Abstract
Lipoprotein lipase (LPL) has a central role in lipoprotein metabolism to maintain normal lipoprotein levels in blood and, through tissue specific regulation of its activity, to determine when and in what tissues triglycerides are unloaded. Recent data indicate that angiopoietin-like protein (Angptl)-4 inhibits LPL and retards lipoprotein catabolism. We demonstrate here that the N-terminal coiled-coil domain of Angptl-4 binds transiently to LPL and that the interaction results in conversion of the enzyme from catalytically active dimers to inactive, but still folded, monomers with decreased affinity for heparin. Inactivation occurred with less than equimolar ratios of Angptl-4 to LPL, was strongly temperature-dependent, and did not consume the Angptl-4. Furthermore, we show that Angptl-4 mRNA in rat adipose tissue turns over rapidly and that changes in the Angptl-4 mRNA abundance are inversely correlated to LPL activity, both during the fed-to-fasted and fasted-to-fed transitions. We conclude that Angptl-4 is a fasting-induced controller of LPL in adipose tissue, acting extracellularly on the native conformation in an unusual fashion, like an unfolding molecular chaperone.
Keywords: chaperone, heparin affinity, protein folding, surface plasmon resonance
Lipoprotein lipase (LPL) is responsible for hydrolysis of triglycerides in plasma lipoproteins, a process generating fatty acids for storage or energy production (for reviews, see refs. 1–3). LPL activity is regulated in a tissue-specific manner according to metabolic demands, important not only for maintaining correct levels of lipoproteins in blood but also for balancing their catabolism so that the right amount of fatty acids is delivered to the right tissue at the right time. LPL is synthesized in many cell types, but mainly in adipocytes and myocytes. The enzyme is secreted and transported to the lumenal surface of blood vessels, where it is anchored by heparan sulfate (HS) proteoglycans. The active form of the enzyme is a noncovalent dimer, composed of two identical subunits (4). Earlier studies indicate that modulation of adipose tissue LPL by the nutritional state is mainly posttranslational (5, 6), with no significant changes in LPL protein mass (7). The mechanism appears to involve changes in the proportion of active to inactive enzyme molecules (7–9).
The angiopoietin-like (Angptl) protein family currently has six members (10, 11). They share a structural motif with the angiopoietins, with an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain, but the Angptls do not bind to Tie receptors. The Angptls are usually oligomeric, and they can be cleaved in vivo to release the two domains (12, 13). Both full-length and cleaved forms are present in plasma (12). At least three of the Angptls have pronounced effects on energy metabolism (10). Of these, Angptl-3 and -4 have been shown to inhibit LPL activity and increase plasma triglycerides (11). These effects are linked to the N-terminal coiled-coil domain (12). Knockout of the gene for either Angptl-3 or -4 in mice leads to activation of the LPL system, as evidenced by increased levels of LPL in plasma after heparin is administered and decreased levels of triglycerides in plasma (14). Overexpression of either Angptl leads to decreased LPL activity and increased plasma triglycerides (14). Tissue-specific overexpression of Angptl-4 in the heart leads to decreased LPL activity and impaired utilization of lipoprotein triglycerides in the heart (15). Inhibition of LPL has been demonstrated in vitro with both Angptl-3 (16) and Angptl-4 (14).
Down-regulation of LPL activity in adipose tissue during fasting requires transcription of a gene separate from the lipase gene (17). Angptl-4 is expressed in adipose tissue, and the expression increases during fasting (18), making Angptl-4 a prime candidate for control of LPL. We have therefore studied the interaction of the coiled-coil domain of Angptl-4 with LPL and find that the inhibition occurs by an unusual mechanism in which the Angptl-4 catalyzes a conformational switch in the LPL molecule from active dimers to catalytically inactive monomers. We have explored whether the changes in Angptl-4 expression occur on the same time scale as the previously known rapid changes of LPL activity on feeding–fasting transitions, and this turned out to be the case.
Results
Expression, Purification, and Characterization of ccd-Angptl-4.
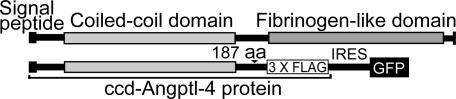
A schematic view of Angptl-4 and of the plasmid containing the coding sequence for the coiled-coil domain of murine Angptl-4 (pIRES-ccd-Angptl-4, amino acids 1–187) is shown in Fig. 1. This plasmid, as well as the empty pIRES-hrGFPII vector, was transiently transfected into 293T cells. Preliminary experiments, shown in Fig. 8a–c, which is published as supporting information on the PNAS web site, demonstrated that conditioned medium containing ccd-Angptl-4 inhibited LPL from two different sources (bovine milk and murine 3T3-L1 cells) and that the inhibition was not due to proteolytic cleavage of the lipase protein.
Fig. 1.
