Abstract
Islet grafts can induce insulin independence in type 1 diabetic patients, but their function is variable with only 10% insulin indepence after 5 years. We investigated whether cultured grafts with defined β cell number help standardize metabolic outcome. Nonuremic C-peptide-negative patients received an intraportal graft with 0.5–5.0 × 106 β cells per kilogram of body weight (kgBW) under antithymocyte globulin and mycophenolate mofetil plus tacrolimus. Metabolic outcome at posttransplant (PT) month 2 was used to decide on a second graft under maintenance mycophenolate mofetil/tacrolimus. Graft function was defined by C-peptide >0.5 ng/ml and reduced insulin needs, metabolic control by reductions in HbA1c, glycemia coefficient of variation, and hypoglycemia. At PT month 2, graft function was present in 16 of 17 recipients of >2 × 106 β cells per kgBW versus 0 of 5 with lower number. The nine patients with C-peptide >1 ng/ml and glycemia coefficient of variation of <25% did not receive a second graft; five of them were insulin-independent until PT month 12. The 12 others received a second implant; it achieved insulin-independence at PT month 12 when the first and second graft contained >2 × 106 β cells per kgBW. Of the 20 recipients of at least one graft with >2 × 106 β cells per kgBW, 17 maintained graft function and metabolic control up to PT month 12. At PT month 12, β cell function in insulin-independent patients ranged around 25% of age-matched control values. Thus, 1-year metabolic control can be reproducibly achieved and standardized by cultured islet cell grafts with defined β cell number.
Keywords: diabetes, islet transplantation, prevention, type 1 diabetes
Islet transplantation can correct type 1 diabetes in both kidney recipients and nonuremic patients (1). However, the degree and duration of clinical success are variable, and only 10% of recipients remain insulin-independent for 5 years (1). Normalization of hyperglycemia is sometimes achieved with a graft from one pancreas (2, 3), but more often requires combined isolates from more donor organs (4–8); these are then injected at one or, more often, two or three time points with different intervals, each involving an additional antibody treatment (2–8). Grafts have not been standardized in terms of cellular composition and β cell mass. This variability in protocol and in implanted β cell mass probably contributes to the variable metabolic outcome and makes it at the same time difficult to undertake comparative protocols in search for a more successful outcome. In the present study we investigated the influence of β cell mass in the implant on β cell function in the recipient during the first year. Data were used to decide whether a second implant would be given and to correlate β cell function in the recipient with metabolic benefit. An immune suppressive protocol was used with one antithymocyte globulin (ATG) course because this was previously found beneficial for survival of a later islet cell allograft while maintaining tolerance to the first graft (9–11). Maintenance immune therapy consisted of a combination of mycophenolate mofetil (MMF) and tacrolimus. At posttransplant (PT) month 12, functional β cell mass was compared with that of age- and body weight (BW)-matched controls, whereas the observed adverse events were analyzed versus those reported in other protocols.
Results
Survival and Function of First β Cell Implant.
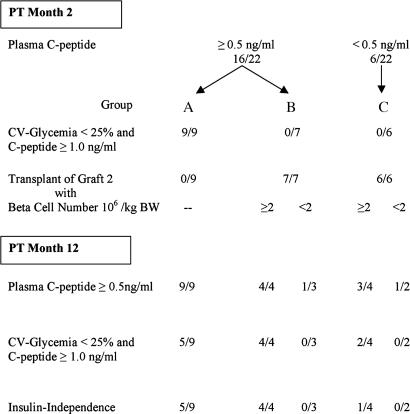
During PT month 2, 16 of 22 recipients exhibited a surviving graft (Fig. 1) with 6-fold higher median plasma C-peptide [1.32 ng/ml, interquartile range (IQR) 0.93–1.87 ng/ml] than in the six other cases (0.22 ng/ml, IQR 0.12–0.29 ng/ml) (P < 0.001). Their mean fasting blood glucose (113 mg/dl, IQR 107–119 mg/dl) was similar (121 mg/dl, IQR 112–137 mg/dl) (P = 0.18), but its coefficient of variation (CV) was significantly lower (17%, IQR 12–22% versus 38%, IQR 35–41%) (P = 0.001). Comparison of both groups for recipient and graft characteristics demonstrated a significant difference in the number of donor β cells per kilogram of body weight (BW) (P = 0.002) (Table 3, which is published as supporting information on the PNAS web site). Plasma C-peptide and CV of fasting glycemia (CVgl) strongly correlated with β cell number per kilogram of BW (Fig. 2). The index of a surviving graft (plasma C peptide ≥0.5 ng/ml) was achieved with grafts containing minimally 2 × 106 β cells per kilogram of BW (Fig. 2); all but one patient with 2-month C-peptide <0.5 ng/ml had received fewer β cells per kilogram of BW.
Fig. 1.
Flow diagram of islet β cell transplants in 22 nonuremic type 1 diabetic patients. Survival of a β cell implant in C-peptide-negative (<0.09 ng/ml) recipients was defined by PT plasma C-peptide ≥0.5 ng/ml.
