Abstract
A hallmark of rheumatoid arthritis is the formation of an aggressive, tumor-like structure called pannus that erodes the joint. A major cellular component of the pannus is the fibroblast-like synoviocyte (FLS), whose morphology strikingly resembles that of a transformed cell, but underlying mechanisms of this “transformation” are not known. Here, using animal models of rheumatoid arthritis, we show that arthritic FLS contain a substantial (>30%) fraction of bone marrow-derived precursors that can differentiate in vitro into various mesenchymal cell types, but inflammation prevents the multilineage differentiation. We show that the transcription factor NF-κB plays the key role in the repression of osteogenic and adipogenic differentiation of arthritic FLS. Furthermore, we show that specific activation of NF-κB profoundly enhances proliferation, motility, and matrix-degrading activity of FLS. We thus propose that arthritic FLS are mesenchymal stem cells whose differentiation is arrested at early stages of differentiation by activation of NF-κB.
Keywords: fibroblast-like synoviocytes, mesenchymal stem cell, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease that mainly affects joints. Hallmarks of RA pathology are chronic inflammation and pronounced synovial hyperplasia. Normal synovial membrane is a delicate tissue structure that lines the joint cavity and is comprised of one to two layers of cells. Two major populations of resident synoviocytes can be distinguished: macrophage-like (or type A) synoviocytes and fibroblast-like (or type B) synoviocytes (FLS). Onset of RA causes dramatic morphological changes in the synovial membrane, including a thickening of the intimal lining and the formation of an aggressive, tumor-like structure called pannus that invades and erodes the cartilage and the subchondral bone (1).
The pannus is comprised of many cell types, including inflammatory, immune, and mesenchymal cells. The latter, referred to as RA FLS, play a major role in the invasive joint destruction by producing large quantities of enzymes degrading the joint matrix. The RA FLS resemble immature, transformed fibroblasts and are in many ways different from normal FLS. Compared with normal FLS, RA FLS have higher proliferation rates and are highly invasive. Unlike normal FLS, RA FLS invade and degrade cartilage when coengrafted into appropriate animal strains (2, 3).
The mechanisms underlying the tumor-like phenotype of RA FLS are poorly understood. There have been attempts to explain the tumor-like phenotype of RA FLS by dedifferentiation of normal FLS, presumably through an accumulation of genetic and epigenetic abnormalities, similar to that occurring in cancer (4). In support of this hypothesis, a number of protooncogenes, including myc, ras, and fos, are overexpressed in RA FLS (5), albeit no common genetic abnormalities in these genes have been identified. Somatic mutations in the tumor-suppressor gene p53 were associated with RA FLS (6), but a role for these mutations to the “pseudotransformed” RA FLS phenotype is not known. Another point against the “transformation” hypothesis is the slowing of the rapid growth of RA FLS after several passages in culture (7).
The origin of RA FLS is also matter of debate. According to one theory, RA FLS stem from normal FLS. According to another hypothesis, RA FLS are subintimal fibroblasts that migrated to the RA synovium and acquired the altered morphology (8). More recently, it was found that a fraction of the heterogeneous RA FLS population has properties one usually associates with mesenchymal stem cells (MSC). When stimulated in culture with appropriate stimuli, a proportion of RA FLS can differentiate into chondrocytes, osteoblasts, adipocytes, and muscle cells (9, 10). Interestingly, systemically injected purified MSC were shown to home to different joint compartments, e.g., muscles, cartilage, and bones (11, 12).
In the present work we used animal models of RA to elucidate contribution of bone marrow (BM)-derived MSC to synovial hyperplasia in arthritis and to examine the molecular mechanisms underlying the abnormal phenotype of arthritic FLS. We show that BM-derived MSC are present among FLS isolated from normal joints and that the fraction of the BM-derived mesenchymal cells dramatically increases with the onset of arthritis. Furthermore, we demonstrate that differentiation of arthritic FLS into osteoblasts or adipocytes can be prevented by inflammatory cytokines, e.g., IL-1, and the activation of the transcription factor NF-κB appears necessary and sufficient for the suppression of differentiation. Furthermore, specific activation of the NF-κB pathway alone dramatically increases FLS proliferation and produces a highly invasive cell phenotype characterized by a profound increase in cell motility and by constitutive secretion of active matrix metalloproteinases (i.e., MMP-13). These data suggest that synovial hyperplasia in arthritis occurs because of an impairment of a normal physiological mechanism that provides the repair of joint tissues. That is, we propose that RA FLS are bona fide BM-derived pluripotent mesenchymal cells that are recruited to the arthritic joint in large quantities and are arrested at various stages of differentiation by inflammation. The activation of NF-κB appears to play the central role in the differentiation block, as well as in the maintenance of the high invasiveness and abnormal motility of the recruited cells.
Results
Normal FLS Contain a Minor Fraction of BM-Derived Mesenchymal Cells.