A schematic view of the plasmid used to express the coiled-coil domain of murine Angptl-4. (Upper) Overview of the structure of angiopoietin-like proteins. (Lower) The pIRES-hrGFPII expression vector containing the coding sequences for the coiled-coil domain of Angptl-4 (ccd-Angptl-4, residues 1–187) fused at the 3′ end with a 3× FLAG-tag sequence.
ccd-Angptl-4 was purified as described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. The purified protein gave a single band on SDS/PAGE under reducing conditions (Fig. 9a, which is published as supporting information on the PNAS web site). Western blotting with anti-FLAG antibodies of reduced and nonreduced samples showed that the preparation contained a mixture of monomers, disulfide-linked trimers, and higher oligomers (Fig. 9b. Other studies (see below) showed that ccd-Angptl-4 bound to heparin-Sepharose and was eluted at ≈0.4 M NaCl. This property was used for concentration of ccd-Angptl-4 from the expression media.
Effects of Stoichiometry Between Purified ccd-Angptl-4 and LPL.
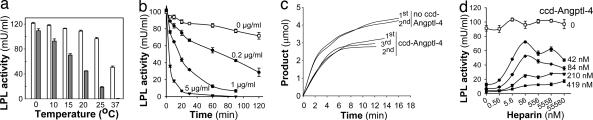
Incubation of LPL with purified ccd-Angptl-4 at a weight ratio of 1:13 caused a temperature-dependent loss of catalytic activity (Fig. 2a). After 1 hour at 37°C, the LPL activity was almost completely lost. At 25°C, ≈20% of the activity remained, whereas, at 0°C, there was only a small reduction of activity. At 37°C, there was significant loss of LPL activity also in samples without ccd-Angptl-4, whereas, at or below 25°C, most of the activity remained. To estimate the affinity and the stoichiometry of the reaction, LPL was incubated without and with three different concentrations of ccd-Angptl-4 (0.2, 1.0, and 5.0 μg/ml), corresponding to molar ratios of 2.6, 13, and 64 of ccd-Angptl-4 monomers to LPL dimers (Fig. 2b). The loss of activity was more rapid with higher concentrations of ccd-Angptl-4, but the effect was substantial already at the lowest concentration. Because our preparation contained a mixture of monomers, trimers, and higher oligomers of ccd-Angptl-4, the molar concentrations calculated from the monomer molecular weight are overestimations. Our experiments therefore demonstrate that significant inactivation occurred at less than equimolar ratios of ccd-Angptl-4 species to LPL dimers and indicate high affinity between ccd-Angptl-4 and LPL.
Fig. 2.
Inhibition of LPL by purified ccd-Angptl-4 depended on temperature and concentration, but the ccd-Angptl-4 was not consumed, and the inhibition was not relieved by heparin. (a) LPL (350 ng/ml) was incubated at the indicated temperatures with (filled bars) or without (open bars) ccd-Angptl-4 (1 μg/ml) in 20 mM Tris·Cl/0.15 M NaCl, pH 7.4, in the presence of 10% FCS. After 1 h, the remaining LPL activity was determined. (b) LPL was incubated without or with the indicated concentrations of ccd-Angptl-4 at 25°C under the same conditions as in a. Samples from the mixtures were analyzed for remaining LPL activity at the indicated times. (c) Inactivation was studied in real time by following hydrolysis of triacetin (in 0.15 M NaCl, pH 7.4) by LPL (6 μg/ml). The released acetic acid was titrated with NaOH by using a pH-stat. The spontaneous hydrolysis of triacetin (without addition of LPL) was subtracted. Upper curves show LPL without ccd-Angptl-4; lower curves show LPL in the presence of ccd-Angptl-4. The molar ratio of ccd-Angptl-4 (calculated from the monomer molecular mass) to LPL dimers was ≈1:1 for the first recording (1st). After LPL had been fully inactivated, a second addition of the same amount of active LPL was added to the reactions (2nd), and the progress of hydrolysis was again followed with time. A third (3rd) addition of LPL was made to the reaction vessel with ccd-Angptl-4. (d) LPL (4 nM) was incubated without or with the indicated concentrations of ccd-Angptl-4 at 25°C under the same conditions as in a, in the presence of the indicated concentrations of heparin.
In the next experiment, we studied the inactivation of LPL in real time. For this experiment, we used the nearly soluble substrate triacetin in 0.15 M NaCl, with no other additions. The released acetic acid was titrated with NaOH by using a pH-stat. The reaction rate decreased with time, demonstrating that LPL was not fully stable under these conditions even without ccd-Angptl-4 (Fig. 2c). ccd-Angptl-4 at a very low concentration (molar ratio of ccd-Angptl-4, calculated from the monomer molecular mass, to LPL dimers was ≈1:1) increased the bend of the titration curve, indicating that the loss of LPL activity was accelerated. This acceleration was even more pronounced at higher molar ratios of ccd-Angptl-4 to LPL, demonstrating a similar concentration dependency as in Fig. 2b (data not shown). The experiments showed that the inactivation of LPL by ccd-Angptl-4 started immediately and did not depend on other factors, for instance serum, apolipoprotein CII, or heparin, which are normally present in assays for LPL. To investigate whether ccd-Angptl-4 was consumed, a second addition of an equal amount of active LPL was made to the reaction when most of the initial LPL activity was gone (Fig. 2c). The shape of the curve was similar to that after the first addition of LPL, indicating that ccd-Angptl-4 had a catalytic effect on the inactivation process. The same result was obtained after a third addition of active enzyme (Fig. 2c). Heparin had effects on the inactivation, but not even a 100-fold molar excess of heparin over LPL could fully prevent the inactivation at any of the concentrations of ccd-Angptl-4 used (Fig. 2d).