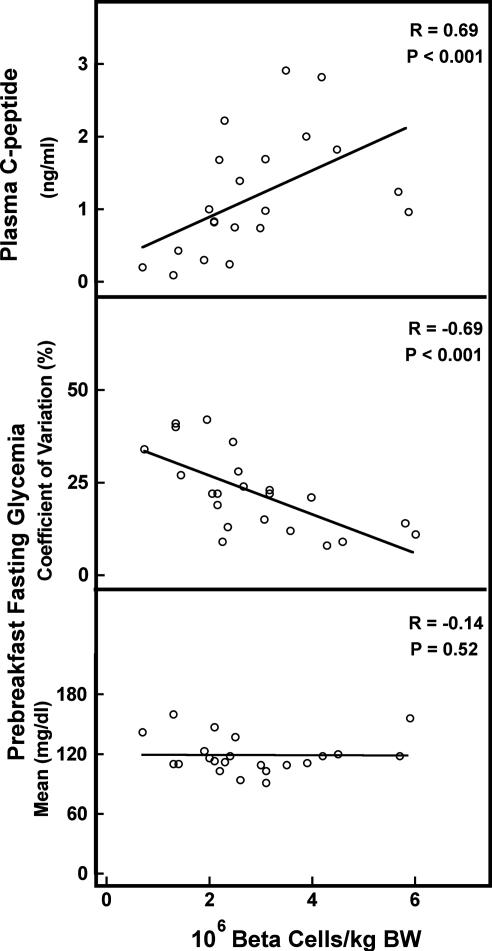
Fig. 2.
Correlation between β cell number in the first graft and metabolic outcome at PT month 2 as measured by plasma C-peptide (Top), CV (Middle), and mean (Bottom) prebreakfast glycemia. A positive correlation was found between β cell number and plasma C-peptide (R = 0.69, P < 0.001) and CVgl (R = −0.69, P < 0.001) but not with mean glycemia (R = −0.14, P = 0.52).
Patients with 2-month C-peptide ≥1 ng/ml and CVgl <25% (n = 9) did not receive a second graft (group A). At PT month 12, they all exhibited a surviving graft (C-peptide ≥0.5 ng/ml), but only five maintained the 2-month values of C-peptide and CVgl: these five became insulin-independent and remained so up to PT month 12 (Fig. 1). Of the four others in group A, two were transiently insulin-independent (PT months 3–9), but for all four insulin dose at PT month 12 was significantly lower than at start (median 53% lower) with similar HbA1c values than preimplantation (median 7.7%). These four cases did not receive fewer β cells (3.7 × 106 per kilogram of BW; IQR 3.4–4.3; P = 0.33) than the five that were insulin-independent at PT month 12 (2.6 × 106 per kilogram of BW; IQR 2.3–4.2).
Survival and Function of Second β Cell Graft.
In 13 patients a second graft was implanted during PT month 3, namely in seven patients with 2-month C-peptide 0.5–1 ng/ml and CVgl >25% (group B), and in six with 2-month C-peptide <0.5 ng/ml (group C) (Fig. 1). At PT month 12, all patients in group B had C-peptide ≥0.5 ng/ml, indicating that a second graft implanted under maintenance immune suppression did not result in massive destruction of the first implant. In fact, when the second graft contained >2 × 106 β cells per kilogram of BW (4 of 7 patients), it increased β cell functions in all cases as shown by the higher PT month 12 plasma C-peptide (1.88 ng/ml, IQR 1.70–2.17 ng/ml) and lower CVgl (9%, IQR 8–10%) (Fig. 1); these four patients also became insulin-independent and remained so up to PT month 12. Such an increase in β cell function was not achieved in patients receiving a second graft with <2 × 106 β cells per kilogram of BW (3 of 7 patients in group B) (Fig. 1). In group C patients, a second graft with ≥2 × 106 β cells per kilogram of BW resulted in graft function at PT month 12 in 3 of 4 recipients (Fig. 1) with low CVgl in two and a state of insulin independence in one; one other patient was insulin-independent between months 5 and 10. Comparison of recipient and graft characteristics did not reveal differences between the insulin-independent and the insulin-treated groups or among the patient groups that maintained insulin independence after either one (n = 5) or two (n = 5) grafts. All four patients who received two grafts with ≥2 × 106 β cells per kilogram of BW were insulin-independent at PT month 12, whereas this was the case for only five of nine receiving one such graft (Fig. 1).
Metabolic Effects of Surviving β Cell Graft.