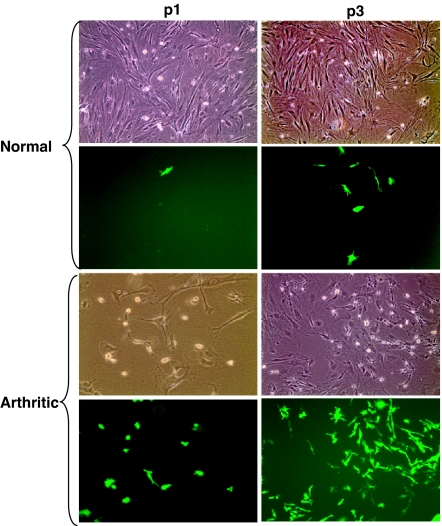
When purified MSC are injected in circulation or i.p., progenitors of these cells can be found in different joint compartments, including muscles and bone (11, 13). However, because the normal physiological levels of MSC in circulation are extremely low, the high concentrations of administered MSC may produce artificial results. Thus, we used another model, i.e., BM transplantation (BMT) of BM cells from GFP-transgenic donor mice into lethally irradiated GFP-negative recipients (14, 15). To assess GFP-positive mesenchymal cells in joints, primary FLS from synovial explants were isolated at 2 months after transplantation. As the rapidly dividing FLS overgrow nondividing macrophage-like synoviocytes, the vast majority of cell culture after two to three passages is represented by mesenchymal cells (1). We found that, at a third passage, GFP-positive cells had a fibroblast-like appearance and comprised 1.2 ± 0.2% of the FLS population (Fig. 1). Thus, a minor fraction of FLS in normal joints is represented by BM-derived mesenchymal precursors.
Fig. 1.
The presence of BM-derived cells in the populations of normal and arthritic FLS. BM cells from GFP transgenic donor mice were transferred into lethally irradiated GFP-negative recipient mice. Two months later, primary FLS were established by an enzymatic dispersal of synovial explants of the recipient BMT mice. p1 and p3, the first and third passages of primary FLS. The first and third rows represent phase-contrast microscopy; the second and fourth rows represent fluorescent microscopy. Normal, primary FLS that were obtained from normal joints of the BMT mice. At the third passage, GFP-positive cells comprised 1.2 ± 0.2% of the population of normal FLS (mean ± SEM of two experiments). Arthritic, primary FLS that were obtained from arthritic BMT recipient mice as described in Materials and Methods. At the third passage, GFP-positive cells comprised 33.7 ± 1.6% of the population of arthritic FLS (mean ± SEM of three experiments). (Original magnification: ×100.)
Migration of BM-Derived Mesenchymal Cells into the Joint Dramatically Increases in Arthritis.
Antigen-induced arthritis in the GFP BMT mice was induced by immunizing the animals with intradermal injection of methylated BSA (mBSA) followed by an intraarticular (i.a.) injection of mBSA 3 weeks later. Three more days later, FLS cells were isolated by dispersal of synovial explants from the antigen-induced arthritis joints. At the third passage, GFP-positive cells comprised a substantial fraction (33.7 ± 1.6%) of the arthritic FLS (Fig. 1). Therefore, arthritis development resulted in a dramatically increased influx of BM-derived mesenchymal cells into synovium.
Inflammatory Cytokines Prevent Differentiation of Arthritic FLS Through the Activation of NF-κB.
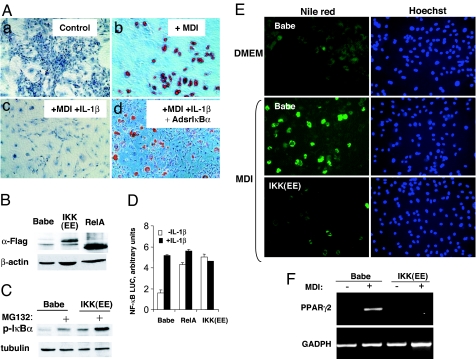
One hallmark of RA joints is a high concentration of inflammatory cytokines TNFα and IL-1β. It is known that both TNFα and IL-1β can inhibit the multilineage differentiation of transformed MSC lines, e.g., C2C12 and C3H10T1/2 cells (16, 17). Because primary cultures of RA FLS have a fraction of pluripotent cells that can differentiate into chondrocytes, osteoblasts, adipocytes, and muscle cells (9, 10), we examined whether inflammation could also affect differentiation of arthritic FLS. In these experiments we used primary FLS derived from rats (rFLS) with PG-PS-induced arthritis (instead of mouse FLS from the BMT GFP mice of which we had rather limited supply). We found that, upon incubation in an adipogenic medium, a proportion of rFLS had differentiated into adipocytes. The adipocyte differentiation was practically abolished by addition of IL-1β to the adipogenic medium (Fig. 2A a–c).
Fig. 2.