Influence of ccd-Angptl-4 on Heparin Affinity and Dimer Structure of LPL.
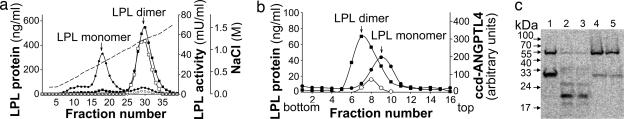
To investigate whether exposure to ccd-Angptl-4 caused a change in the heparin affinity of LPL, we incubated 125I-labeled, active LPL with ccd-Angptl-4 to almost complete inactivation (8.5% of the LPL activity remained at the end of the incubation). When this sample was separated on a heparin-Sepharose column, most of the LPL protein eluted in the position characteristic for LPL monomers, at 0.6 M NaCl (Fig. 3a). Some LPL eluted even earlier in the gradient and probably represented aggregates of inactive LPL monomers. Incubation of LPL without ccd-Angptl-4 under the same conditions did not result in loss of activity, and most of the LPL protein eluted in the position characteristic for active dimers. (Fig. 3a). ccd-Angptl-4 eluted early in the gradient, at ≈0.4 M NaCl (see legend, Fig. 3a). To more directly investigate the monomer/dimer or higher oligomer status of LPL after inactivation, we used sucrose-density gradient ultracentrifugation. The sedimentation pattern for LPL, which had been almost fully inactivated by incubation with ccd-Angptl-4, was compared with the patterns for LPL incubated without ccd-Angptl-4 and the pattern for a reference mixture of active, dimeric LPL and inactive LPL monomers (Fig. 3b). The sample without the inhibitor sedimented, as expected, as LPL dimers. In contrast, ≈80% of the ccd-Angptl-4-inactivated LPL protein sedimented in a symmetrical peak corresponding to LPL monomers. The rest of the LPL protein was lost, probably because of aggregation and sedimentation to the bottom of the tube (Fig. 3b). Analyses of ccd-Angptl-4 in the fractions, by SDS/PAGE and Western blots, showed that most of the ccd-Angptl-4 protein sedimented in a position between dimeric and monomeric LPL (Fig. 3b), corresponding to an approximate molecular mass of 70 kDa, presumably trimers. These results indicated that ccd-Angptl-4 did not remain bound to LPL after inactivation.
Fig. 3.
Inhibition of LPL by ccd-Angptl-4 involved conversion of active, dimeric LPL to inactive LPL monomers. (a) Partially inactivated LPL was separated by chromatography on heparin-Sepharose. For this experiment, a mixture of 200 ng of 125I-labeled and 1.8 μg of unlabeled LPL was incubated with 2.4 μg of ccd-Angptl-4 (circles) or buffer (20 mM Tris·Cl/0.15 M NaCl) (squares) for 30 min at 20°C in the presence of 10% delipidated FCS. The mixtures were then applied on a 1-ml Hi-Trap heparin-Sepharose column and eluted by a gradient of NaCl (dashed line). Fractions of 0.5 ml were collected, and LPL radioactivity (filled symbols) and LPL activity (open symbols) was measured. The positions of the peaks for a standard mixture of dimeric and monomeric LPL are indicated by arrows. ccd-Angptl-4, run in the absence of LPL and detected by Western blots with anti-FLAG antibodies, eluted at ≈0.4 M NaCl (data not shown). (b) Partially inactivated LPL was separated by sucrose-density gradient ultracentrifugation. For this experiment, 17.5 ng of 125I-labeled LPL and 900 ng of unlabeled LPL in 10% delipidated FCS were incubated for 30 min at 20°C with 2.5 μg of ccd-Angptl-4 (filled circles) or buffer (20 mM Tris·Cl/0.15 M NaCl) (filled squares) in a total volume of 0.5 ml. Then 0.2 ml of each sample was applied to linear sucrose-density gradients. The curves represent mean values from two tubes run in parallel for each sample. The profile for sedimentation of ccd-Angptl-4 was determined by scanning of a Western blot of samples from the fractions visualized by an anti-FLAG antibody (open circles). (c) The conformation of partially inactivated LPL was probed by limited tryptic digestion. For this experiment, samples of 125I-labeled LPL dimers and monomers isolated by heparin-Sepharose chromatography after thermal inactivation at 37°C or inactivation by treatment with ccd-Angptl-4 (as in a) were incubated for 15 min with trypsin and were then analyzed by SDS/PAGE under reducing conditions. The bands were detected by imaging. Lane 1 is a reference sample of active dimeric LPL treated with trypsin. Lane 2 is the peak of inactive LPL (first peak in a) treated with trypsin. Lane 4 is the peak of active dimeric LPL (second peak in a) treated with trypsin. Lanes 3 and 5 are analogous to lanes 2 and 4, but, in this case, LPL was partially inactivated by thermal treatment before separation on heparin-Sepharose and trypsin treatment.