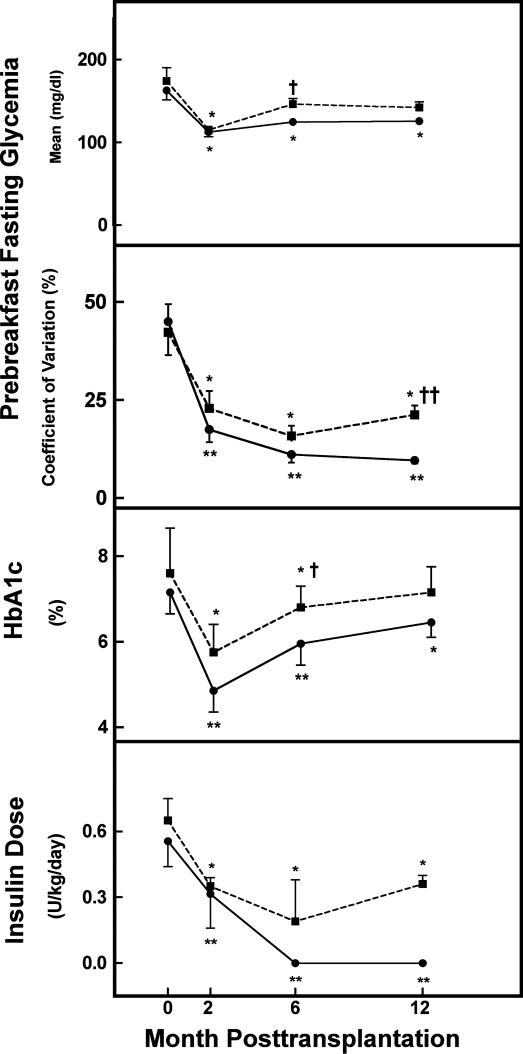
In 18 recipients, β cell function (as evidenced by plasma C-peptide ≥0.5 ng/ml) was maintained up to PT month 12 (Fig. 1). This occurrence was associated with beneficial metabolic effects in the form of (i) lower mean fasting glycemia for lower doses of insulin treatment (Fig. 3), (ii) a significantly lower CVgl (Fig. 3), and (iii) fewer hypoglycemic episodes. The percentage of glucose measurements <70 mg/dl decreased from 11% (IQR 8–16%) before transplantation to 0% (IQR 0–3%) (P = 0.001) between 6 and 12 months, and the percentage of severe hypoglycemia (<50 mg/dl) decreased from 3% (IQR 1–5%) to 0% (IQR 0–0%) (P < 0.001). None of the recipients exhibited a hypoglycemic episode requiring assistance. These beneficial effects were significantly higher in patients who were insulin-independent at PT month 12 than in those who were on insulin treatment (Fig. 3); they never presented a glycemia <70 mg/dl (0%, IQR 0–0%) whereas this occurred in 3% (IQR 2–8%) (P = 0.03) of insulin-treated patients; their HbA1c level was also lower (Fig. 3). No correlation was noticed between the degree of metabolic normalization at PT month 12 and the total β cell number that was transplanted at one or at two occasions; however, the small number of patients per subgroup limits the significance of this analysis. For the same reason we could not assess whether a positive correlation existed between long-term metabolic function and the number of cytokeratin-19 positive cells, as recently reported by Street et al. (12).
Fig. 3.
Longitudinal follow-up of mean and CVgl, HbA1c, and daily insulin dose in the 18 type 1 diabetic patients with 1 year surviving grafts. Patients are divided in two groups according to their state of insulin (in)dependence at PT month 12: n = 10 insulin-independent (solid line) and n = 8 insulin-dependent (dashed line) patients. Data are means ± SEM. Statistical difference versus pretransplantation: ∗, P < 0.01; ∗∗, P < 0.001. Statistical difference between groups: †, P < 0.01; ††, P < 0.001.
Comparison of Functional β Cell Mass in Insulin-Independent Recipients with That in Matched Normal Controls.
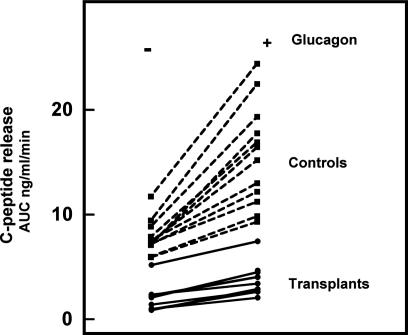
At PT month 12, fasting glycemia was slightly higher in insulin-independent recipients than in age- and BW-matched controls (107 mg/dl versus 83 mg/dl; P < 0.001) whereas the corresponding plasma C-peptide levels were slightly lower (Table 1). The difference in functional β cell mass was quantified by expressing C-peptide release during a glucose clamp test, first in the absence and then in the presence of glucagon. In insulin-independent graft recipients, no secretory response was detected during the first 10 min of glucose stimulation; it was clearly present after prolonged hyperglycemia (120–150 min) but corresponded only to 26% of the values in the normal controls (Fig. 4 and Table 1); administration of glucagon did further increase C-peptide levels but not their relative proportion with respect to the control levels (Table 1). When individual values were plotted, all recipients were found to exhibit a markedly lower β cell secretory capacity than controls, both in the absence and in the presence of glucagon (Fig. 4). Nine of the 10 recipients were clustered at 20% of the median control and one was at 60% (Fig. 4). These data can be considered as an index for a markedly lower functional β cell mass in insulin-independent recipients at PT month 12.
Table 1.
β cell secretory function of insulin-independent recipients at PT month 12
| β cell secretory function | Controls (n = 12) | Transplants (n = 10) | P value |
|---|---|---|---|
| Basal | |||
| Glycemia | 83 (79–88) | 107 (100–123) | <0.001 |
| AUC C-peptide | 0.94 (0.88–1.17) | 0.72 (0.64–0.83) | 0.008 |
| Glucose-induced clamp, | |||
| 0–10 min | |||
| Glycemia | 144 (128–151) | 173 (159–185) | 0.001 |
| AUC C-peptide | 1.52 (1.34–1.83) | 0.70 (0.62–0.79) | <0.001 |
| 120–150 min | |||
| Glycemia | 191 (176–198) | 199 (168–213) | 0.81 |
| AUC C-peptide | 7.32 (7.14–8.11) | 2.08 (1.00–2.28) | <0.001 |
| 150–160 min (plus glucagon) | |||
| Glycemia | 199 (178–208) | 196 (178–214) | 0.97 |
| AUC C-peptide | 15.80 (11.93–18.15) | 3.71 (2.72–4.39) | <0.001 |
Data represent median glycemia (mg/dl) and AUC C-peptide (ng/ml per min) with IQR in parentheses. AUC, area under curve.