NF-κB activation is necessary and sufficient to inhibit the adipogenic differentation of arthritic rFLS. (A) NF-κB is required for the antidifferentiating activity of IL-1 in the adipogenic differentiation of arthritic rFLS. Primary FLS from rats with PG-PS-induced arthritis were infected with a control, “empty” adenovirus (Control) (a–c) or with adenovirus expressing superrepressor IκBα (AdsrIκBα) (d). Cells were incubated in DMEM (a) or in an adipogenesis-inducing medium (MDI) (b), or in the MDI medium in the presence of IL-1β (100 units/ml) (c and d). After 21 days, differentiated cells were visualized by staining the accumulated lipids with an Oil red stain. Representative data of three experiments are shown. (Original magnification: ×100.) (B–D) Constitutive activation of NF-κB by retrovirus-mediated gene transfer of a dominant-positive IKKβ and RelA. Primary rFLS were transduced with a control RV Babe (control Babe) or with vectors that expressed cDNA of FLAG-tagged constitutively active IKKβ mutant [IKK(EE)], or FLAG-tagged RelA cDNA. After puromycin selection, NF-κB activation was assessed. (B) The expression of exogenous IKK(EE) and RelA proteins was assessed by immunoblotting with anti-FLAG Ab. (C) The expression of IKK(EE) results in accumulation of a phosphorylated form of IκBα. The control (Babe) and IKK(EE) cells were detected by using antiphospho IκBα Ab. Where indicated, cells were pretreated with a proteasomal inhibitor, MG132, to allow for accumulation of the phosphorylated IκBα. (D) Ectopic expression of IKK(EE) and RelA induces constitutive activation of NF-κB-driven gene expression. Transduced rFLS were transiently cotransfected with an NF-κB-inducible reporter vector expressing the firefly luciferase and a constitutively active reporter expressing the Renilla luciferase. Normalized values of NF-κB activation from duplicated samples are shown. Representative data of two experiments are shown. (E and F) Constitutive activation of NF-κB inhibits the adipogenic differentiation of arthritic rFLS. (E) RV-transduced cells (as in B) were incubated in a control medium (DMEM) or in an adipogenesis-inducing medium (MDI). After 21 days, the adipogenic differentiation was assessed by staining accumulated lipids with Nile red. The staining was visualized by fluorescent microscopy (Left). (Original magnification: ×100.) Cells were counterstained with the nuclear stain Hoechst 33342 (Right). (F) The expression of an adipogenic differentiation marker (PPARγ2) was assessed in cells shown in B by RT-PCR.
The transcription factor NF-κB is a common downstream target of both TNFα and IL-1β that has been implicated into multiple aspects of RA joint pathology (18, 19). NF-κB activation is also known to inhibit multiple pathways of MSC differentiation, including the chondrocytic, myoblast, and adipocytic differentiation (16, 17). The role for NF-κB in osteogenic differentiation is less known, and some observations suggest that the activation of NF-κB may rather promote the osteogenic differentiation (20). To assess the potential role for NF-κB in differentiation of arthritic FLS, we infected the cells with an adenovirus vector constitutively expressing “superrepressor” IκBα (21), a nondegradable form of IκBα that potently inhibits NF-κB (22). In the presence of srIκBα, IL-1β failed to block the adipogenic differentiation, indicating that NF-κB activation was required for the “antidifferentiating” activity of IL-1β (Fig. 2A c and d).
Because NF-κB is one of many downstream targets of inflammatory cytokines, we assessed whether NF-κB activation would be sufficient to prevent differentiation of FLS. To selectively activate NF-κB, we used two approaches. In one approach, cells were transduced with a retroviral vector (RV) expressing RelA, a transcriptionally active subunit of NF-κB (Fig. 2B). In another approach, cells were transduced with a RV expressing a constitutively active form of IκB kinase β [IKK(EE)] (23) that catalyzes the phosphorylation-dependent degradation of IκB (Fig. 2 B and C). Both approaches caused a sustained NF-κB activation, as evidenced by elevated NF-κB-dependent reporter gene transcription (Fig. 2D).
Initially we examined the role for NF-κB in adipogenic differentiation of rFLS. When incubated in an adipogenesis-inducing medium (MDI), a fraction of rFLS with an empty pBabe vector underwent adipogenic differentiation, as evidenced by lipid accumulation (Fig. 2E) and by the expression of PPARγ2, a marker of adipogenic differentiation (Fig. 2F). The adipogenic differentiation was potently inhibited by ectopic expression of CA IKKβ (Fig. 2 E and F) and of RelA (data not shown).
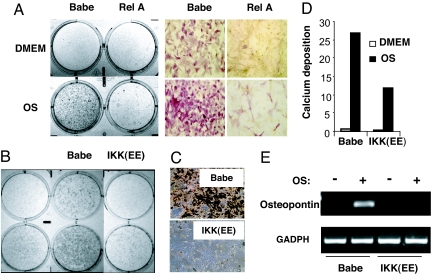
To determine whether this antidifferentiation role of NF-κB was restricted to the particular (adipogenic) differentiation pathway, we examined the osteogenic differentiation of rFLS. We found that the selective activation of NF-κB also prevented the osteogenic differentiation. When cultured in an osteogenic medium, a substantial fraction of rFLS transduced with the control (Babe) retrovirus underwent the osteogenic differentiation, as evidenced by increased enzymatic activity of alkaline phosphatase (ALP) (Fig. 3A and B), by calcium deposition (Fig. 3 C and D), and by increased expression of the osteoblast differentiation markers osteopontin (Fig. 3E) and osteocalcin (data not shown). The osteogenic differentiation was potently inhibited by ectopic expression of either RelA or CA IKKβ (Fig. 3). Therefore, the activation of NF-κB appeared sufficient to inhibit differentiation of rFLS into osteoblasts and adipocytes.