Conformational Changes in LPL upon Inactivation with ccd-Angptl-4 Detected by Limited Proteolysis.
We used limited proteolysis with trypsin to compare the extent of conformational changes in LPL monomers induced by ccd-Angptl-4 to those found on dissociation of LPL to monomers by other methods (treatment with guanidinium chloride or elevated temperature). Active LPL is cleaved in the middle by trypsin, generating fragments of ≈30 kDa (19). The experiment in Fig. 3c demonstrated that the cleavage pattern of LPL, inactivated by ccd-Angptl-4 and isolated by heparin-Sepharose chromatography (lane 2), was similar to that for LPL monomers produced by thermal dissociation (lane 3), with a major band at ≈20 kDa. Remaining active LPL, eluting at higher salt concentration from the column (lanes 4 and 5), was cleaved in the middle like the starting material (lane 1).
Direct Studies of the Interaction Using Surface Plasmon Resonance.
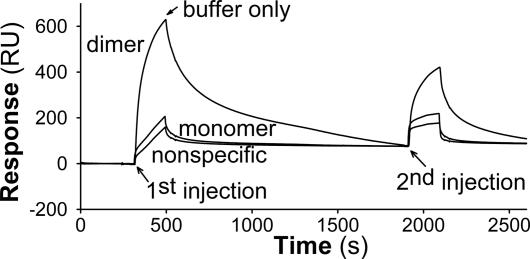
For this experiment, biotinylated LPL was immobilized to streptavidin-derivatized sensor chips, and ccd-Angptl-4 was injected into the flow cells (Fig. 4). There was avid binding of ccd-Angptl-4 to active LPL, as indicated by the rapid rise of response with time. When the flow was changed to buffer only, the bound ccd-Angptl-4 dissociated, as demonstrated by the decrease in response. A second injection of ccd-Angptl-4 over the same flow cells resulted in lower binding of ccd-Angptl-4 to the sensor chip than on the first injection, indicating that the material on the chip now had lower affinity for ccd-Angptl-4. Binding of ccd-Angptl-4 to chips with LPL monomers was only slightly higher than background binding (to empty streptavidin-derivatized chips) and was not much changed on the second injection (Fig. 4). These observations suggest that the first injection of ccd-Angptl-4 had induced changes in some of the LPL dimers, and, therefore, there was less binding of ccd-Angptl-4 to the sensor chip on the second injection. Experiments were also performed in the presence of BSA (2 mg/ml) in the running buffer, at 25°C and 4°C. BSA reduced the nonspecific binding, but, at both temperatures, binding to immobilized dimeric LPL was similar to that in Fig. 4. Interestingly, at 4°C, binding was similar on the second injection of ccd-Angptl-4, indicating that, at low temperature, binding did not cause immediate monomerization (data not shown).
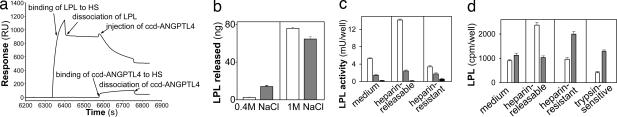
Fig. 4.
ccd-Angptl-4 bound with high affinity to dimeric, but less to monomeric, LPL as determined by surface plasmon resonance. Biotinylated dimeric and monomeric LPL were bound to streptavidin-coated sensor chips to a level of 5,100 response units (RU) and 5,300 RU, respectively. ccd-Angptl-4 (13.8 μg/ml) was injected over these two flow cells and over a flow cell that contained only streptavidin (1st injection). At the indicated time (arrow) the flow was changed to buffer without ccd-Angptl-4 to study dissociation of the ligand. Then a second injection of the same ccd-Angptl-4 solution was made (2nd injection). The buffer contained 20 mM Hepes and 0.15 M NaCl and the temperature was 25°C.
Effects of ccd-Angptl-4 on LPL Bound to Heparin/HS.
It is currently assumed that LPL carries out its physiological function while bound to oligosaccharide chains of endothelial HS proteoglycans (1, 3). We therefore questioned whether ccd-Angptl-4 could interact with LPL bound to heparin/HS and, if so, whether the interaction would lead to restructuring of the lipase. For this experiment, ccd-Angptl-4 was injected over flow cells containing dimeric LPL bound to immobilized HS. Binding of ccd-Angptl-4 to the dimeric LPL should result in an increase of the response. In contrast, a decrease in response was observed (Fig. 5a). This finding suggests that ccd-Angptl-4 caused dissociation of LPL from the immobilized HS. The effect was probably larger than can be seen from the response, because the results from a parallel flow cell with HS in the absence of LPL showed, as expected, that ccd-Angptl-4 itself bound to HS.