Fig. 4.
Glucose-clamp induced C-peptide release in the absence and presence of glucagon. Data represent individual values of area under the curve (AUC) (ng/ml per min) measured in 10 insulin-independent graft recipients at PT month 12 (solid lines) and in 12 age- and BW-matched nondiabetic controls (dashed lines).
Adverse Events.
None of the recipients presented complications of the graft infusion (i.e., no bleeding, thrombosis, or sepsis) or life-threatening adverse events. Only one of 24 patients developed HLA antibodies. Immune suppressive treatment caused a series of side effects (listed in Table 4, which is published as supporting information on the PNAS web site) of which the following are the most prevalent or serious: during the ATG course, 8 of 24 patients presented fever (37.5–39°C) for 1 or 2 days. MMF caused gastrointestinal symptoms (mainly pyrosis, 9 of 24) that were reduced or disappeared after lowering the dose; they caused the dropout of one patient. One patient was hospitalized for cerebellar ataxia that disappeared after reducing the tacrolimus dose.
Alanine transaminases were increased in 8 of 24 recipients 1–3 weeks after the first graft (2 of 12 after laparoscopy and 6 of 12 after transcutaneous injection); this was not the case after the second graft (0 of 14) (P = 0.03). One patient developed CMV hepatitis (18-fold rise in alanine transaminases at PT week 6 with a progressively increasing number of CMV DNA copies and CMV seroconversion) that was treated with ganciclovir. Red blood cells transiently decreased in 18 of 24 recipients of the first graft; six of them received a blood transfusion at week 2. Leucopenia (<3,000/mm3) was seen in 17 of 24 patients at PT month 3 but in only 6 of 24 patients at PT month 12 (P = 0.003), presumably because the MMF dose was reduced. Creatinine clearance was 16% lower from PT week 2 on without further decrease during the 1-year follow-up; none presented a serum creatinine >2 mg/dl. Albuminuria decreased in 8 of 8 patients with pretransplant micro- or macroalbuminuria: i.e., from 108 (47–368) mg every 24 h (median, IQR) at start to 29 (22–65), 21 (15–48), and 25 (21–86) mg every 24 h at PT months 2, 6, and 12, respectively; corresponding values for estimated glomerular filtration rate (Cockroft–Gault formula) were 87 (78–100) ml/min at start and 71 (61–91), 70 (61–85), and 72 (64–82) at PT months 2, 6, and 12, respectively. Total cholesterol was significantly reduced at PT month 12 and also in the six patients with pretransplant cholesterol >190 mg/dl. Twenty-two patients lost weight during the first 6 PT months.
Discussion
The present study demonstrates that cultured human islet cell preparations can be used to prepare grafts with reproducible metabolic outcome after intraportal transplantation in preuremic type 1 diabetic patients. Grafts are characterized by their cell number and cellular composition. Donor β cell mass is quantified by the number of insulin-positive cells as was first described for rat islet cell grafts (13, 14) and later used for human allografts in patients with a prior kidney transplant (9). The number of β cells injected per kilogram of BW closely correlated with metabolic outcome parameters, namely plasma C-peptide levels and the ability to suppress variations in fasting glycemia. We have not found such a correlation when using islet equivalents (IEQ) (15) to express graft size (data not shown). In a previous article we discussed reasons why IEQ is a less adequate index for β cell mass (9); most dithizone-positive particles contain β cells but also variable percentages of nonendocrine cells and extracellular volume, which are thus included in the IEQ measurement, leading to variable overestimations of β cell mass. Moreover, in cultured preparations dithizone sometimes stains nonislet structures while it becomes weaker and more difficult to distinguish in β cells as a result of their degranulation. Defining the grafts on the basis of their β cell number and purity overcomes these limitations and provides a direct measurement of β cell mass and of its degree of contamination. Use of this cellular definition led us to the name of β cell grafts, just as the term islet preparation relates to a characterization as the number of isolated islets or counted IEQ. In opting for cultured preparations, more quality control tests can be performed before transplantation. Grafts with defined β cell mass help to set standards for comparative trials and should facilitate multicenter studies. This finding could be particularly useful when assessing the therapeutic potential of insulin-positive cells generated from stem or precursor cells. Use of culture also allows to test its ability to reduce the immunogenicity of human β cell grafts, as was the case in rodents (16, 17). On the other hand, culture has the disadvantage of reducing the yield in β cells per donor organ, thus increasing the number of donors needed per transplantation. However, this factor did not reduce the number of transplanted patients in our program; on the contrary, because we rarely have access to optimal donor pancreases, our trial depended on the ability to collect donor cells from organs that would otherwise be discarded because of insufficient quality or quantity. The relatively small β cell yield from such organs necessitated collection from several donors, thus prolonging total culture time.