Fig. 3.
Constitutive activation of NF-κB inhibits osteogenic differentiation of arthritic rFLS. RV-transduced cells (as in Fig. 2B) were incubated in a control medium (DMEM) or in an osteogenesis-inducing medium (OS). (A) The activity of ALP in the control (Babe) and in RelA-transduced cells after 14 days of incubation. (B) ALP activity in the control and IKK(EE)-transduced cells after 14 days of incubation. (C) Mineral deposition in the control (Babe) and in IKK(EE)-transduced cells after 21 days of incubation, as assessed by silver staining. (Original magnification in A and C: ×100.) (D) Calcium deposition in cells shown in C was quantitatively assessed and normalized on protein content. (E) The expression of an osteogenic differentiation marker (osteopontin) in cells shown in B as assessed by RT-PCR.
Constitutive NF-κB Activation Accelerates FLS Proliferation.
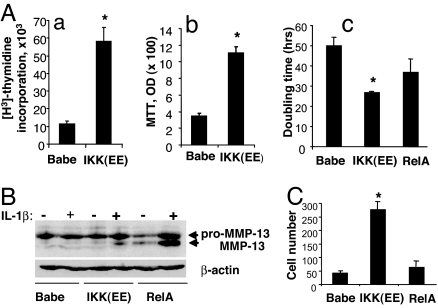
In an earlier work we showed that NF-κB activation in primary FLS was required for transmitting mitogenic responses to platelet-derived growth factor (24). Here we found that, in the absence of growth factors, NF-κB activation alone was sufficient to accelerate FLS proliferation, as evidenced by an increase in DNA synthesis rate and by MTT assay (Fig. 4A a and b). The FLS with constitutively active NF-κB had shorter doubling time (26.8 ± 0.5 h in CA IKKβ-RV transduced FLS vs. 50 ± 4 h in control FLS transduced with an empty RV; P < 0.005) (Fig. 4Ac). The overexpression of RelA also somewhat shortened the doubling time (37 ± 7 h vs. 50 ± 4 h in control cells), but this was not a statistically significant difference (P = 0.167) (Fig. 4Ac).
Fig. 4.
Constitutive activation of NF-κB accelerates cell growth and enhances the invasiveness of arthritic rFLS. (A) Constitutive activation of NF-κB accelerates cell growth. Cells were transduced as described for Fig. 2B, and proliferation was assessed by determining thymidine incorporation (a) or by MTT proliferation assay (b). (Ac) To directly assess cell growth, cells were plated at a low density and counted after 5 days in culture. The numbers of cells was adjusted to plating efficacy that was assessed 1 day after plating. Doubling times were calculated assuming an exponential cell growth. Representative data of three experiments are shown. ∗, P < 0.05 vs. Babe control. (B) The production of preproactive and active forms of MMP-13 protein by transduced cells was assessed by immunoblotting in lysates of cells that were unstimulated or stimulated for 4 h with IL-1β (100 units/ml). The blot was reprobed with β-actin Ab. (C) Constitutive activation of NF-κB enhances invasiveness. Cell motility was assessed in a transwell cell migration assay as described in Materials and Methods. The cells that migrated through the filter were stained and counted. One representative experiment from three independent experiments is shown.
Constitutive NF-κB Activation Stimulates the Production of Active MMP-13 by FLS.
One mechanism whereby RA FLS contribute to destruction of the cartilage and bone is through secretion of large quantities of MMPs. Promoters of many MMPs (e.g., MMP-1, MMP-3, and MMP-13) contain NF-κB binding sites that are required for their transcriptional activation by inflammatory cytokines (25). The MMP-13 is particularly important in RA pathology (25, 26). Like most MMPs, the MMP-13 is secreted as a proenzyme that has to be clipped to become an active enzyme. In our hands, IL-1β caused a moderate (if any) increase in the production of pre-MMP-13 in FLS, but constitutive activation of NF-κB (in CA IKKβ- or RelA-overexpressing cells) strongly potentiated the induction of MMP-13 by IL-1β (Fig. 4B). Quite unexpectedly, the constitutive activation of NF-κB also strongly enhanced the production of active MMP-13, the effect particularly pronounced in RelA-overexpressing cells, wherein large amounts of active MMP-13 were evident even in the absence of IL-1 stimulation (Fig. 4B). This suggests NF-κB-dependent mechanism(s) that act independent of the transcriptional activation of MMP-13 gene transcription by accelerating the processing of the MMP-13 proenzyme.
Constitutive NF-κB Activation Enhances FLS Motility.
Cell motility is another factor determining cells' invasiveness. For example, increased motility casually relates to increased invasiveness and metastasis of cancer cells. We used a transwell migration assay to assess an impact of constitutively active NF-κB on FLS motility. The overexpression of RelA had a weak effect (≈1.5-fold increase of the motility), whereas the overexpression of CA IKKβ had a dramatic effect on the motility (a 6.5-fold increase over the control cells) (Fig. 4C). Combined with data on enhanced production and processing of MMP-13, these results suggest that activation of NF-κB strongly enhances the invasiveness of FLS.