Fig. 5.
Binding of LPL to immobilized HS/heparin or to cell surfaces did not protect the enzyme from inactivation by ccd-Angptl-4. (a) Biotinylated HS was immobilized to two flow cells of a streptavidin-coated sensor chip. Dimeric LPL (50 μg/ml) was bound to one of these flow cells (upper curve). ccd-Angptl-4 (17 μg/ml) was simultaneously injected over the flow cell with already bound LPL and the flow cell with only HS (lower curve). The times for the injections and for the wash with running buffer (dissociation phase) are shown by arrows. (b) Heparin-Sepharose beads, preloaded with 125I-labeled LPL, were incubated with conditioned medium from 293T cells transfected with pIRES-hrGFPII vector (open bars) or pIRES-ccd-Angptl-4 (filled bars). The beads were then sequentially eluted with buffer containing 0.4 and 1 M NaCl. The amount of LPL released in each fraction was determined from the radioactivity that was recalculated to LPL mass (ng). The data are means of five parallel samples. (c and d) LPL activity (c) and LPL mass (d) in fractions obtained from 3T3-L1 preadipocytes loaded with 125I-labeled LPL and then incubated with (filled bars) or without (open bars) ccd-Angptl-4 for 1 h at 25°C. The results represent mean values of 6 wells for activity and 18 wells for radioactivity ± SD. One thousand cpm corresponded to 6.8 ng of LPL. Activity of endogenously produced LPL in cells incubated without [125I]LPL was also measured (filled bars). Surface exposure of the heparin-resistant radioactivity remaining with the cells was probed by brief digestion with trypsin and measurement of release into the medium. Less than 47 ± 8.7 cpm and 68 ± 14 cpm, respectively, was released by the buffer without trypsin.
In the next experiment, 125I-labeled LPL was allowed to bind to heparin-Sepharose beads, which were then incubated for 1 h with conditioned media from 293T cells transfected with pIRES-ccd-Angptl-4 or empty pIRES-hrGFPII vector. The beads were then rinsed and successively eluted with buffers containing 0.4 and 1.0 M NaCl, respectively. After exposure of the heparin-bound LPL to the medium containing ccd-Angptl-4, more of the lipase was eluted at the lower salt concentration and less at the higher salt concentration (Fig. 5b), indicating that the heparin affinity had been reduced for part of the heparin-bound LPL. Together with data from the surface plasmon resonance experiments described above, these results indicate that ccd-Angptl-4 was also able to dissociate LPL to monomers when the enzyme was bound to HS or to heparin.
Effects of ccd-Angptl-4 on LPL Bound to Cells.
We studied the effect of purified ccd-Angptl-4 on LPL bound to cells. For this experiment, 125I-labeled LPL was bound to 3T3-L1 preadipocytes at 25°C to reduce internalization. Because the cells were still preadipocytes, their endogenous production of active LPL was low compared with the exogenous LPL added (Fig. 5c, black bars). Incubation of the cells with medium containing ccd-Angptl-4 at 25°C caused a dramatic decline in LPL activity (Fig. 5c) both in the medium and in the cell-bound (heparin-releasable) fraction. In contrast, the level of LPL protein in the medium was higher with cells treated with ccd-Angptl-4 than with control cells (Fig. 5d). These results showed that ccd-Angptl-4 acted on the active, HS-bound pool of LPL, causing inactivation and, most likely, monomerization of the enzyme. There was a significant decrease of cell-associated radioactivity in the heparin-releasable fraction and an increase in the heparin-resistant fraction (Fig. 5d). Analysis of trypsin-sensitivity of the heparin-resistant fractions revealed that more radioactivity was released from cells incubated with ccd-Angptl-4 compared with control cells. This indicated that the main part of the inactivated LPL remained on the cell surface and was not internalized.
Correlations Between Changes in Angptl-4 mRNA and LPL Activity in Rat Adipose Tissue.
Finally, to explore whether Angptl-4 mRNA levels in adipose tissue can change rapidly enough to account for the known rapid modulation of LPL activity in adipose tissue (2, 17), two in vivo experiments were carried out. In one experiment (Fig. 6a–c), food was removed from fed rats. In the other experiment (Fig. 6d–f), food was returned to fasted rats. In both cases, the time was 6 h. We included groups of rats that were given actinomycin D to block transcription, resulting in a reduction of the Angptl-4 mRNA abundance in epididymal adipose tissue by >65% in fed (Fig. 6a) and >85% in fasted rats (Fig. 6d), indicating that the turnover time for this mRNA was rapid. LPL activity in tissue homogenates increased after actinomycin injection (75% in fed and ≈300% in fasted rats). LPL mRNA abundance (Fig. 6b and e) did not change significantly, in accord with earlier observations that this mRNA turns over relatively slowly (17). These results show that, when the block of transcription caused Angptl-4 mRNA to decrease, LPL activity increased.
