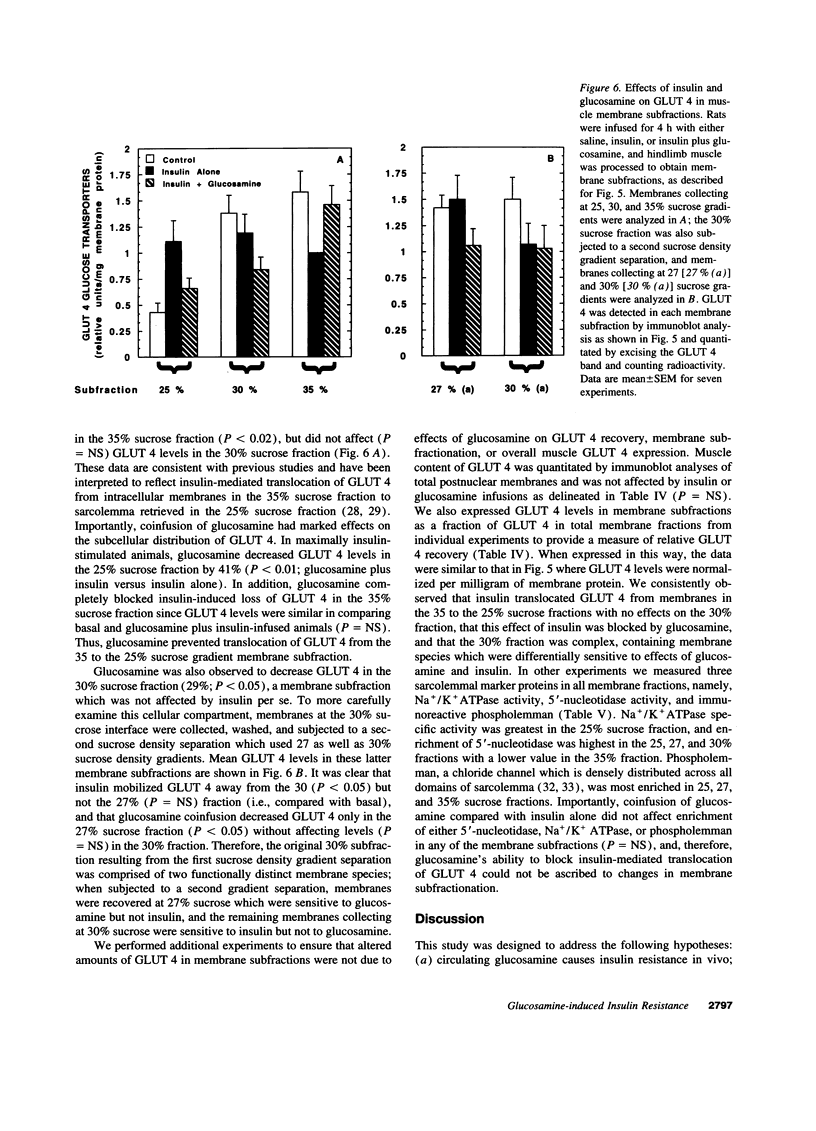
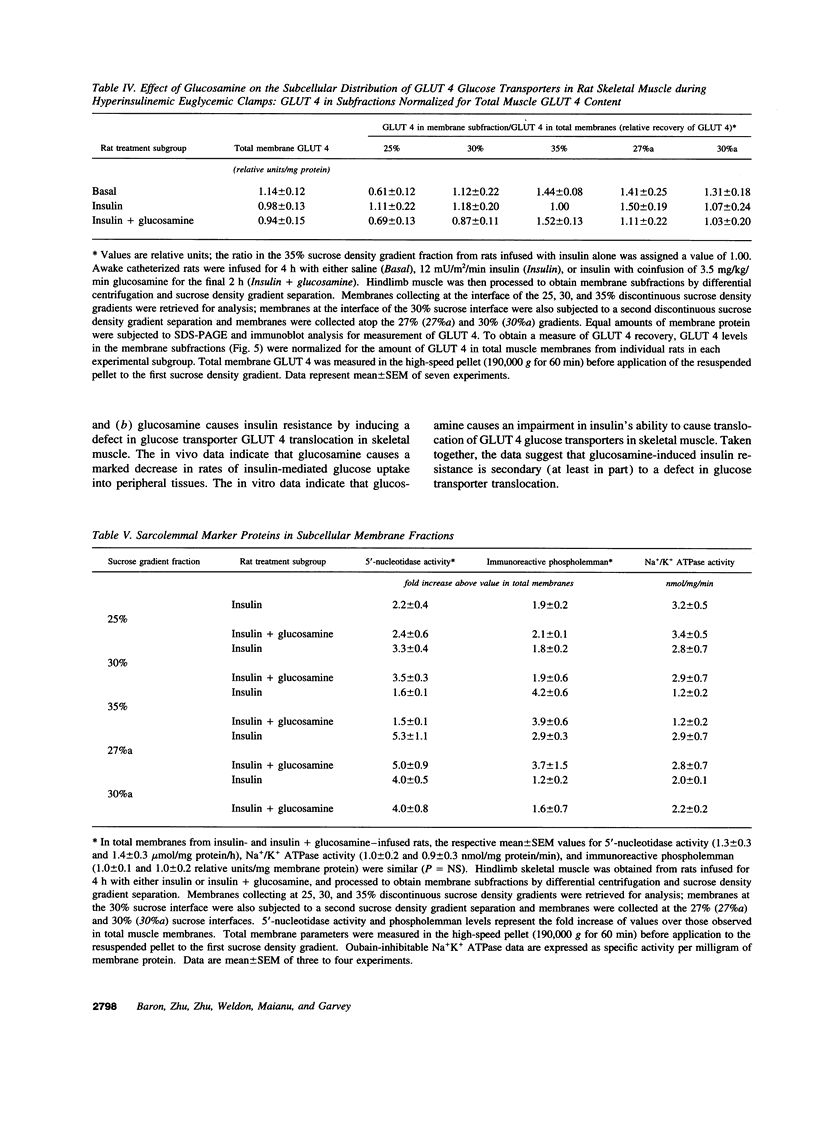
Glucosamine (Glmn), a product of glucose metabolism via the hexosamine pathway, causes insulin resistance in isolated adipocytes by impairing insulin-induced GLUT 4 glucose transporter translocation to the plasma membrane. We hypothesized that Glmn causes insulin resistance in vivo by a similar mechanism in skeletal muscle. We performed euglycemic hyperinsulinemic clamps (12 mU/kg/min + 3H-3-glucose) in awake male Sprague-Dawley rats with and without Glmn infusion at rates ranging from 0.1 to 6.5 mg/kg/min. After 4h of euglycemic clamping, hindquarter muscles were quick-frozen and homogenized, and membranes were subfractionated by differential centrifugation and separated on a discontinuous sucrose gradient (25, 30, and 35% sucrose). Membrane proteins were solubilized and immunoblotted for GLUT 4. With Glmn, glucose uptake (GU) was maximally reduced by 33 +/- 1%, P < 0.001. The apparent Glmn dose to reduce maximal GU by 50% was 0.1 mg/kg/min or 1/70th the rate of GU on a molar basis. Control galactosamine and mannosamine infusions had no effect on GU. Relative to baseline, insulin caused a 2.6-fold increase in GLUT 4 in the 25% membrane fraction (f), P < 0.01, and a 40% reduction in the 35%f, P < 0.05, but had no effect on GLUT 4 in the 30% f, P= NS. Addition of Glmn to insulin caused a 41% reduction of GLUT 4 in the 25%f, P < 0.05, a 29% fall in the 30%f, and prevented the reduction of GLUT 4 in the 35% f. The 30%f membranes were subjected to a second separation with a 27 and 30% sucrose gradient. Insulin mobilized GLUT 4 away from the 30%f, P < 0.05, but not the 27% f. In contrast, Glmn reduced GLUT 4 in the 27%f, P < 0.05, but not the 30%f. Thus Glmn appears to alter translocation of an insulin-insensitive GLUT 4 pool. Coinfusion of Glmn did not alter enrichment of the sarcolemmal markers 5'-nucleotidase, Na+/K+ATPase, and phospholemman in either 25, 30, or 35% f. Thus Glmn completely blocked movement of Glut 4 induced by insulin. Glmn is a potent inducer of insulin resistance in vivo by causing (at least in part) a defect intrinsic to GLUT 4 translocation and/or trafficking. These data support a potential role for Glmn to cause glucose-induced insulin resistance (glucose toxicity).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daniels M. C., Kansal P., Smith T. M., Paterson A. J., Kudlow J. E., McClain D. A. Glucose regulation of transforming growth factor-alpha expression is mediated by products of the hexosamine biosynthesis pathway. Mol Endocrinol. 