Discussion
The RA FLS differ in many aspects from the normal FLS. One of the most pronounced phenotypic features is the high invasiveness of RA FLS that correlates with the elevated production of inflammatory cytokines and MMPs. Under appropriate conditions, this phenotype can be sustained for many weeks: for example, unlike their normal counterparts, RA FLS, being coengrafted with a piece of cartilage in renal cavity of permissive mouse, invade and erode the cartilage (2, 5). The highly invasive phenotype as well as elevated expression of protooncogenes, matrix proteinases, and accumulation of DNA mutations in RA FLS are similar to that occurring in transformed fibroblasts, hence the attempts to explain the RA FLS phenotype by a pseudotransformation of normal FLS, presumably stemming from the chronic exposure of these cells to the inflammatory environment of RA joints (1, 4). According to this theory, the RA FLS represent dedifferentiated progenitors of resident FLS. However, the pseudotransformation hypothesis has many difficulties, e.g., the molecular mechanisms underlying this “dedifferentiation” are yet to be shown. We propose here an alternative explanation.
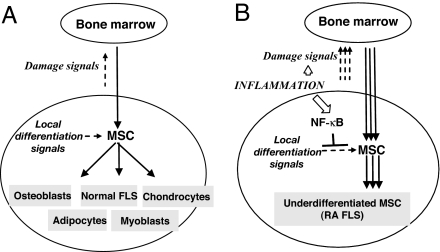
Under normal physiological conditions, the musculoskeletal system is subjected to persistent mechanical stress. To keep the physiological wearing and tearing in check, there are repair mechanisms that renew the damaged tissues, and BM-derived MSC appear central to this process. For example, it is well established that muscle damage stimulates influx of MSC into the muscle compartment, the influx being commensurate with the damage. The recruited MSC differentiate into myoblasts thereby repairing the damage (13). Because MSC can also differentiate into chondrocytes, osteoblasts, adipocytes, and other mesenchymal cell types, similar mechanisms should operate to provide the repair of the bone, cartilage, and other tissues of the joint under the normal physiological conditions. The recruited MSC receive microenvironmental clues that instruct cells to differentiate into corresponding cell types (chondrocytes, osteoblasts, “normal” FLS, etc.). The intensity of the damage signals should be commensurate with wearing and tearing of joint tissues so that MSC influx would be appropriately regulated (Fig. 5A).
Fig. 5.
Constitutive activation of NF-κB in arthritic joints maintains the highly invasive, undifferentiated phenotype of arthritic FLS.
We postulate that diverse arthritopathogenic stimuli, e.g., chronic viral or bacterial infection or persistent bacterial products, may produce persistent and elevated levels of damage signals, presumably through inducing chronic inflammation in the joint space. The persisting damage signals will perpetuate MSC influx to the joint. On the other hand, inflammatory milieu in arthritic joints induces and maintains NF-κB activation in the recruited MSC, thereby blocking the microenvironmental differentiation signals and sustaining cells in an underdifferentiated state (Fig. 5B). Therefore, according to this model, the RA FLS appear bona fide MSC that are arrested at various stages of differentiation into different lineages.
According to our hypothesis, RA FLS appear as underdifferentiated precursors, rather than dedifferentiated progenitors, of resident FLS cells. In line with that, we believe that “pannocytes,” another primary cell type isolated from the pannus/cartilage interface of RA joints (whose transient phenotype resembles both chondrocytes and fibroblasts) (10), may be considered as MSC arrested in intermediate stages of chondrogenic differentiation. Although we focused on only the osteogenic and adipogenic differentiation of primary FLS, experimental data by others (in transformed MSC cell lines) suggest that inflammation-inducible NF-κB activation can prevent multiple (e.g., myogenic and chondrogenic) differentiation pathways (16, 17). Thus, we expect that a thorough examination of primary RA FLS cell cultures may reveal other transient phenotypes, e.g., fibroblasts/osteoblasts, fibroblasts/adipocytes, fibroblasts/myoblasts, etc.
Another corollary of our model is that neutralizing the damage signals may have therapeutic effects in RA. This brings the question about the nature of the damage signals in the normal and in RA joints. Among the soluble cytokines that may serve as a damage signal, one evident candidate is the inflammatory cytokine TNFα. For example, it has been shown that, in mice with collagen-induced arthritis, the canals connecting the joint space with the BM compartment are enlarged, resulting in an enhanced migration of BM cells to the joint, whereas TNFα inhibitors inhibited the migration (12). However, this does not rule out a role for other chemokines in regulating the recruitment of BM-derived MSC into the joints (through the canals or from the circulation). It would be also of interest to investigate the role of the chemokine receptors that mediate the recruitment of leukocytes to RA joints and to assess a possible involvement of these receptors in the recruitment of the mesenchymal cells. NF-κB may play an important role in the generation of the damage signals, owing to the essential role for NF-κB in chronic inflammation (27).