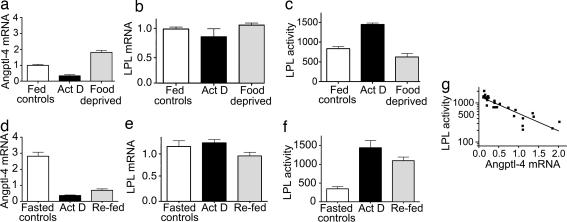
Fig. 6.
The levels of Angptl-4 mRNA in adipose tissue changed rapidly with nutritional state. (a and d) Angptl-4 mRNA abundance. (b and e) LPL mRNA abundance. (c and f) LPL activity. (a–c) The fed-to-fasted transition. For this experiment, food was removed from two groups of rats in the morning. The rats in one of these groups were given an i.p. injection of Actinomycin D (2 mg/kg of body weight). The rats were killed 6 h later. (d–f) The same parameters for the fasted-to-fed transition. For this experiment, food was removed from the rats at 4 p.m. the day before the experiment. The next morning, food was returned to two groups of rats. The rats in one of these groups were given an i.p. injection of Actinomycin D. The rats were killed 6 h later. There were three to six rats in each group. Data for LPL activity are shown as mean ± SEM. Data for expression of Angptl-4 and LPL mRNA are shown as mean ± SD. (g) The relation between Angptl-4 mRNA abundance and LPL activity for all individual rats. Note that the y axis is logarithmic. Correlation analysis for the log of LPL activity vs. Angptl mRNA abundance returned P < 0.0001, Pearson r = −0.88.
The nutritional transitions also caused rapid changes of Angptl-4 mRNA abundance, which increased by ≈80% in the fed-to-fasted transition (Fig. 6a) and decreased by ≈75% in the fasted-to-fed transition (Fig. 6d). LPL mRNA abundance (Fig. 6b and e) did not change significantly, whereas LPL activity changed in the expected directions. The activity decreased in the fed-to-fasted transition, although this decrease did not reach statistical significance, presumably because of the short time for food withdrawal (6 h). LPL activity increased by >200% in the fasted-to-fed transition.
These results are in concert with the hypothesis that Angptl-4 suppresses LPL activity by a conformational switch mechanism and that changes in the expression of Angptl-4 modulate adipose tissue LPL activity in response to changes in nutritional state. To further explore this hypothesis, we plotted the LPL activity against the Angptl-4 mRNA abundance for all individual rats in the experiments (Fig. 6g). There was a highly significant (P < 0.0001), inverse (r = −0.88) correlation of log LPL activity to Angptl-4 mRNA over the entire span of data, irrespective of whether they came from feeding–fasting transitions or from blockade of transcription.
Discussion
We demonstrate here that the N-terminal coiled-coil domain of Angptl-4 binds to LPL and converts the catalytically active, dimeric form of the enzyme to catalytically inactive monomers. It is known from many studies that LPL is unstable (4). Folding of LPL to the active form during biosynthesis requires support from molecular chaperones, such as calnexin and calreticulin, in the endoplasmatic reticulum (20). Recent studies have shown that there is rapid exchange of subunits between LPL dimers (21) and that the intermediate monomer is metastable under in vivo conditions. Our data suggest that Angptl-4 catalyzes a conformational switch from active to inactive LPL. More research is needed to understand this complex system, but it appears that active LPL is a spring-loaded molecule and that Angptls can unleash the spring to control the activity of the enzyme in extracellular locations.
The affinity of Angptl-4 for active LPL is relatively high because binding was already observed at low concentrations of ccd-Angptl-4. Studies in real time, using a soluble substrate, showed that nearly equimolar amounts of ccd-Angptl-4 caused an immediate increase of the inactivation rate for LPL. Separation of active and inactive forms of LPL by chromatography on heparin-Sepharose and by sucrose-density gradient ultracentrifugation demonstrated that the loss of activity involved formation of LPL monomers. These monomers behaved similarly to monomers produced by other methods, such as thermal inactivation or exposure to dissociating agents like urea or guanidinium chloride (21). This behavior included loss of catalytic activity, reduced affinity for heparin, and a similar cleavage pattern upon limited proteolysis with trypsin. Some conformational changes occur upon monomerization (4, 21), but the monomers are still folded, representing a relatively stable form of LPL that is slightly more flexible and exposes more hydrophobic areas compared with the dimeric form (21). The data from surface plasmon resonance and sucrose gradients indicate that ccd-Angptl-4 did not remain bound to the inactive LPL monomer. On direct comparison of binding by surface plasmon resonance the monomers displayed much lower affinity for ccd-Angptl-4 than did the active LPL dimers. Upon repeated additions of active LPL to an equimolar amount of ccd-Angptl-4 (calculated on the basis of the monomer molecular mass, an overestimation), inactivation of the enzyme occurred as efficiently in the second and third round as in the first. Our data therefore support a model where ccd-Angptl-4 has a catalytic effect, promoting inactivation of LPL, but without being itself firmly bound to LPL or consumed during the process. At 4°C, binding still occurred, but monomerization was slower.
