Abstract
A primary human skeletal muscle culture (HSMC) system, which retains cellular integrity and insulin responsiveness for glucose transport was employed to evaluate glucose transport regulation. As previously reported, cells cultured from non-insulin-dependent diabetic (NIDDM) subjects displayed significant reductions in both basal and acute insulin-stimulated transport compared to nondiabetic controls (NC). Fusion/differentiation of NC and NIDDM HSMC in elevated media insulin (from 22 pM to 30 microM) resulted in increased basal transport activities but reduced insulin-stimulated transport, so that cells were no longer insulin responsive. After fusion under hyperinsulinemic conditions, GLUT1 protein expression was elevated in both groups while GLUT4 protein level was unaltered. Fusion of HSMC under hyperglycemic conditions (10 and 20 mM) decreased glucose transport in NC cells only when combined with hyperinsulinemia. Hyperglycemia alone down-regulated transport in HSMC of NIDDM, while the combination of hyperglycemia and hyperinsulinemia had greater effects. In summary: (a) insulin resistance of glucose transport can be induced in HSMC of both NC and NIDDM by hyperinsulinemia and is accompanied by unaltered GLUT4 but increased GLUT1 levels; and (b) HSMC from NIDDM subjects demonstrate an increased sensitivity to impairment of glucose transport by hyperglycemia. These results indicate that insulin resistance in skeletal muscle can be acquired in NC and NIDDM from hyperinsulinemia alone but that NIDDM is uniquely sensitive to the additional influence of hyperglycemia.
Full text
PDF

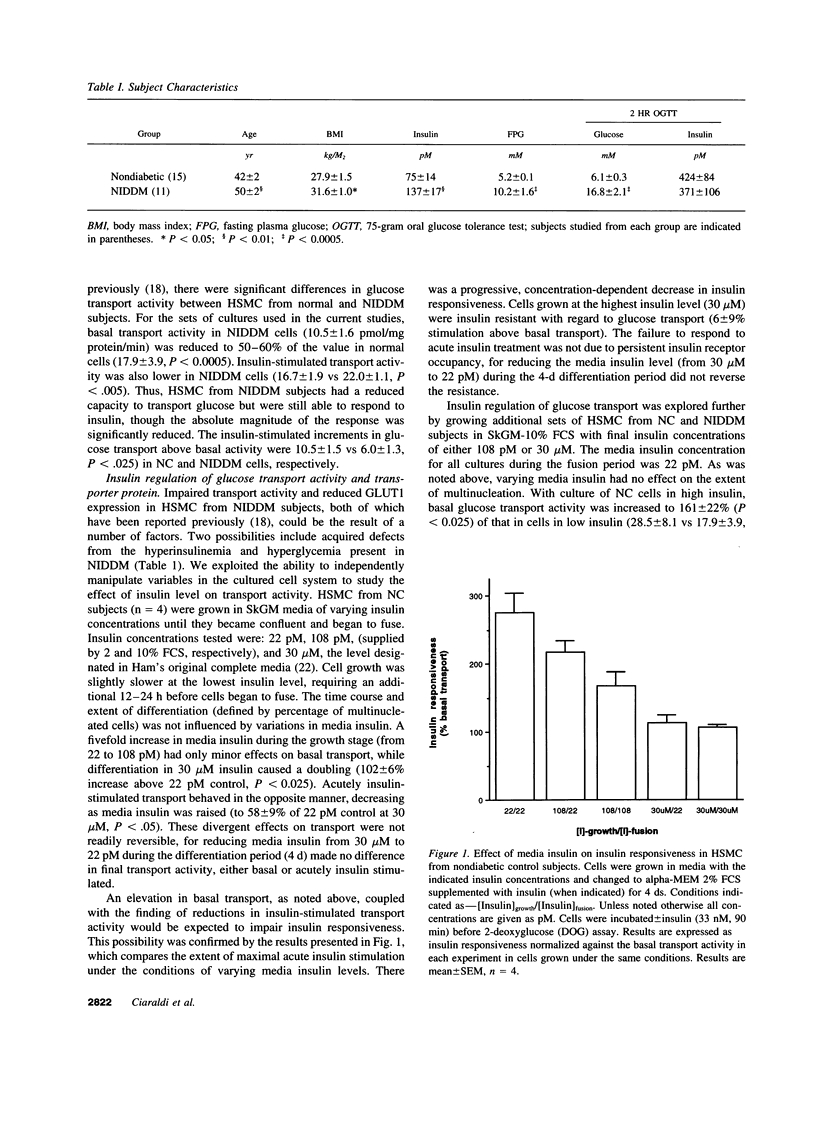
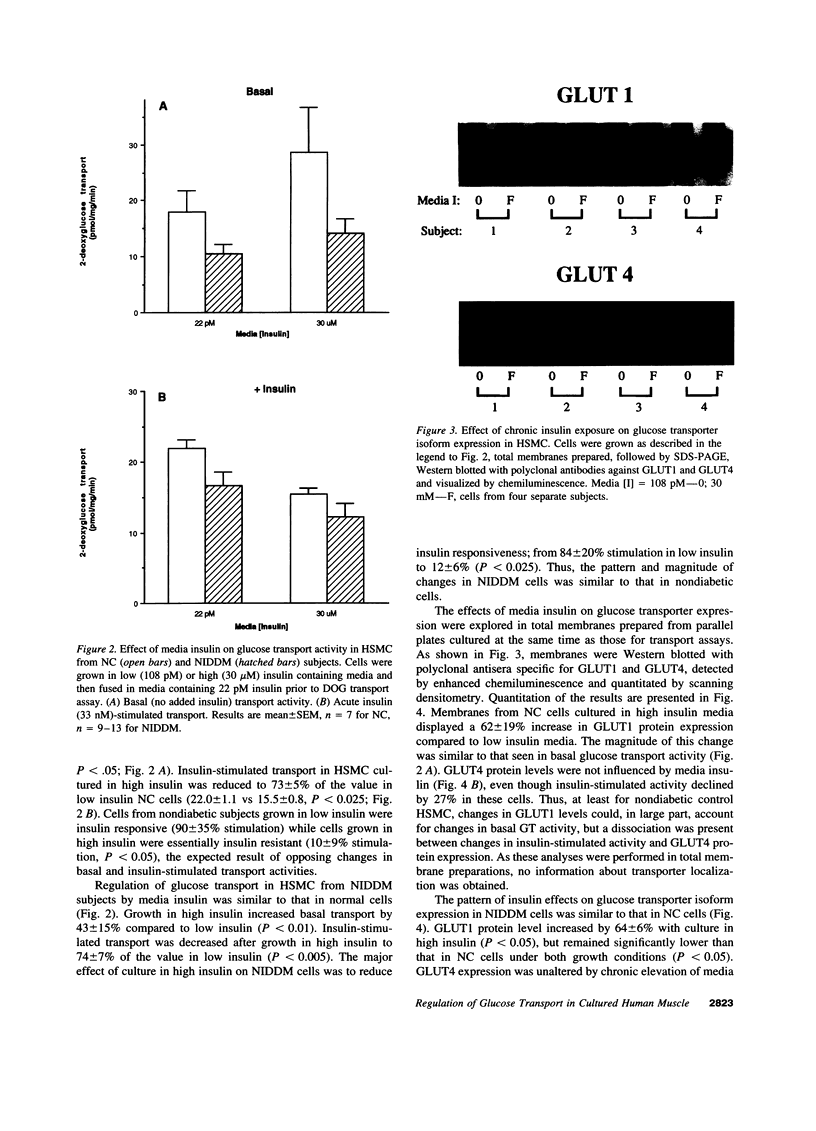
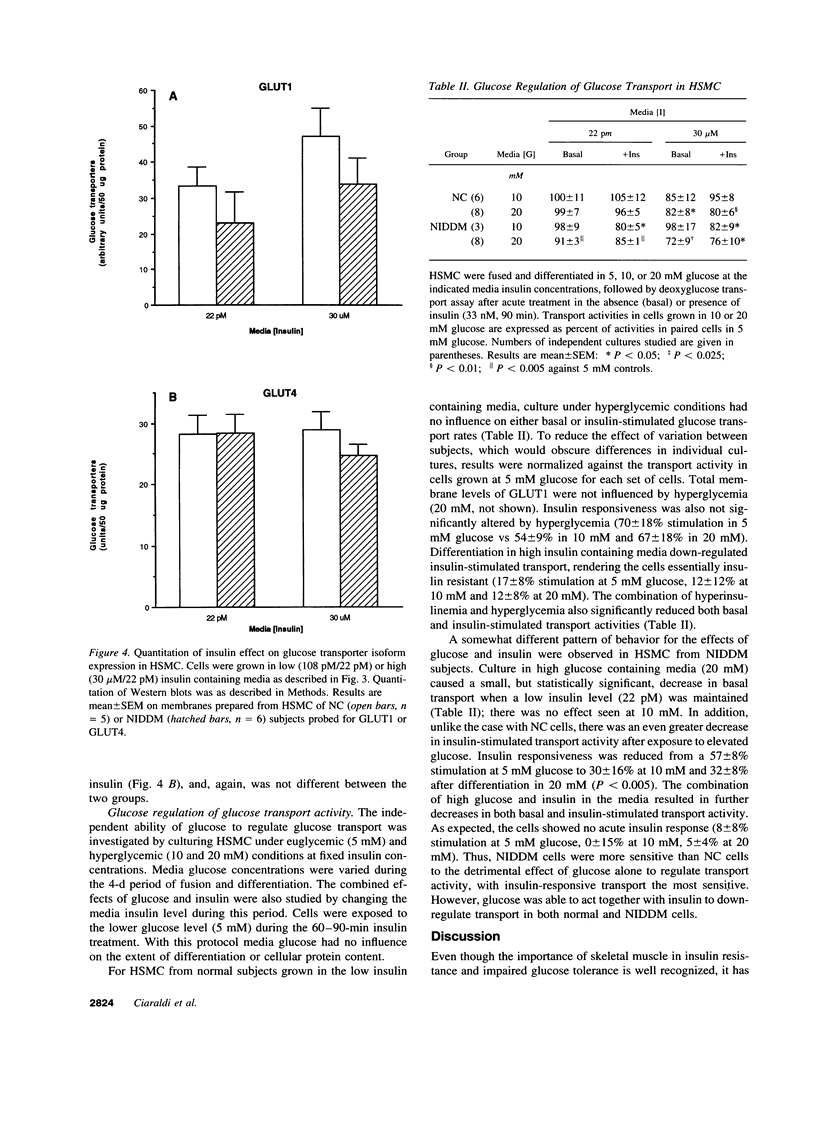



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Lund S., Vestergaard H., Junker S., Kahn B. B., Pedersen O. Expression of the major insulin regulatable glucose transporter (GLUT4) in skeletal muscle of noninsulin-dependent diabetic patients and healthy subjects before and after insulin infusion. J Clin Endocrinol Metab. 1993 Jul;77(1):27–32. doi: 10.1210/jcem.77.1.8325952. [DOI] [PubMed] [Google Scholar]