Our results indicate a central role for NF-κB in RA FLS differentiation. A pivotal role of NF-κB in inflammatory responses has been well established, so that NF-κB became synonymous with chronic inflammation and is considered a hallmark of RA pathology. Others and we have shown the numerous roles for NF-κB in many aspects of RA pathology (inflammation, T cell activation, FLS proliferation, and apoptosis) (18). Here we demonstrate yet another mechanism whereby NF-κB contributes to RA pathology. We show that NF-κB activation prevents the multilineage pathways of RA FLS differentiation while dramatically increasing the proliferation, the motility, and the production of active MMP-13. Therefore, NF-κB is central to the invasive phenotype one associates with RA FLS.
There is an evident implication of our study for the development of treatments targeting RA-associated bone resorption. Bone tissue turnover is controlled by the balance of two processes, i.e., the bone resorption (mediated by osteoclasts) and bone synthesis (mediated by osteoblasts). It is well known that NF-κB activation in hematopoietic precursors promotes the osteoclast differentiation (e.g., by mediating RANK-induced signaling) and increases the osteolytic activity of osteoclasts (28). Opposite to that, our results show that NF-κB activation in mesenchymal FLS inhibits the osteoblast differentiation of arthritic FLS. Thus, using NF-κB inhibitors, on one hand, should block the osteoclast differentiation, and, on the other hand, it should promote osteoblast differentiation and thus may provide very efficacious treatment of RA-associated bone resorption.
Interestingly, although NF-κB suppression had similar effects on osteogenic and adipogenic differentiation pathways, we found that, by acting on different steps of the NF-κB pathway in FLS, we had produced somewhat different phenotypes. For example, inducing constitutive activation of NF-κB by overexpressing RelA had most pronounced effect on MMP-13 secretion, whereas overexpressing CA IKKβ most profoundly affected cell motility and proliferation. Thus, it should be possible to tailor distinct NF-κB inhibitors to specific therapeutic applications by interfering at different points of the signaling pathways that control NF-κB activation (19).
The precise molecular mechanisms whereby NF-κB activation impinges on the multiple differentiation pathways in arthritic FLS remain to be identified. The fact that NF-κB activation was equally important for different differentiation pathways suggests that there may be a unified mechanism that acts in these pathways. In this regard, recent publications indicate that NF-κB can modulate the differentiation of mesenchymal-derived cell lineages via RNA sequence-dependent, posttranscriptional down-regulation of key developmental regulators, including Sox9 and MyD (17).
In summary, our study suggests that the abnormal phenotype of RA FLS may stem from an aberrant activation of mechanisms that normally serve to provide physiological repair of joint tissues. Arthritogenic stimuli provide aberrant production of damage signals that cause sustained influx of BM-derived MSC to the joint. Thus, the RA FLS appear as a heterogeneous population of underdifferentiated MSC that are arrested at intermediate stages of differentiation by the inflammatory milieu. The sustained activation of NF-κB plays a central role in blocking the multilineage differentiation and in maintaining the highly invasive phenotype by the RA FLS.
Materials and Methods
All tissue culture materials were from Gibco (Grand Island, NY). Unless stated otherwise, all other chemicals were from Sigma (St. Louis, MO). Human recombinant IL-1β and TNFα were from Roche Applied Science (Indianapolis, IN). MG132 was from Peptides International (Louisville, KY). All work in animals was carried out in strict accordance with the guidelines for humane care and use of laboratory animals in University of North Carolina (Chapel Hill).
BMT.
The eGFP transgenic mice (on C57BL/6 background) wherein eGFP was ubiquitously expressed under the control of the chicken β-actin promoter/CMV immediate early enhancer were a gift from Jon Serody (University of North Carolina, Chapel Hill, NC) (29). The BM cells were obtained from the eGFP transgenic mice at the age of 2–3 months by flushing the cavity of tibias and femurs. A single-cell suspension was obtained by centrifuging in Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden). Recipient C57BL/6 mice (aged 8 weeks; Charles River Laboratories, Wilmington, MA) were lethally irradiated with 9.5-Gy 60Co γ rays. Approximately 20 h after irradiation they received 1 × 106 donor GFP positive BM cells intravenously. At 60 days after transplantation, GFP-positive cells comprised >80% of BM cells and >92% of circulating leukocytes in the recipient animals, as determined by FACS analysis.
mBSA-Induced Arthritis.
Antigen-induced arthritis in mice was induced as described (30). Two months after the BMT, the recipient mice were immunized intradermally at the base of the tail with 100 μg of mBSA emulsified in 100 μl of complete Freud's adjuvant containing 5 mg/ml H37RA Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI). Three weeks later, the primed mice were i.a. injected with mBSA (20 μg) at the knee joints. Three days after the i.a. injection, primary FLS cultures were established from synovial explants by enzymatic dispersal (24).
rFLS Cell Culture.
Arthritic rFLS were isolated from joints of rats with PG-APS-induced arthritis, as described (22). Synovium explants were obtained at a chronic phase of arthritis, digested with collagenase IV in serum-free DMEM for 3 h at 37°C, filtered through a nylon mesh, and incubated overnight in a humidified 5% CO2 atmosphere at 37°C in a DMEM culture medium supplemented with 10% FBS, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. After the removal of nonadherent cells, adherent cells were cultivated in a fresh medium. Cells were split at a 1:3 ratio. Cells from passages 3–8 were used in these experiments.