Grafts were only 40–50% pure in endocrine cells with nongranulated cells as major “contaminant” that probably corresponds to duct cells given their positivity for cytokeratin-19 and their ultrastructural features. This purity is markedly lower than in rodent islet experiments, which can be responsible for a less favorable outcome (14). There are presently no techniques available that achieve a higher degree of purity in β cell numbers that are needed for transplantation. The number of insulin-positive cells injected varied from 0.5 to 6 million per kilogram of BW. It was found that minimally 2 million per kilogram of BW were needed to achieve signs of functioning grafts, during PT month 2, i.e., in 16 of 17 recipients of such grafts versus 0 of 5 recipients of a smaller β cell number, plasma C-peptide >0.5 ng/ml plasma and CVgl was reduced. Because none of these 16 recipients were insulin-independent at PT month 2, the question rose whether a second transplant would be needed to reach insulin independence with time. The PT month-2 values of plasma C-peptide and CVgl were used as basis to decide on a second graft to be performed during PT month 3 without a second antibody course and without changing MMF and tacrolimus maintenance doses. No second transplant was conducted in the nine patients with C-peptide >1 ng/ml and CVgl <25% because this C-peptide level had been found to precede a state of insulin independence in islet cell after kidney recipients (9) and because this CVgl expresses stable fasting glycemia (group A, stable fasting glycemia). A second graft was administered in the other seven subjects (plasma C-peptide 0.5–1 ng/ml and CVgl 17–23%; group B, intermediate fasting glycemia control). The six patients with C-peptide <0.5 ng/ml and CVgl 34–41% (group C, no fasting glycemia control) also received a second graft. All patients were then evaluated 12 months after the first transplant. It was found that an allograft of cultured β cells can survive when transplanted in a recipient who is only on maintenance immune suppression for an islet cell allograft that was performed >2 months before. Furthermore, reintroduction of foreign β cells did not lead to the destruction of a surviving first graft; on the contrary, in all four recipients showing a functioning first graft, a second graft improved fasting glycemia control and resulted in insulin independence when containing >2 million β cells per kilogram of BW. Within the constraints of the limited numbers of recipients, this outcome seems better than that after one injection of >2 million β cells per kilogram of BW, where stable fasting glycemia control was maintained in only five of nine patients, with all five being insulin-independent at PT month 12. A longer follow-up period is now needed to assess whether this apparent advantage of a second graft remains. Studies with larger β cell numbers should also be planned considering the fact that the numbers transplanted in the present series of patients are on the lower side of those needed to maintain long-term normoglycemia in isogenic rat recipients (10 million β cells per kilogram of BW) (14); interestingly, contamination with nonendocrine cells interfered with long-term normalization in this rat model (14).
Achieving stable fasting glycemia, as expressed by a CVgl <25%, represents an index of immediate clinical benefit for the patients because it is associated with a reduced risk for (severe) hypoglycemic events (refs. 18 and 19 and the present study). If maintained long-term it should also correlate with a delay or an arrest in the development of chronic diabetes lesions. The therapeutic significance of islet cell transplantation will depend on the duration over which grafts stabilize blood glucose control. A likely predictor for this metabolic control function is the secretory capacity of the β cell implant during a hyperglycemic clamp test because such a test can be correlated with the surviving β cell mass (20). We therefore performed this test in graft recipients that were insulin-independent at PT month 12 and compared C-peptide release with that in age- and BW-matched nondiabetic controls. The data indicated that the secretory capacity of the β cell implant was only 25% of that in normal controls, with little interindividual variations. Because there is presently little evidence for a growing β cell mass or function in recipients of human islet cell grafts, this size may progressively become insufficient in subsequent months and years. A progressive decline in graft function has already been reported by Ryan et al. (21), who performed Kaplan–Meier analysis on patients transplanted in Edmonton. Studies are thus needed to investigate how a larger functional β cell mass can be achieved and maintained in the diabetic recipients. Among the variables to be examined are donor, graft, and recipient characteristics, as well as the immune suppressive treatment. We believe that such comparative analysis can benefit from the presently proposed protocol because this achieves a reproducible metabolic outcome during the first year PT.
Further trials will have to weigh the benefit of islet cell transplantation against its adverse events. In the present study no serious adverse event was recorded during or after any of the 37 transplantations, irrespective of whether the portal vein was reached by laparoscopy (22) or percutaneously (23). A transient rise in liver transaminases occurred between weeks 1 and 3 after the first implantation, as was also seen in previous studies (24, 25), but this was not observed after the second one, suggesting a correlation with the start of immune suppressive drugs. Our immune suppressive therapy consisted of induction with ATG and maintenance doses of MMF plus tacrolimus. This regimen did not cause worsening of albuminuria, blood pressure, and cholesterol levels as was the case with the Edmonton protocol using sirolimus plus tacrolimus (8, 26). It was associated with a weight loss and with gastrointestinal side effects that were reduced after lowering the dose of MMF. Use of tacrolimus may impair kidney function, but this was not detected during the first year; a longer follow-up is needed.
Methods
Graft Recipients.