- Andréasson K., Galuska D., Thörne A., Sonnenfeld T., Wallberg-Henriksson H. Decreased insulin-stimulated 3-0-methylglucose transport in in vitro incubated muscle strips from type II diabetic subjects. Acta Physiol Scand. 1991 Jun;142(2):255–260. doi: 10.1111/j.1748-1716.1991.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Del Prato S., Saccomani M. P., Bonora E., Gulli G., Ferrannini E., Bier D., Cobelli C., DeFronzo R. A. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest. 1993 Jul;92(1):486–494. doi: 10.1172/JCI116592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Saccomani M. P., Seely L., Zych K. S., Ferrannini E., Cobelli C., DeFronzo R. A. Glucose transport in human skeletal muscle. The in vivo response to insulin. Diabetes. 1993 Jan;42(1):191–198. doi: 10.2337/diab.42.1.191. [DOI] [PubMed] [Google Scholar]
- Bonen A., Clark M. G., Henriksen E. J. Experimental approaches in muscle metabolism: hindlimb perfusion and isolated muscle incubations. Am J Physiol. 1994 Jan;266(1 Pt 1):E1–16. doi: 10.1152/ajpendo.1994.266.1.E1. [DOI] [PubMed] [Google Scholar]
- Bornemann A., Ploug T., Schmalbruch H. Subcellular localization of GLUT4 in nonstimulated and insulin-stimulated soleus muscle of rat. Diabetes. 1992 Feb;41(2):215–221. doi: 10.2337/diab.41.2.215. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Bouch C., Venkatesan N., Karjala R. G. Impaired glucose transport in skeletal muscle but normal GLUT-4 tissue distribution in glucose-infused rats. Am J Physiol. 1994 Dec;267(6 Pt 1):E808–E813. doi: 10.1152/ajpendo.1994.267.6.E808. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Del Prato S., Leonetti F., Simonson D. C., Sheehan P., Matsuda M., DeFronzo R. A. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994 Oct;37(10):1025–1035. doi: 10.1007/BF00400466. [DOI] [PubMed] [Google Scholar]
- Dimitrakoudis D., Ramlal T., Rastogi S., Vranic M., Klip A. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J. 1992 Jun 1;284(Pt 2):341–348. doi: 10.1042/bj2840341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Koranyi L., Bourey R., Schalin-Jäntti C., Widén E., Mueckler M., Permutt A. M., Groop L. C. Insulin resistance in type 2 (non-insulin-dependent) diabetic patients and their relatives is not associated with a defect in the expression of the insulin-responsive glucose transporter (GLUT-4) gene in human skeletal muscle. Diabetologia. 