Flow Cytometry and Cell Sorting.
eGFP expression in BM cells and in circulating leukocytes was analyzed by using MoFlo cell sorter (Cytomation, Fort Collins, CO). Data were analyzed with Summit software (Cytomation).
Retroviral Transduction and Adenoviral Infection.
VSV-G pseudotyped recombinant Moloney murine leukemia virus retroviruses were generated in E-23 cells packaging cell line that stably expressed gag and pol proteins (31). The packaging cells were transiently cotransfected with an expression plasmid pVSVG carrying cDNA of the vesicular stomatitis virus envelope protein and pBabe vector carrying cDNA of the transgene [human IKKβEE (177/181EE) or wt RelA] and a Puro resistance marker gene (32). The titers of the retrovirus in the supernatants were in the range of 3 × 105 to 3 × 106 cfu/ml. Cells were transduced by incubation with retrovirus-containing supernatants in the presence of Polybrene and selected in a medium containing 6 μg/ml puromicin. Recombinant Ad5-based vector carrying the cDNA of superrepressor IκBα (32/36 AA) was described previously (22). Cells were infected with the adenovirus at the multiplicity of infection of 200 particles per cell.
Adipogenic and Osteogenic Differentiation.
Postconfluent FLS were incubated in adipogenic induction medium (MDI) as described elsewhere (33). To induce osteogenic differentiation, FLS at 90% confluence were incubation in osteogenesis-inducing medium containing with 50 μg/ml ascorbic acid, 100 nM dexamethasone, 10 mM β-glycerol phosphate, and 10% FBS (33). On day 14, cells were fixed and ALP expression was assessed with Diagnostic Kit 86 from Sigma. At day 21, mineral deposition was assessed by staining with 2% silver nitrate according to the von Kossa method (34). In parallel samples, deposited calcium was extracted in 0.5 N HCl for 24 h at 4°C and measured by using Sigma Diagnostic Kit 587 according to the manufacturer's instructions.
Immunoblotting.
Cell lysates were resolved on a 10% SDS/PAGE gel and transferred onto PVDF membranes. Immunodetection was performed with Abs to IκBα Santa Cruz Biotechnology (Santa Cruz, CA); to FLAG epitope and anti-β-actin; to phospho-IκBα and anti-tubulin (Cell Signaling Technology, Danvers, MA), and with secondary HRP-conjugated anti-rabbit or anti-mouse IgG Ab (Santa Cruz Biotechnology). The detection was visualized by using an ECL detection kit (PerkinElmer, Boston, MA).
Reporter Gene Assay.
NF-κB activity in FLS was assessed by using an NF-κB reporter gene construct, as described (22). For normalization, cells were cotransfected with a constitutive Renilla luciferase reporter (Promega, Madison, WI). Transfections were performed with Effectene reagent (Qiagen, Valencia, CA). Two days after transfection, the firefly and Renilla luciferase activities were determined by dual luciferase assay system (Promega).
RT-PCR.
Total RNA was extracted from cells by using TRIzol extraction (Gibco Life Science, Rockville, MD) and was reversely transcribed with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). PCR amplification was done with the following primers: PPARγ2, 5′-GTGACTTGGTGGTGATCTAG-3′ (forward primer) and 5′-CGTTACAAGATTTATTCACACCA-3′ (reverse primer); osteopontin, 5′-TGT CTCCCGGTGAAAGTG-3′ (forward primer) and 5′-AGTCCATAAGCCAAGCT ATC-3′ (reverse primer); GAPDH, 5′-TGAAGGTCGGTGTCAACGGATTTG-3′ (forward primer) and 5′-GTACATCCGTACTCCAGGTGGTG-3′ (reverse primer). PPARγ2 was amplified by 34 cycles of PCR at an annealing temperature of 55°C. GAPDH and ostopontin were amplified at an annealing temperature of 60°C by using 24 and 22 cycles, respectively. PCR products were resolved and visualized by 1.5% agarose gel electrophoresis.
Cell Proliferation Assays.
For [3H]thymidine incorporation, exponentially growing FLS were plated into 12-well plates at 104 cells per well. Three days later, [3H]thymidine (1 μCi/ml) was added for 8 h. Harvested cells were washed, precipitated with 5% trichloroacetic acid at 4°C, washed with ice-cold 95% ethanol, dried, and counted with a radioactivity counter.
MTT Assay.
Exponentially growing FLS were plated in into 12-well plates in duplicate (4 × 104 cells per well). After 48 h, cell proliferation was measured by a modified MTT assay (35).
in vitro Invasion Assay.
The invasion assay was adapted from a procedure described previously (36). Matrigel (Becton Dickinson, San Jose, CA) was diluted to 0.4 mg/ml in a serum-free DMEM and layered on the top of polycarbonate filter insert (6.5-mm diameter, 8.0 μm; Costar, Cambridge, MA) to form a gel at 37°C. FLS (2 × 104 cells/200 μl) were loaded on the Matrigel-coated inserts and transferred to a 24-well plate with 600 μl of 0.5% FBS DMEM in the lower compartment. After 3 days of incubation, the cells were fixed in an ethanol/crystal violet solution. The number of cells that crossed the transwell membrane was counted under a light microscope. All experiments were done in duplicate.