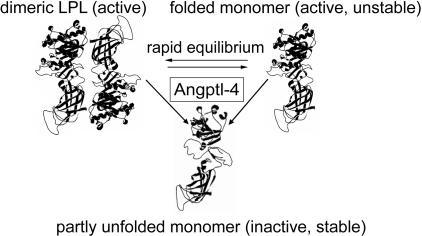
Fig. 7 is a schematic overview of the possible pathways of the inactivation. We cannot determine whether Angptl-4 binds to the LPL dimers as such or to the active monomers that are intermediates in the subunit exchange between LPL dimers (21), because these intermediates cannot be isolated. An important feature of the interaction is, however, that ccd-Angptl-4 does not remain bound to LPL, but is free to inhibit new molecules of the enzyme. Hence, the action of Angptl-4 in the system is that of a catalyst.
Fig. 7.
Schematic illustration of possible mechanisms for action of Angptl-4 on LPL. The active form of LPL is a noncovalent dimer with propensity to dissociate to inactive, but still folded, monomers. Kinetic analyses have shown that inactivation most likely involves a short-lived intermediate active monomer (21, 23). It is possible that Angptl-4 interacts preferentially with this intermediate state and thereby drives LPL from the active dimer to inactive monomers. It is also possible that Angptl-4 interacts with the active LPL dimers and promotes their dissociation to inactive monomers or dissociation to the active intermediates, which, in turn, refold to inactive monomers. The schematic representation of the LPL subunit is based on the model generated by van Tilbeurgh et al. (24).
It is clear from the experiments with LPL bound to cells, heparin-Sepharose beads, and surface plasmon resonance sensor chips covered with heparin, that Angptl-4 can inactivate LPL when the enzyme is bound to heparin/HS. An interesting observation was that ccd-Angptl-4 itself bound to heparin. A heparin-binding consensus sequence had been reported in Angptl-3 (12), but this sequence is absent in Angptl-4 (13). Considering LPL bound to HS proteoglycans on the surface of endothelial cells, the heparin affinity of Angptl-4 could localize the inhibitor to the neighborhood of LPL. It is not known how Angptl-4 is distributed within the adipose tissue or what the contribution of Angptl-4 or -3 from other organs may be for regulation of LPL activity in adipose tissue. The down-regulation of LPL activity on fasting appears to occur extracellularly, whereas the intracellular LPL activity is not changed (9). It is therefore likely that Angptl-4 and LPL meet in the subendothelial space and/or at the endothelium, resulting in inactivation.
Angptl-4 is present in several LPL-rich tissues, and its expression is activated by ligands of all peroxisome proliferator-activated receptors. This finding is in line with the hypothesis that Angptl-4 may be involved in the modulation of LPL activity in response to changes in nutritional state (11). For this result, the time frame for changes in Angptl-4 expression must match the rapid changes that occur in adipose tissue LPL activity during feeding–fasting transitions (2, 17). Our data from the in vivo experiments show that this is the case. When transcription was blocked by actinomycin D, Angptl-4 mRNA abundance rapidly decreased, and LPL activity increased. When fed rats were deprived of food, Angptl-4 mRNA increased, and LPL activity decreased. When food was returned to fasted rats, Angptl-4 mRNA decreased by 75% in 6 h, whereas LPL activity increased by >200%. These data show that Angptl-4 expression in adipose tissue responds rapidly and can act as a prime controller of the activity of LPL. The picture that emerges is unusual and interesting. LPL is an extracellular enzyme that is produced and released at relatively constant rates, but its activity is rapidly modulated according to physiological demands by a mechanism whereby Angptl-4 can render the enzyme inactive. This modulation occurs by direct, but transient, interaction, similar to the action of molecular chaperones, and causes a conformational switch in LPL that is practically irreversible.
Methods
Details of reagents, cell cultures, expression, and purification of the coiled-coil domain of murine Angptl-4, measurement of LPL activity, measurement of Angptl-4 and LPL mRNA, sucrose-density gradient centrifugation, effects of ccd-Angptl-4 on dimeric LPL bound to heparin-Sepharose beads or on LPL bound to cells, and of conditions for animal experiments are given in Supporting Materials and Methods.
Chromatography on Heparin-Sepharose.
Heparin-Sepharose chromatography was carried out on an ÄKTA-purifier system (GE Healthcare, Buckinghamshire, U.K.). Briefly, a HiTrap Heparin HP 1-ml column was equilibrated with 20 mM Tris·Cl, 0.15 M NaCl, and 20% glycerol. After loading of samples, the column was washed with equilibration buffer and eluted by a linear gradient of NaCl (from 0.15 to 2.0 M) in the same buffer.
Limited Proteolysis of LPL by Trypsin.
Treatment of LPL with trypsin was carried out at 4°C in 20 mM Tris·Cl, 0.4 M NaCl, and 20% glycerol. To stop the reaction, SDS was added to a final concentration of 2%, and the samples were immediately heated at 95°C. After reduction with β-mercaptoethanol, the samples were subjected to SDS/PAGE.