1992 Feb;35(2):143–147. doi: 10.1007/BF00402546. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Dohm G. L., Elton C. W., Rovira A., Chen J. J., Leggett-Frazier N., Atkinson S. M., Jr, Thomas F. T., Long S. D., Caro J. F. Muscle insulin resistance in uremic humans: glucose transport, glucose transporters, and insulin receptors. Am J Physiol. 1991 Jul;261(1 Pt 1):E87–E94. doi: 10.1152/ajpendo.1991.261.1.E87. [DOI] [PubMed] [Google Scholar]
- Galante P., Maerker E., Scholz R., Rett K., Herberg L., Mosthaf L., Häring H. U. Insulin-induced translocation of GLUT 4 in skeletal muscle of insulin-resistant Zucker rats. Diabetologia. 1994 Jan;37(1):3–9. doi: 10.1007/BF00428770. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Hancock J. A., Golichowski A. M., Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992 Apr;41(4):465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Matthaei S., Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987 Jan 5;262(1):189–197. [PubMed] [Google Scholar]
- Ham R. G., St Clair J. A., Webster C., Blau H. M. Improved media for normal human muscle satellite cells: serum-free clonal growth and enhanced growth with low serum. In Vitro Cell Dev Biol. 1988 Aug;24(8):833–844. doi: 10.1007/BF02623656. [DOI] [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Abrams L., Nikoulina S., Ciaraldi T. P. Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects. Comparison using human skeletal muscle cell cultures. Diabetes. 1995 Aug;44(8):936–946. doi: 10.2337/diab.44.8.936. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Thorburn A. W., Beerdsen P., Gumbiner B. Dose-response characteristics of impaired glucose oxidation in non-insulin-dependent diabetes mellitus. Am J Physiol. 1991 Jul;261(1 Pt 1):E132–E140. doi: 10.1152/ajpendo.1991.261.1.E132. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., Cushman S. W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990 Oct 25;265(30):18172–18179. [PubMed] [Google Scholar]