Potential graft recipients (age 18–65 years) were recruited at participating hospitals according to the following inclusion criteria: (i) type 1 diabetes with plasma C-peptide <0.09 ng/ml at glycemia of 120–200 mg/dl, both in the absence and the presence of i.v. glucagon stimulation; (ii) large within-person between-day variation in fasting self-monitored plasma glucose (as defined by CV ≥25%) together with episodes of hypoglycemia (27, 28); (iii) HbA1c >7% despite intensive insulin therapy; (iv) one or more signs of chronic diabetes lesions (hypoglycemic unawareness, microalbuminuria despite optimal dose of ACE inhibitor, retinopathy). Exclusion criteria were as follows: BW >90 kg; active smoking; pregnancy; disturbed liver function tests; history of hepatic disease, thrombosis, pulmonary embolism, tuberculosis, or malignancy; presence of HLA antibodies; and EBV antibody negative status. After signing an informed consent, candidate recipients were listed at Eurotransplant and selected on the basis of listing date, blood group compatibility with available graft, and status of physical and mental health at the time of implant. Twenty-four patients were consecutively enrolled for the present protocol; two of them were removed from the 12-month metabolic analysis, one after developing CMV hepatitis at PT week 5 and the other by his own decision to drop out at PT week 26 because of gastrointestinal side effects; they were not removed from the safety analysis. None of these patients were treated with metformin or insulin sensitizers either before or during the study. Their baseline characteristics are shown in Table 2.
Table 2.
Recipient and graft characteristics
| Recipients | |
| Age, years | 43 (39- 49) |
| Gender | 13 M/9 F |
| Body weight, kg | 70 (64–75) |
| BMI, kg/m2 | 24 (22–26) |
| Diabetes | |
| Age at clinical onset, years | 19 (12–24) |
| Duration of disease, years | 25 (18–33) |
| Positivity for ICA/GADA/IA2-A/I(A)A | 7/12/9/19 |
| HbA1c, % | 7.6 (7.0–8.1) |
| Insulin dose, units/kg/day | 0.7 (0.5–0.8) |
| Fasting glycemia | |
| Mean, mg/dl | 174 (145–188) |
| CV, % | 43 (40–47) |
| <70 mg/dl, % | 7 (4–12) |
| <50 mg/dl, % | 1 (0–2) |
| All glycemia | |
| <70 mg/dl, % | 10 (8–16) |
| <50 mg/dl, % | 3 (1–5) |
| Retinopathy | 17 |
| Microalbuminuria | 7 |
| Donor tissue | |
| Donor age, years | 50 (43–56) |
| Pancreas cold ischemia time, h | 9 (7–13) |
| Pancreas weight, g | 89 (70–107) |
| Grafts | |
| Culture time, days | 6 (3–11) |
| Cellular composition, % | |
| β cells | 33 (26–42) |
| α cells | 9 (5–13) |
| Nongranulated cells | 45 (37–53) |
| Acinar cells | 1 (1–4) |
| Dead cells | 7 (5–11) |
Data represent median for 22 recipients with IQR in parentheses. All recipient data were measured before transplantation, except glycemia, which represents measurements at home during the preceding year. The CVgl correlated with the percentage of fasting hypoglycemia <70 mg/dl (R = 0.71, P < 0.001) and <50 mg/dl (R = 0.73, P < 0.001). None of the recipients had a CVgl < 25%. M, male; F, female.
Immune Suppression and Monitoring.
The immune suppression protocol was comparable to that found to sustain survival of cultured β cell grafts when implanted in patients who were on maintenance immune therapy for a prior kidney transplantation (9). It consisted of a short course of ATG and a maintenance treatment with an antimetabolite and a calcineurin inhibitor (9). ATG (Fresenius HemoCare, Redmond, WA) was started 1–4 days before transplantation at a dose of 9 mg/kg and continued at 3 mg/kg for 6 days except when T lymphocyte count was <50/mm3. Total cumulative dose of ATG ranged from 18 to 31 mg/kg (median 24 mg/kg). MMF (2 g/day; gift of Roche, Brussels, Belgium) was started together with the first ATG injection and maintained at this dose. Tacrolimus was started the day before the last ATG administration, and its dose was adjusted to maintain blood trough levels of 8–10 ng/ml during the first 3 months and 6–8 ng/ml afterward. In case a second β cell graft was implanted, no additional course of ATG was given and the MMF dose was not changed, but tacrolimus levels were maintained at 8–10 ng/ml for the subsequent 3 months. Three hours before a β cell implant, one dose of 500 mg of methylprednisolone was given i.v.
Preparation of β Cell Grafts.
Pancreases from brain-dead heart-beating donors were procured by hospitals affiliated with the Eurotransplant Foundation (Leiden, The Netherlands) according to local medical, legal, and ethical guidelines for organ donation. Organs were transported to the Beta Cell Bank in Brussels for preparation of cultured β cell suspensions according to standardized procedures (9); the Beta Cell Bank is recognized and monitored as a transplant tissue bank by the Belgian Ministry of Social Affairs, Health, and Environment. Islet cell-enriched fractions were cultured as described previously (29) by using serum-free Ham's F10 medium/0.5% human albumin/135 mg/dl glucose/2 mM glutamine (50 μl of tissue in 45 ml of medium suspended in a T175 Starsted culture flask with a vented cap). After 2–20 days (median 6 days; IQR 3–11 days) the preparations were analyzed for their β cell number and purity (9, 29). Data were used to select preparations that, after combination, would constitute a graft with 0.5–5 × 106 β cells per kilogram of recipient BW suspended in 40–85 ml of Ham's F10 medium with 0.5% human albumin. The final cellular composition of each β cell graft was determined on samples that were taken just before implantation (9). For each preparation, whether taken at the start of culture or during culture, or from the final graft, triplicate samples for DNA assay were taken, each being assayed in duplicate; when calculated for 30 consecutive grafts, the CV among these aliquots was 9% (5–14%), and that among duplicate samples was <5%. The total number of cells in a fraction was calculated by dividing its DNA content (in picograms) by 6.5 pg per cell, the average cellular DNA content measured in sorted single human adult β cells and duct cells. The number of β cells was then determined on the basis of the percentage of insulin-positive cells counted in duplicate samples of this fraction. The characteristics of the 35 grafts used in this study are listed in Table 2; the number of donors per graft was four (median; IQR of three to five). Compared with freshly isolated islet fractions (4), these preparations exhibit a higher percent β cells and contain virtually no acinar cells. Culture led to fragmentation of large tissue particles and reassociation of small particles. In view of these characteristics and the selected criteria for quantifying the grafts, we define them as β cell preparations.