Statistical Analysis.
Unless otherwise indicated, all results are shown as means ± SEM. Statistical differences between groups were determined by using ANOVA.
Acknowledgments
We thank Jonathan Serody for the gift of eGFP transgenic mice, John Olsen (University of North Carolina, Chapel Hill, NC) for providing cells and reagents for preparation of RVs, Sergei Romanov and Alex Kinev for help in generating retroviruses, and all members of S.S.M.'s laboratory for their support and assistance. The research was supported by National Institutes of Health Grants 5-P60 AR-30701-14 and AR/AI-44030 and by an Investigator Award (to S.S.M.) from The Arthritis Foundation.
Abbreviations
- FLS
fibroblast-like synoviocyte(s)
- rFLS
rat FLS
- BM
bone marrow
- BMT
BM transplantation
- RA
rheumatoid arthritis
- MSC
mesenchymal stem cell
- mBSA
methylated BSA
- MMP
matrix metalloproteinase
- i.a.
intraarticular(ly)
- RV
retroviral vector
- ALP
alkaline phosphatase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Firestein GS. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Pap T, Muller-Ladner U, Gay RE, Gay S. Arthritis Res. 2000;2:361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards JC. Arthritis Res. 2000;2:344–347. doi: 10.1186/ar110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firestein GS. Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Ladner U, Kriegsmann J, Gay RE, Gay S. Rheum Dis Clin North Am. 1995;21:675–690. [PubMed] [Google Scholar]
- 6.Yamanishi Y, Boyle DL, Rosengren S, Green DR, Zvaifler NJ, Firestein GS. Proc Natl Acad Sci USA. 2002;99:10025–10030. doi: 10.1073/pnas.152333199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL. J Clin Invest. 1989;83:1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JCW. In: Mechanisms and Models in Rheumatoid Arthritis. Henderson B, Edwards JCW, Pettipher ER, editors. London: Academic; 1995. pp. 153–162. [Google Scholar]
- 9.Yamasaki S, Nakashima T, Kawakami A, Miyashita T, Tanaka F, Ida H, Migita K, Origuchi T, Eguchi K. Rheumatology (Oxford) 2004;43:448–452. doi: 10.1093/rheumatology/keh092. [DOI] [PubMed] [Google Scholar]
- 10.Zvaifler NJ, Tsai V, Alsalameh S, von Kempis J, Firestein GS, Lotz M. Am J Pathol. 1997;150:1125–1138. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa S, Toritsuka Y, Wakitani S, Denno K, Tomita T, Owaki H, Kimura T, Shino K, Ochi T. J Rheumatol. 1996;23:2098–2103. [PubMed] [Google Scholar]
- 12.Marinova-Mutafchieva L, Williams RO, Funa K, Maini RN, Zvaifler NJ. Arthritis Rheum. 2002;46:507–513. doi: 10.1002/art.10126. [DOI] [PubMed] [Google Scholar]
- 13.LaBarge MA, Blau HM. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 14.Wu YP, McMahon E, Kraine MR, Tisch R, Meyers A, Frelinger J, Matsushima GK, Suzuki K. Am J Pathol. 2000;156:1849–1854. doi: 10.1016/S0002-9440(10)65058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Stroke. 2002;33:1362–1368. doi: 10.1161/01.str.0000014925.09415.c3. [DOI] [PubMed] [Google Scholar]
- 16.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 17.Sitcheran R, Cogswell PC, Baldwin AS., Jr Genes Dev. 2003;17:2368–2373. doi: 10.1101/gad.1114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makarov SS. Arthritis Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarov SS. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 20.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, et al. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 21.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 22.Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delhase M, Hayakawa M, Chen Y, Karin M. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 24.Romashkova JA, Makarov SS. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti MP, Brinckerhoff CE. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mengshol JA, Vincenti MP, Brinckerhoff CE. Nucleic Acids Res. 2001;29:4361–4372. doi: 10.1093/nar/29.21.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes PJ, Karin M. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 28.Goldring SR. J Rheumatol Suppl. 2002;65:44–48. [PubMed] [Google Scholar]
- 29.Serody JS, Burkett SE, Panoskaltsis-Mortari A, Ng-Cashin J, McMahon E, Matsushima GK, Lira SA, Cook DN, Blazar BR. Blood. 2000;96:2973–2980. [PubMed] [Google Scholar]
- 30.Setoguchi K, Misaki Y, Terauchi Y, Yamauchi T, Kawahata K, Kadowaki T, Yamamoto K. J Clin Invest. 2001;108:1667–1675. doi: 10.1172/JCI13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson LG, Mewshaw JP, Ni H, Friedmann T, Boucher RC, Olsen JC. J Virol. 1998;72:8861–8872. doi: 10.1128/jvi.72.11.8861-8872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenstern JP, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 34.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 36.Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC, Huizinga TW. Ann Rheum Dis. 2002;61:975–980. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]