- Häring H. U., Kellerer M., Mosthaf L. Modulation of insulin receptor signalling: significance of altered receptor isoform patterns and mechanism of hyperglycaemia-induced receptor modulation. Diabetologia. 1994 Sep;37 (Suppl 2):S149–S154. doi: 10.1007/BF00400838. [DOI] [PubMed] [Google Scholar]
- Klip A., Li G., Logan W. J. Induction of sugar uptake response to insulin by serum depletion in fusing L6 myoblasts. Am J Physiol. 1984 Sep;247(3 Pt 1):E291–E296. doi: 10.1152/ajpendo.1984.247.3.E291. [DOI] [PubMed] [Google Scholar]
- Klip A., Marette A. Acute and chronic signals controlling glucose transport in skeletal muscle. J Cell Biochem. 1992 Jan;48(1):51–60. doi: 10.1002/jcb.240480109. [DOI] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Olefsky J. M., Brechtel G., Wallace P., Baron A. D. Kinetics of in vivo muscle insulin-mediated glucose uptake in human obesity. Diabetes. 1990 Aug;39(8):965–974. doi: 10.2337/diab.39.8.965. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalande M. E., Ling V., Miller R. G. Hoechst 33342 dye uptake as a probe of membrane permeability changes in mammalian cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):363–367. doi: 10.1073/pnas.78.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M. Crosstalk among multiple signal-activated phospholipases. Trends Biochem Sci. 1992 Oct;17(10):393–399. doi: 10.1016/0968-0004(92)90007-v. [DOI] [PubMed] [Google Scholar]
- Marette A., Richardson J. M., Ramlal T., Balon T. W., Vranic M., Pessin J. E., Klip A. Abundance, localization, and insulin-induced translocation of glucose transporters in red and white muscle. Am J Physiol. 1992 Aug;263(2 Pt 1):C443–C452. doi: 10.1152/ajpcell.1992.263.2.C443. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994 Feb 1;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen B. F., Hansen S. A. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J. 1988 Jun 15;252(3):733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabia V., Lam L., Burdett E., Leiter L. A., Klip A. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest. 1992 Oct;90(4):1386–1395. doi: 10.1172/JCI116005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabia V., Ramlal T., Klip A. Glucose uptake in human and animal muscle cells in culture. Biochem Cell Biol. 1990 Feb;68(2):536–542. doi: 10.1139/o90-076. [DOI] [PubMed] [Google Scholar]