Transplantation.
Nine patients received one graft. The thirteen others received a second graft 3 months later (IQR 2–5 months). The graft suspension was infused into the portal vein over 5–6 min. Access to the portal vein occurred through a catheter inserted in the umbilical vein after laparoscopic repermeabilization (n = 20) (9, 22) or through s.c. transhepatic punction under ultrasound guidance (n = 15) (23).
Function of β Cell Implants and Metabolic Control.
Graft recipients were followed for their fasting blood glucose level and its CV (home monitoring), their plasma C-peptide levels at glycemia 120–200 mg/dl, and their blood HbA1c concentration. Hypoglycemic episodes were documented by home glucose monitoring during the year before and after transplantation and expressed as percentage of all measurements. They are tabulated as all measurements <70 mg/dl as proposed by the American Diabetes Association Workgroup on Hypoglycemia (30) and as all measurements <50 mg/dl, which is considered as a higher risk for acute complications (31, 32). Survival of a β cell implant in C-peptide-negative (< 0.09/ml) recipients was defined by plasma C-peptide ≥0.5 ng/ml measured in postbreakfast samples (33). A state of C-peptide negativity was based on measurements in postbreakfast samples as well as after glucagon injection. Graft recipients who were insulin-independent at PT month 12 were submitted to a glucose clamp test in which C-peptide release was measured first at basal glycemia (median 107 mg/dl; IQR 99–120 mg/dl) and then during sustained glucose stimulation (180 mg/dl, 15–160 min), first in the absence of glucagon (at minutes 120, 135, and 150) and then over 5 and 10 min after a glucagon injection at minute 150. Plasma C-peptide levels were used to calculate the area under the curve per minute. The same test was conducted in 12 age- and BW-matched normal volunteers. It was also recently used to follow the insulin secretory capacity in type 1 diabetic patients during the first 18 months after diagnosis (20).
Daily insulin dose was adjusted to keep blood glucose levels between 70 and 180 mg/dl without frequent episodes of symptomatic hypoglycemia. Although the plan was to taper insulin dose only after PT month 2, this was started earlier when patients with graft function presented hypoglycemic episodes (<70 mg/dl). Insulin treatment was increased or reintroduced after two consecutive HbA1c measurements >7.0% (normal HbA1c < 6.0%).
Medical Follow-Up.
Patients were consulted weekly until PT week 6, every two weeks between PT week 6 and 12, and monthly thereafter. They underwent a systematic interrogation by questionnaire and general physical examination for adverse events. Blood was taken for hematology, kidney, and liver tests and CMV PCR. Adverse events were graded with the National Cancer Institute Common Toxicity Criteria system version 3.0 (34). In the safety analysis we included grade two to five events. Plasma C-peptide (C-peptide TRFIA; PerkinElmer, Turku, Finland) and corresponding glycemia, as well as HbA1c concentrations, were measured in the central laboratory of the Belgian Diabetes Registry. Tacrolimus trough levels (Tacrolimus II, IMx Abbott; Abbott Laboratories, Wiesbade, Germany), lymphocyte subsets (Epics XL flow cytometer; Beckman Coulter, Miami, FL), and HLA antibodies (Lambda Antigen Tray Elisa, Mixed Class 1 and 2; One Lambda, Canoga Park, CA) were also measured centrally.
Statistical Methods.
Data are presented as median (quartile range). Comparison of patient subgroups used the Mann–Whitney test for quantitative variables and Fisher's exact test for binary variables. Statistical differences between repeated measurements were examined with the Friedman test and, if significant, with Wilcoxon's signed-rank test. Analysis of correlations was performed by using the Spearman rank-correlation test. Differences were considered significant for P < 0.05.
Supplementary Material
Acknowledgments
We thank Koen Verbeeck and Sabrina Uyttenhove for their excellent collaboration in organizing this project, and we acknowledge the loyal support of the Beta Cell Bank, the Belgian Diabetes Registry, the Diabetes Research Center of Vrije Universiteit Brussel, the Clinical Biology Department of Academisch Ziekenhuis (Vrije Universiteit Brussel), the physicians and nurses of the patients included in this study, and the Eurotransplant Foundation and its transplant surgeons and coordinators. This study was supported by a Center Grant from the Juvenile Diabetes Research Foundation, Fonds voor Wetenschappelijk Onderzoek (FWO)–Flanders, and Vrije Universiteit Brussel. B.K. and C.M. are Senior Clinical Investigators of the FWO.
Abbreviations
- PT
posttransplant
- IQR
interquartile range
- ATG
antithymocyte globulin
- BW
body weight
- CV
coefficient of variation
- CVgl
CV of fasting glycemia
- MMF
mycophenolate mofetil
- IEQ
islet equivalent.
Footnotes
The authors declare no conflict of interest.