1993 Aug;7(8):1041–1048. doi: 10.1210/mend.7.8.8232303. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem. 1983 Jan;128(1):159–163. doi: 10.1016/0003-2697(83)90356-1. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Dohm G. L., Leggett-Frazier N., Elton C. W., Tapscott E. B., Pories W. P., Caro J. F. Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest. 1992 Feb;89(2):701–705. doi: 10.1172/JCI115638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Birnbaum M. J. Cellular insulin action and insulin resistance. Baillieres Clin Endocrinol Metab. 1993 Oct;7(4):785–873. doi: 10.1016/s0950-351x(05)80237-x. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Lima F. B., Birnbaum M. J. Expression of a glucose transporter gene cloned from brain in cellular models of insulin resistance: dexamethasone decreases transporter mRNA in primary cultured adipocytes. Mol Endocrinol. 1989 Jul;3(7):1132–1141. doi: 10.1210/mend-3-7-1132. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Hancock J. A., Golichowski A. M., Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992 Apr;41(4):465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Zhu J. H., Hancock J. A., Golichowski A. M. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes. 1993 Dec;42(12):1773–1785. doi: 10.2337/diab.42.12.1773. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Matthaei S., Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987 Jan 5;262(1):189–197. [PubMed] [Google Scholar]
- Ginsberg H., Rayfield E. J. Effect of insulin therapy on insulin resistance in type II diabetic subjects. Evidence for heterogeneity. Diabetes. 1981 Sep;30(9):739–745. doi: 10.2337/diab.30.9.739. [DOI] [PubMed] [Google Scholar]
- Greenfield M. S., Doberne L., Rosenthal M., Schulz B., Widstrom A., Reaven G. M. Effect of sulfonylurea treatment on in vivo insulin secretion and action in patients with non-insulin-dependent diabetes mellitus. Diabetes. 1982 Apr;31(4 Pt 1):307–312. doi: 10.2337/diab.31.4.307. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- Hirshman M. F., Goodyear L. J., Wardzala L. J., Horton E. D., Horton E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990 Jan 15;265(2):987–991. [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Shulman G. I., DeFronzo R. A., Cushman S. W., Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest. 1991 Feb;87(2):561–570. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., Holloszy J. O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys Res Commun. 1990 Oct 30;172(2):728–736. doi: 10.1016/0006-291x(90)90735-6. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Shapiro G., Scarlett J. A., Griffin J., Olefsky J. M. The acute and chronic effects of sulfonylurea therapy in type II diabetic subjects. Diabetes. 1984 Apr;33(4):346–354. doi: 10.2337/diab.33.4.346. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lima F. B., Bao S., Garvey W. T. Biological actions of insulin are differentially regulated by glucose and insulin in primary cultured adipocytes. Chronic ability to increase glycogen synthase activity. Diabetes. 1994 Jan;43(1):53–62. doi: 10.2337/diab.43.1.53. [DOI] [PubMed] [Google Scholar]
- Lima F. B., Thies R. S., Garvey W. T. Glucose and insulin regulate insulin sensitivity in primary cultured adipocytes without affecting insulin receptor kinase activity. Endocrinology. 1991 May;128(5):2415–2426. doi: 10.1210/endo-128-5-2415. [DOI] [PubMed] [Google Scholar]
- Marshall S., Bacote V., Traxinger R. R. Complete inhibition of glucose-induced desensitization of the glucose transport system by inhibitors of mRNA synthesis. Evidence for rapid turnover of glutamine:fructose-6-phosphate amidotransferase. J Biol Chem. 1991 Jun 5;266(16):10155–10161. [PubMed] [Google Scholar]
- Marshall S., Bacote V., Traxinger R. R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991 Mar 15;266(8):4706–4712. [PubMed] [Google Scholar]
- Marshall S., Garvey W. T., Traxinger R. R. New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. FASEB J. 1991 Dec;5(15):3031–3036. doi: 10.1096/fasebj.5.15.1743436. [DOI] [PubMed] [Google Scholar]
- Palmer C. J., Scott B. T., Jones L. R. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991 Jun 15;266(17):11126–11130. [PubMed] [Google Scholar]
- Revers R. R., Kolterman O. G., Scarlett J. A., Gray R. S., Olefsky J. M. Lack of in vivo insulin resistance in controlled insulin-dependent, type I, diabetic patients. J Clin Endocrinol Metab. 1984 Feb;58(2):353–358. doi: 10.1210/jcem-58-2-353. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen B. F., Hansen S. A. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J. 1988 Jun 15;252(3):733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. A., Sens D. A., Buse M. G. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor. Diabetes. 1993 Sep;42(9):1333–1346. doi: 10.2337/diab.42.9.1333. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A., DeFronzo R. A. Glucose toxicity. Diabetes Care. 1990 Jun;13(6):610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Smith D., Rossetti L., Ferrannini E., Johnson C. M., Cobelli C., Toffolo G., Katz L. D., DeFronzo R. A. In vivo glucose metabolism in the awake rat: tracer and insulin clamp studies. Metabolism. 1987 Dec;36(12):1167–1174. doi: 10.1016/0026-0495(87)90244-7. [DOI] [PubMed] [Google Scholar]
- Sternlicht E., Barnard R. J., Grimditch G. K. Mechanism of insulin action on glucose transport in rat skeletal muscle. Am J Physiol. 1988 May;254(5 Pt 1):E633–E638. doi: 10.1152/ajpendo.1988.254.5.E633. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxinger R. R., Marshall S. Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine. Role of hexosamine biosynthesis in enzyme regulation. J Biol Chem. 1991 Jun 5;266(16):10148–10154. [PubMed] [Google Scholar]
- Traxinger R. R., Marshall S. Insulin regulation of pyruvate kinase activity in isolated adipocytes. Crucial role of glucose and the hexosamine biosynthesis pathway in the expression of insulin action. J Biol Chem. 1992 May 15;267(14):9718–9723. [PubMed] [Google Scholar]
- Traxinger R. R., Marshall S. Role of amino acids in modulating glucose-induced desensitization of the glucose transport system. J Biol Chem. 1989 Dec 15;264(35):20910–20916. [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Horn R. S., Albert K. A., Adler A., Walaas O. Phosphorylation of multiple sites in a 15,000 dalton proteolipid from rat skeletal muscle sarcolemma, catalyzed by adenosine 3',5'-monophosphate-dependent and calcium/phospholipid-dependent protein kinases. Biochim Biophys Acta. 1988 Jan 18;968(1):127–137. doi: 10.1016/0167-4889(88)90052-3. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Helve E., Koivisto V. A. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes. 1987 Aug;36(8):892–896. doi: 10.2337/diab.36.8.892. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Vuorinen-Markkola H., Koranyi L., Bourey R., Tordjman K., Mueckler M., Permutt A. M., Koivisto V. A. Defect in insulin action on expression of the muscle/adipose tissue glucose transporter gene in skeletal muscle of type 1 diabetic patients. J Clin Endocrinol Metab. 1992 Sep;75(3):795–799. doi: 10.1210/jcem.75.3.1517369. [DOI] [PubMed] [Google Scholar]