- Sargeant R., Mitsumoto Y., Sarabia V., Shillabeer G., Klip A. Hormonal regulation of glucose transporters in muscle cells in culture. J Endocrinol Invest. 1993 Feb;16(2):147–162. doi: 10.1007/BF03347669. [DOI] [PubMed] [Google Scholar]
- Sasson S., Edelson D., Cerasi E. In vitro autoregulation of glucose utilization in rat soleus muscle. Diabetes. 1987 Sep;36(9):1041–1046. doi: 10.2337/diab.36.9.1041. [DOI] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Yki-Järvinen H., Koranyi L., Bourey R., Lindström J., Nikula-Ijäs P., Franssila-Kallunki A., Groop L. C. Effect of insulin on GLUT-4 mRNA and protein concentrations in skeletal muscle of patients with NIDDM and their first-degree relatives. Diabetologia. 1994 Apr;37(4):401–407. doi: 10.1007/BF00408478. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Wallace P., Henry R. R. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest. 1990 Feb;85(2):522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Donovan J. A., Doering T. P., Sandra A., Klip A., Pessin J. E. Insulin and glucose-dependent regulation of the glucose transport system in the rat L6 skeletal muscle cell line. J Biol Chem. 1989 Apr 15;264(11):6587–6595. [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Sarabia V., Koivisto U. M., Bilan P. J., Pessin J. E., Klip A. Glucose transport activity in L6 muscle cells is regulated by the coordinate control of subcellular glucose transporter distribution, biosynthesis, and mRNA transcription. J Biol Chem. 1990 Jan 25;265(3):1516–1523. [PubMed] [Google Scholar]
- Young D. A., Uhl J. J., Cartee G. D., Holloszy J. O. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem. 1986 Dec 5;261(34):16049–16053. [PubMed] [Google Scholar]
- Young D. A., Uhl J. J., Cartee G. D., Holloszy J. O. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem. 1986 Dec 5;261(34):16049–16053. [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]