Surface Plasmon Resonance.
Binding studies were performed by surface plasmon resonance on a BIAcore 2000 instrument, using sensor chips CM5 (Biacore, Uppsala, Sweden). The experiments were carried out at 25°C and at 4°C in 10 mM Hepes buffer, pH 7.4, containing 0.15 M NaCl, as described, with biotinylated LPL bound to streptavidin-coated chips or with LPL on chips with biotinylated heparin bound to streptavidin (22). For these studies, ccd-Angptl-4 was dialyzed overnight against the running buffer. Some experiments were run in the presence of 2 mg of BSA per milliliter. Binding is expressed in response units.
Supplementary Material
Acknowledgments
We thank Dr. Elena Makoveichuk for helpful discussions. This work was supported by Swedish Science Council Grants 727 and 12203 and grants from King Gustaf V's and Queen Victoria's research fund and the Kempe Research Foundation.
Abbreviations
- Angptl
angiopoietin-like protein
- ccd-Angptl
coiled-coil domain of Angptl
- LPL
lipoprotein lipase
- HS
heparan sulfate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Olivecrona T, Olivecrona G. In: Lipoproteins in Health and Disease. Betteridge DJ, Illingworth DR, Shepherd J, editors. London: Arnold; 1999. pp. 223–246. [Google Scholar]
- 2.Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Curr Opin Lipidol. 2002;13:471–481. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 4.Osborne JC, Jr, Bengtsson-Olivecrona G, Lee NS, Olivecrona T. Biochemistry. 1985;24:5606–5611. doi: 10.1021/bi00341a048. [DOI] [PubMed] [Google Scholar]
- 5.Enerbäck S, Semb H, Tavernier J, Bjursell G, Olivecrona T. Gene. 1988;64:97–106. doi: 10.1016/0378-1119(88)90484-2. [DOI] [PubMed] [Google Scholar]
- 6.Doolittle MH, Ben-Zeev O, Elovson J, Martin D, Kirchgessner TG. J Biol Chem. 1990;265:4570–4577. [PubMed] [Google Scholar]
- 7.Bergö M, Olivecrona G, Olivecrona T. Biochem J. 1996;313:893–898. doi: 10.1042/bj3130893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G, Brouckaert P, Olivecrona T. Am J Physiol Endocrinol Metab. 2004;286:E711–E717. doi: 10.1152/ajpendo.00257.2003. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Olivecrona G, Olivecrona T. J Biol Chem. 2003;278:11925–11930. doi: 10.1074/jbc.M212736200. [DOI] [PubMed] [Google Scholar]
- 10.Oike Y, Akao M, Kubota Y, Suda T. Trends Mol Med. 2005;11:473–479. doi: 10.1016/j.molmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Li C. Curr Opin Lipidol. 2006;17:152–156. doi: 10.1097/01.mol.0000217896.67444.05. [DOI] [PubMed] [Google Scholar]
- 12.Ono M, Shimizugawa T, Shimamura M, Yoshida K, Noji-Sakikawa C, Ando Y, Koishi R, Furukawa H. J Biol Chem. 2003;278:41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 13.Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. J Biol Chem. 2004;279:2038–2045. doi: 10.1074/jbc.M307583200. [DOI] [PubMed] [Google Scholar]
- 14.Koster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, et al. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Proc Natl Acad Sci USA. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizugawa T, Ono M, Shimamur M, Yoshida K, Ando Y, Koishi R, Ueda K, Inaba T, Minekura H, Kohama T, et al. J Biol Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 17.Bergö M, Wu G, Ruge T, Olivecrona T. J Biol Chem. 2002;277:11927–11932. doi: 10.1074/jbc.M200325200. [DOI] [PubMed] [Google Scholar]
- 18.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Mol Cell Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson-Olivecrona G, Olivecrona T, Jörnvall H. Eur J Biochem. 1986;161:281–288. doi: 10.1111/j.1432-1033.1986.tb10444.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wu G, Tate CG, Lookene A, Olivecrona G. J Biol Chem. 2003;278:29344–29351. doi: 10.1074/jbc.M300455200. [DOI] [PubMed] [Google Scholar]
- 21.Lookene A, Zhang L, Hultin M, Olivecrona G. J Biol Chem. 2004;279:49964–49972. doi: 10.1074/jbc.M407419200. [DOI] [PubMed] [Google Scholar]
- 22.Lookene A, Chevreuil O, Ostergaard P, Olivecrona G. Biochemistry. 1996;35:12155–12163. doi: 10.1021/bi960008e. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Lookene A, Wu G, Olivecrona G. J Biol Chem. 2005;280:42580–42591. doi: 10.1074/jbc.M507252200. [DOI] [PubMed] [Google Scholar]
- 24.van Tilbeurgh H, Roussel A, Lalouel J-M, Cambillau C. J Biol Chem. 1994;269:4626–4633. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.