References
- 1.Robertson RP. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 2.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parky J, et al. J Am Med Assoc. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, et al. Am J Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 6.Hirshberg B, Rother KI, Digon BJ, III, Lee J, Gaglia JL, Hines K, Read EJ, Chang R, Wood BJ, Harlan DM. Diabetes Care. 2003;26:3288–3295. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 7.Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, Frank A, Markmann E, Palanjian M, Brayman K, et al. Ann Surg. 2003;237:741–749. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, et al. Am J Transplant. 2005;5:2037–2046. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 9.Keymeulen B, Ling Z, Gorus FK, Delvaux G, Bouwens L, Grupping A, Hendrieckx C, Pipeleers-Marichal M, Van Schravendijk C, Salmela K, et al. Diabetologia. 1998;41:452–459. doi: 10.1007/s001250050929. [DOI] [PubMed] [Google Scholar]
- 10.Roep BO, Stobbe I, Duinkerken G, van Rood JJ, Lernmark A, Keymeulen B, Pipeleers D, Claas FH, de Vries RR. Diabetes. 1999;48:484–490. doi: 10.2337/diabetes.48.3.484. [DOI] [PubMed] [Google Scholar]
- 11.Stobbe I, Duinkerken G, van Rood JJ, Lernmark A, Keymeulen B, Pipeleers D, De Vries RR, Glass FH, Roep BO. Diabetologia. 1999;42:1379–1380. doi: 10.1007/s001250051456. [DOI] [PubMed] [Google Scholar]
- 12.Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, et al. Diabetes. 2004;53:3107–3114. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 13.Pipeleers DG, Pipeleers-Marichal M, Hannaert JC, Berghmans M, In't Veld PA, Rozing J, Van de Winkel M, Gepts W. Diabetes. 1991;40:908–919. doi: 10.2337/diab.40.7.908. [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Korbutt G, De Paepe M, Gorus F, Kloppel G, Pipeleers DG. Diabetes. 1996;45:1814–1821. doi: 10.2337/diab.45.12.1814. [DOI] [PubMed] [Google Scholar]
- 15.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJ, Socci C, Alejandro R, et al. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 16.Pipeleers DG, Pipeleers-Marichal M, Vanbrabandt B, Duys S. Diabetes. 1991;40:920–930. doi: 10.2337/diab.40.7.920. [DOI] [PubMed] [Google Scholar]
- 17.Pipeleers D, Keymeulen B, Korbutt G. In: Diabetes Annual. Marshall S, Home P, editors. Amsterdam: Elsevier Science; 1994. pp. 299–330. [Google Scholar]
- 18.Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Clin Ther. 2004;26:724–736. doi: 10.1016/s0149-2918(04)90072-0. [DOI] [PubMed] [Google Scholar]
- 19.Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Diabetologia. 2004;47:622–629. doi: 10.1007/s00125-004-1365-z. [DOI] [PubMed] [Google Scholar]
- 20.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 21.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 22.Movahedi B, Keymeulen B, Lauwers MH, Goes E, Cools N, Delvaux G. Transpl Int. 2003;16:186–190. doi: 10.1007/s00147-002-0517-7. [DOI] [PubMed] [Google Scholar]
- 23.Maleux G, Gillard P, Keymeulen B, Pipeleers D, Ling Z, Heye S, Thijs M, Mathieu C, Marchal G. J Vasc Interv Radiol. 2005;16:1693–1697. doi: 10.1097/01.RVI.0000182506.88739.39. [DOI] [PubMed] [Google Scholar]
- 24.Barshes NR, Lee TC, Goodpastor SE, Balkrishnan R, Schock AP, Mote A, Brunicardi FC, Alejandro R, Ricordi C, Goss JA. J Am Coll Surg. 2005;200:353–361. doi: 10.1016/j.jamcollsurg.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Rafael E, Ryan EA, Paty BW, Oberholzer J, Imes S, Senior P, McDonald C, Lakey JR, Shapiro AM. Transplantation. 2003;76:1280–1284. doi: 10.1097/01.TP.0000098822.85924.4C. [DOI] [PubMed] [Google Scholar]
- 26.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, et al. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 27.Chapman TM, Perry CM. Drugs. 2004;64:2577–2595. doi: 10.2165/00003495-200464220-00008. [DOI] [PubMed] [Google Scholar]
- 28.Vague P, Selam JL, Skeie S, De Leeuw I, Elte JW, Haahr H, Kristensen A, Draeger E. Diabetes Care. 2003;26:590–596. doi: 10.2337/diacare.26.3.590. [DOI] [PubMed] [Google Scholar]
- 29.Ling Z, Pipeleers DG. J Clin Invest. 1996;98:2805–2812. doi: 10.1172/JCI119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Workgroup on Hypoglycemia, American Diabetes Association. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 31.Cryer PE, Fisher JN, Shamoon H. Diabetes Care. 1994;17:734–755. doi: 10.2337/diacare.17.7.734. [DOI] [PubMed] [Google Scholar]
- 32.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Lancet. 1994;344:283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 33.Benhamou PY, Oberholzer J, Toso C, Kessler L, Penfornis A, Bayle F, Thivolet C, Martin X, Ris F, Badet L, et al. Diabetologia. 2001;44:859–864. doi: 10.1007/s001250100571. [DOI] [PubMed] [Google Scholar]
- 34.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, et al. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.